Phenotypic Identification and Fine-Mapping of the Rice Narrow-Leaf Mutant nal25
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic and Agronomic Trait Analysis
2.3. Genomic DNA Extraction and PCR
2.4. Genetic Analysis of the nal25 Mutant
2.5. Fine-Mapping of the NAL25 Gene
2.6. Gene Expression Analysis of NAL25
3. Results
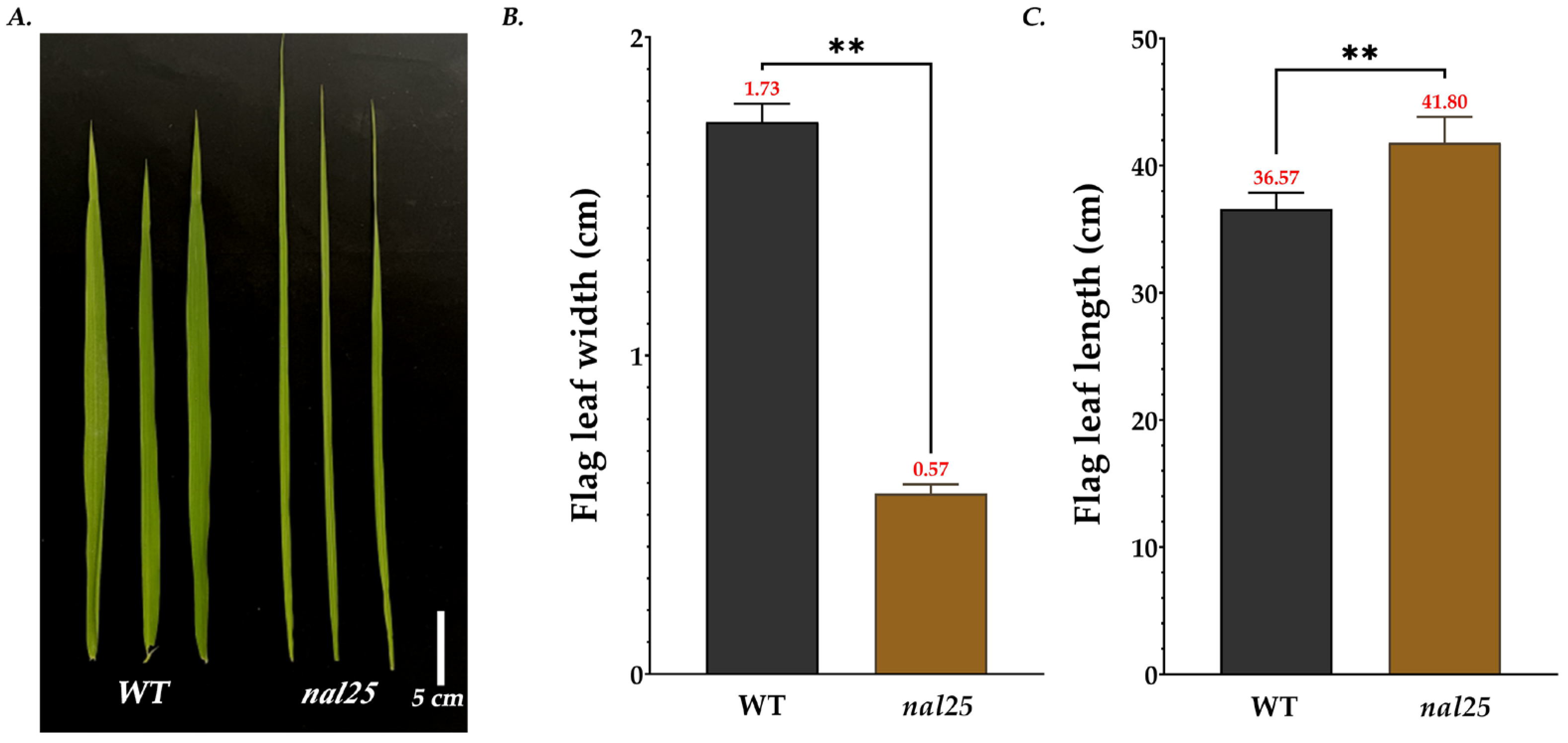
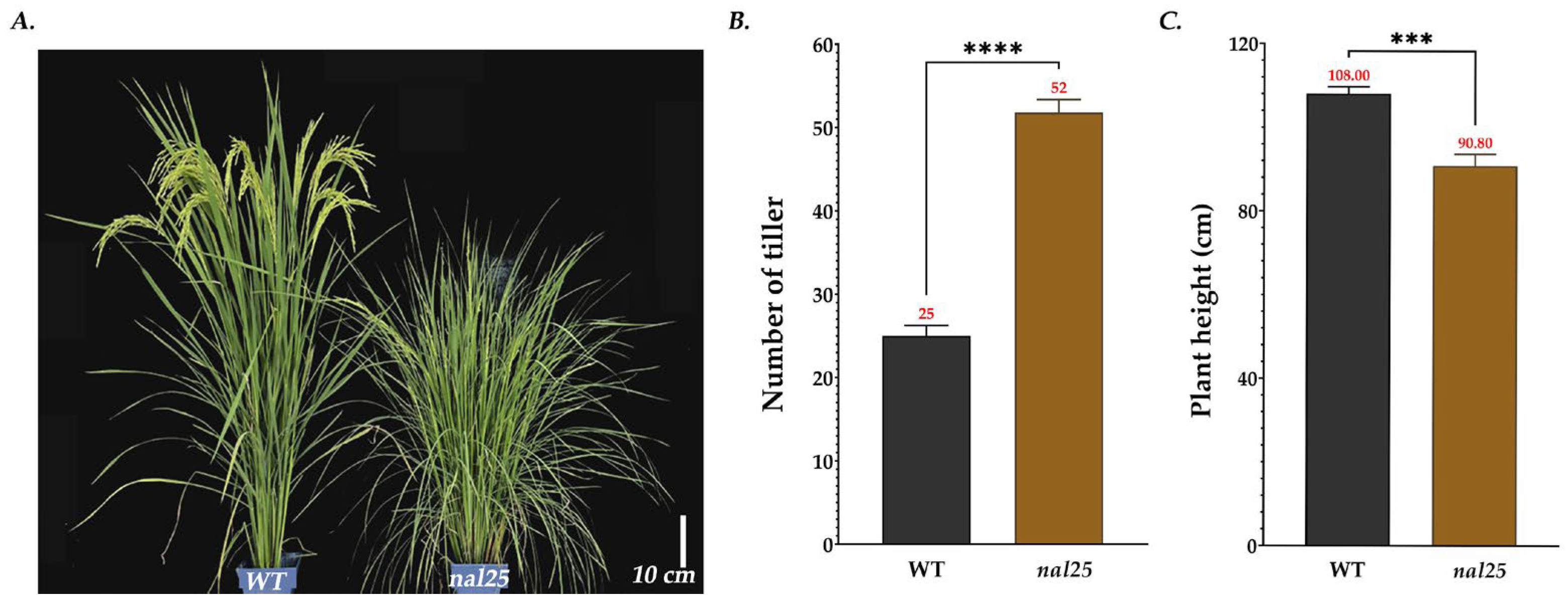
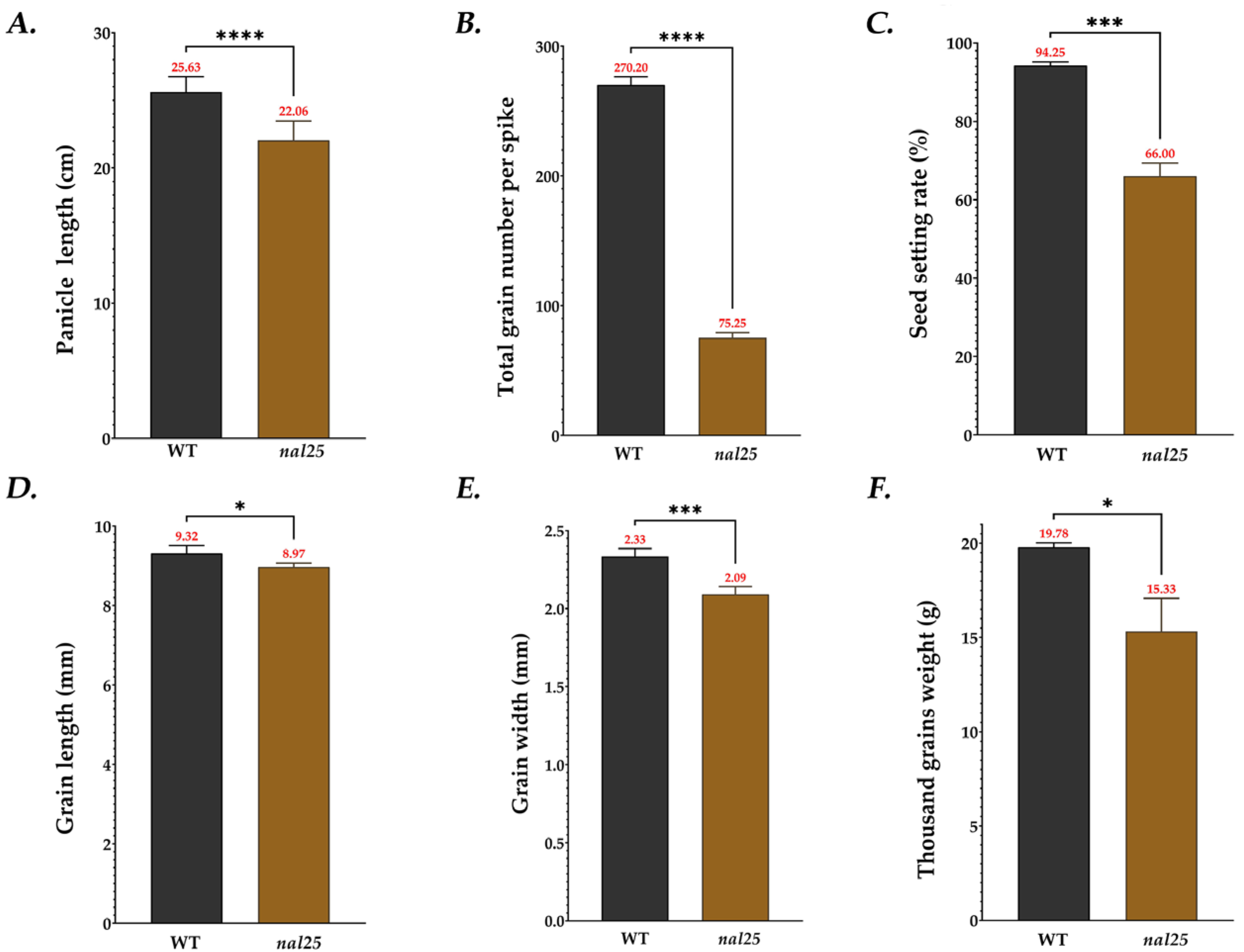
3.1. Agronomic Traits of the Narrow-Leaf Mutant nal25
3.2. Genetic Analysis of the Narrow-Leaf Mutant nal25
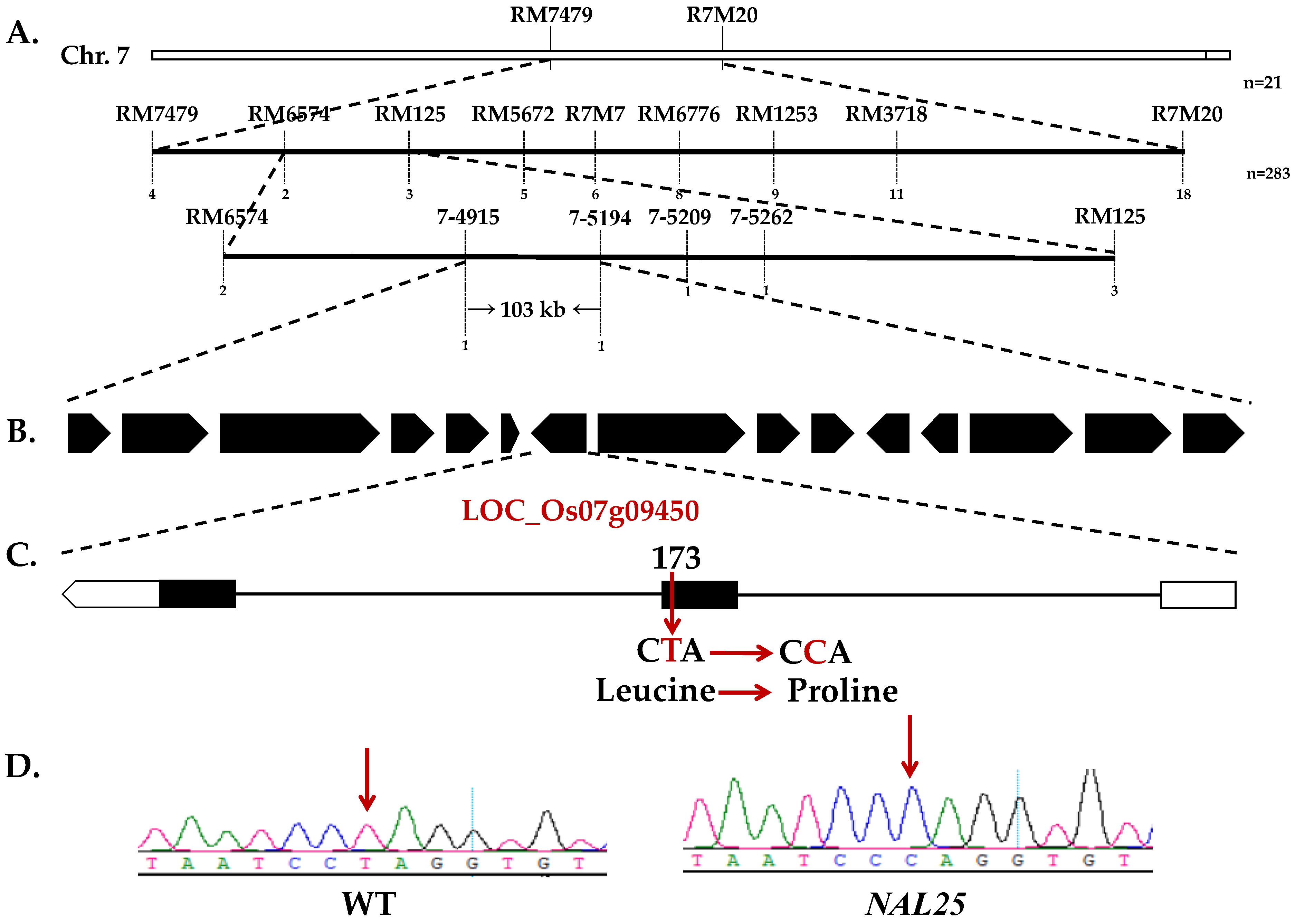
3.3. Fine-Mapping and Candidate Gene Prediction of NAL25
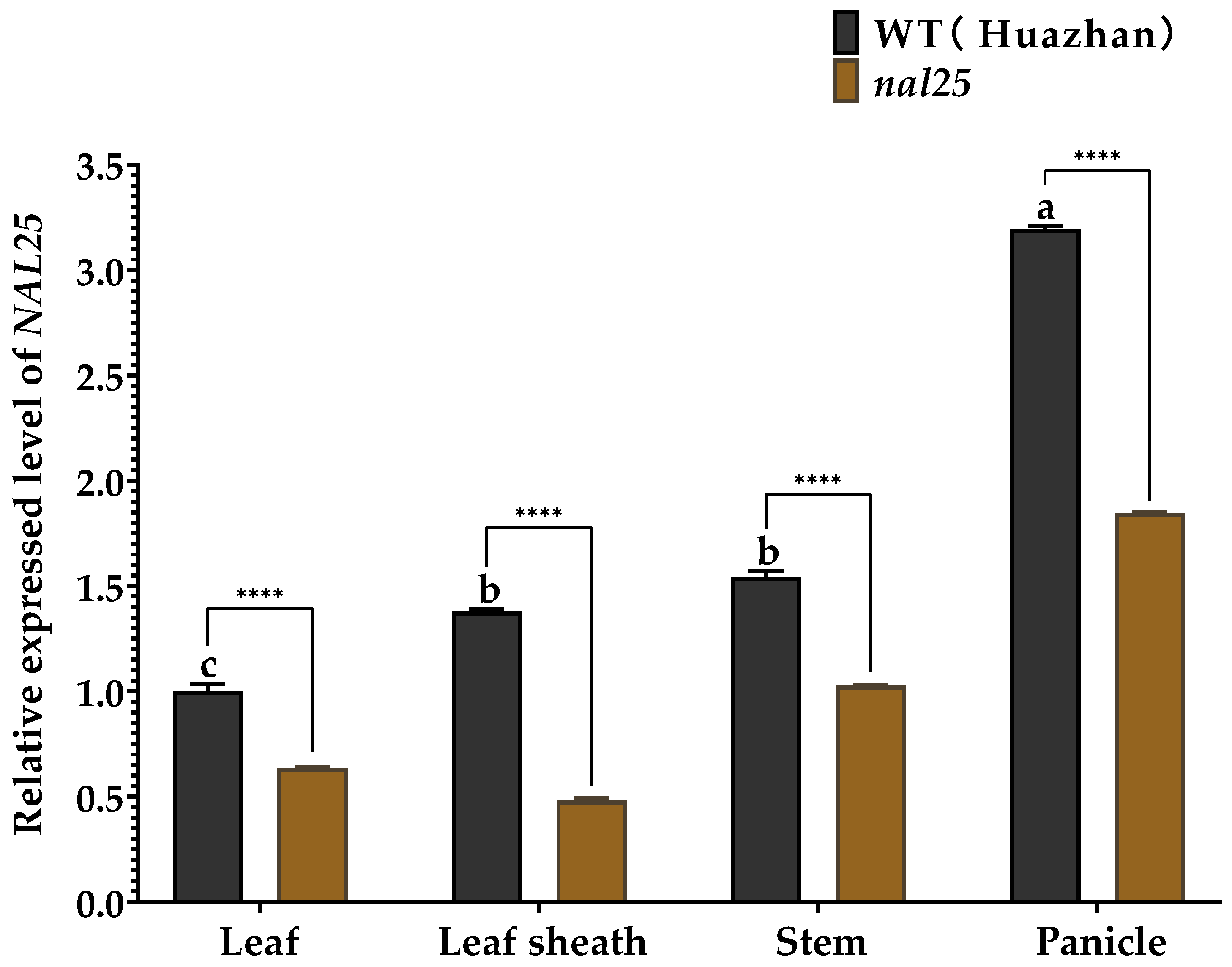
3.4. Expression Analysis of the NAL25 Gene
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Xiao, J.; Chen, L.; Huang, X.; Cheng, Z.; Han, B.; Zhang, Q.; Wu, C. Rice Functional Genomics Research: Past Decade and Future. Mol. Plant 2018, 11, 359–380. [Google Scholar] [CrossRef]
- Guo, T.; Yu, H.; Qiu, J.; Li, J.Y.; Han, B.; Lin, H.X. Advances in rice genetics and breeding by molecular design in China. Sci. Sin. Vitae 2019, 49, 1185–1212. [Google Scholar]
- Xu, N.; Xu, Q.; Xu, Z.J.; Chen, W.F. Research progress on physiological ecology and genetic basis of rice plant architecture. Acta Agron. Sin. 2023, 49, 1735–1746. [Google Scholar] [CrossRef]
- He, M.; Li, X.B.; Huang, J.; Huang, G.F. The Relationship Between Leaf Area Index and Yield of Rice: Research Progress. J. Agric. 2022, 12, 1–5. [Google Scholar]
- Liu, M.L.; Ye, S.H.; Zhang, P.; Yang, Y.L.; Zhai, R.R.; Ye, J.; Wu, M.M.; Zhu, G.F.; Zhang, X.M. Research Progress of Leaf Shape Related Regulation Genes in Rice. Mol. Plant Breed. 2022, 20, 7540–7549. [Google Scholar]
- Subudhi, P.K.; Garcia, R.S.; Coronejo, S.; Leon, T.B.D. A Novel Mutation of the NARROW LEAF 1 Gene Adversely Affects Plant Architecture in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 8106–8127. [Google Scholar] [CrossRef]
- Qi, J.; Qian, Q.; Bu, Q.; Li, S.; Chen, Q.; Sun, J.; Liang, W.; Zhou, Y.; Chu, C.; Li, X. Mutation of the Rice Narrow leaf1 Gene, Which Encodes a Novel Protein, Affects Vein Patterning and Polar Auxin Transport1. Plant Physiol. 2008, 147, 1947–1959. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoo, S.C.; Zhang, H. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013, 198, 1071–1084. [Google Scholar] [CrossRef]
- Fujino, K.; Matsuda, Y.; Ozawa, K.; Nishimura, T.; Koshiba, T.; Fraaije, M.W.; Sekiguchi, H. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol. Genet. Genom. 2008, 279, 499–507. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Chao, W.; Hu, G.; Li, X.; Qian, W.; Si, H.; Sun, Z.; Wang, X.; Fu, Y. Characterization and Fine Mapping of a Novel Rice Narrow Leaf Mutant nal9. J. Integr. Plant Biol. 2013, 55, 1016–1025. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, L.; Chen, L.; Tao, X.; Huang, M.; Wang, H.; Chen, Z.; Xiao, W. Chromosome mapping, molecular cloning and expression analysis of a novel gene response for leaf width in rice. Biochem. Biophys. Res. Commun. 2016, 480, 394–401. [Google Scholar] [CrossRef]
- Luo, L.X.; Xie, Y.L.; Yu, S.J.; Yang, J.; Chen, S.R.; Yuan, X.; Guo, T.; Wang, H.; Liu, Y.Z.; Chen, C.; et al. The DnaJ domain-containing heat-shock protein NAL11 determines plant architecture by mediating gibberellin homeostasis in rice (Oryza sativa). New Phytol. 2023, 237, 2163–2179. [Google Scholar] [CrossRef] [PubMed]
- Uzair, M.; Long, H.; Zafar, S.A.; Patil, S.B.; Chun, Y.; Li, L.; Fang, J.J.; Zhao, J.F.; Peng, L.X.; Yuan, S.J.; et al. Narrow Leaf21, encoding ribosomal protein RPS3A, controls leaf development in rice. Plant Physiol. 2021, 186, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Yan, S.Y.; Jiang, S.; Bai, L.; Liu, Y.C.; Peng, S.S.; Chen, R.B.; Liu, Q.; Xiao, Y.H.; Kang, H.X. Identification of a Rice Leaf Width Gene Narrow Leaf 22 (NAL22) through Genome-Wide Association Study and Gene Editing Technology. Int. J. Mol. Sci. 2023, 24, 4073–4089. [Google Scholar] [CrossRef]
- Wei, X.J.; Tang, S.Q.; Shao, G.N.; Chen, M.L.; Hu, Y.C.; Hu, P.S. Fine mapping and characterization of a novel dwarf and narrow-leaf mutant dnl1 in rice. Genet. Mol. Res. 2013, 12, 3845–3855. [Google Scholar] [CrossRef]
- Ding, Z.Q.; Lin, Z.F.; Li, Q.; Wu, H.; Xiang, C.Y.; Wang, J.F. DNL1, encodes cellulose synthase-like D4, is a major QTL for plant height and leaf width in rice (Oryza sativa L.). Biochem. Biophys. Res. Commun. 2015, 457, 133–140. [Google Scholar] [CrossRef]
- Shen, W.Q.; Sun, J.J.; Xiao, Z.; Feng, P.; Zhang, T.; He, G.H.; Sang, X.C. Narrow and Stripe Leaf 2 Regulates Leaf Width by Modulating Cell Cycle Progression in Rice. Rice 2023, 16, 20–31. [Google Scholar] [CrossRef]
- Ma, L.; Sang, X.C.; Zhang, T.; Yu, Z.Y.; Li, Y.F.; Zhao, F.M.; Wang, Z.W.; Wang, Y.T.; Yu, P.; Wang, N.; et al. ABNORMAL VASCULAR BUNDLES regulates cell proliferation and procambium cell establishment during aerial organ development in rice. New Phytol. 2017, 213, 275–286. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Meng, Y.L.; Zeng, J.; Luo, Y.; Feng, Z.; Bian, L.Y.; Gao, S.Y. Coordination between GROWTH-REGULATING FACTOR1 and GRF-INTERACTING FACTOR1 plays a key role in regulating leaf growth in rice. BMC Plant Biol. 2020, 20, 200–211. [Google Scholar] [CrossRef]
- Sakamoto, T.; Morinaka, Y.; Inukai, Y.; Kitano, H.; Fujioka, S. Auxin signal transcription factor regulates expression of the brassinosteroid receptor gene in rice. Plant J. 2013, 73, 676–688. [Google Scholar] [CrossRef]
- Chu, H.W.; Cheng, C.; Niu, F.A.; Zhou, J.H.; Tu, R.J.; Luo, Z.Y.; Wang, X.Q.; Cao, L.M. Distribution of 8 rice blast-resistant genes in parents of three-line hybrid keng rice. Acta Agric. Shanghai 2018, 34, 8–13. [Google Scholar]
- Yuan, L.P. Hybrid Rice Breeding for Super High Yield. Hybrid Rice 1997, 12, 4–9. [Google Scholar]
- Wang, F.H.; Tang, Z.B.; Wang, Y.; Fu, J.; Yang, W.B.; Wang, S.X.; Wang, Y.T.; Bai, T.; Huang, Z.B.; Yin, H.Q.; et al. Leaf Mutant 7 Encoding Heat Shock Protein OsHSP40 Regulates Leaf Size in Rice. Int. J. Mol. Sci. 2022, 23, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.F.; Yang, X.; Kang, Y.Y.; Chai, X.R.; Meng, L.P. Research Progress on DnaJ Proteins in Plants. Mol. Plant Breed. 2018, 16, 2028–2034. [Google Scholar]
- Sarkar, N.K.; Thapar, U.; Kundnani, P.; Panwar, P.; Grover, A. Functional relevance of J-protein family of rice (Oryza sativa). Cell Stress Chaperones 2013, 18, 321–331. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Shao, L.Y.; Yan, X.; Peng, H.; Ouyang, J.X.; Li, S.B. Expression and function analysis of a rice OsHSP40 gene under salt stress. Genes Genom. 2019, 41, 175–182. [Google Scholar] [CrossRef]
- Liu, Y.T.; Li, M.T.; Yu, J.L.; Ma, A.; Wang, J.; Yun, D.J.; Xu, Z.Y. Plasma membrane-localized Hsp40/DNAJ chaperone protein facilitates OsSUVH7-OsBAG4-OsMYB106 transcriptional complex formation for OsHKT1; 5 activation. J. Integr. Plant Biol. 2023, 65, 265–279. [Google Scholar] [CrossRef]
- Távora, F.T.P.K.; Meunier, A.C.; Vernet, A. Murielle Portefaix, M.; Milazzo, J.; Adreit, H.; Tharreau, D.; Lfranco, O.; Mehta, A. CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae. Rice Sci. 2022, 29, 535–544. [Google Scholar] [CrossRef]
- Zhong, X.H.; Yang, J.X.; Shi, Y.L.; Wang, X.L.; Wang, G.L. The DnaJ protein OsDjA6 negatively regulates rice innate immunity to the blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2018, 19, 607–614. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhong, X.H.; Shi, Y.L.; Liu, Z.; Jiang, N.; Liu, J.; Ding, B.; Li, Z.Q.; Kang, H.X.; Ning, Y.S.; et al. A fungal effector targets a heat shock-dynamin protein complex to modulate mitochondrial dynamics and reduce plant immunity. Sci. Adv. 2020, 6, eabb7719. [Google Scholar] [CrossRef]
- Zhu, X.B.; Liang, S.H.; Yin, J.J.; Yuan, C.; Wang, J.; Li, W.T.; He, M.; Wang, J.H.; Chen, W.L.; Ma, B.T.; et al. The DnaJ OsDjA7/8 is essential for chloroplast development in rice (Oryza sativa). Gene 2015, 574, 11–19. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Qian, Q.; Zhang, G.H. Research Advance in Molecule Regulation Mechanism of Leaf Morphogenesis in Rice (Oryza sativa L.). Acta Agron. Sin. 2013, 39, 767–774. [Google Scholar] [CrossRef]
- Huang, L.J.; Luo, J.; Wang, Y.; Li, N. From Green Revolution to Green Balance: The Nitrogen and Gibberellin Mediated Rice Tiller Growth. Plant Signal. Behav. 2021, 16, 1917838. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Ma, B.; Liu, G.; Wang, J.; Wang, J.; Gao, R.; Li, J.; Liu, J.; Xu, J.; et al. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat. Commun. 2017, 8, 14789. [Google Scholar] [CrossRef]
- Wang, F.; Han, T.; Song, Q.; Ye, W.; Song, X.; Chu, J.; Li, J.; Chen, Z.J. The Rice Circadian Clock Regulates Tiller Growth and Panicle Development Through Strigolactone Signaling and Sugar Sensing. Plant Cell 2020, 32, 3124–3138. [Google Scholar] [CrossRef]
| Reagent | Usage/μL | Reaction Parameters | ||||
|---|---|---|---|---|---|---|
| 2× Master Mix | 10.0 | Stage 1 | Initial denaturation | Reps:1 | 95 °C | 30 s |
| Primer1 (10 μM) | 0.4 | Stage 2 | Cycle reaction | Reps:40 | 95 °C | 3~10 s |
| Primer2 (10 μM) | 0.4 | 60 °C | 10~30 s | |||
| cDNA | 0.2 | Stage 3 | Melting curve | Rep:1 | 95 °C | 15 s |
| Sterilized double-distilled water | Add to 20.0 | 60 °C | 60 s | |||
| 95 °C | 15 s | |||||
| Name | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| RM7479 | GCTCTGGTTAGTGATCATGG | ACATGGTGGCTTAGGAGTG |
| RM6574 | AACCTCGAATTCCTTGGGAG | TTCGACTCCAAGGAGTGCTC |
| 7-4915 | ATTCAATCCAACCTCTCACAAT | CTTGAAGTTCTTGGGGTTCAT |
| 7-4934 | TACTGAGAGAGAGGGCTTGAGA | AACTAGTGGCATATTCGCTGAT |
| 7-5037 | TATGTGTGTGTGTGTGTGTGTG | TCAACAAATTTGGAAACAAAAA |
| 7-5194 | ATTAGCAGCAGCTTTGTATTCG | ATAGGTCCATGGTTGAACAAAA |
| 7-5209 | AGCATCGATAAGTTGAGGAGAC | GCCTGTTAGGAATGGGAAGT |
| 7-5262 | GGCTAAAAGTGGATAGATGCAG | ACACACCAAGATTGTCAGATCA |
| RM125 | ATCAGCAGCCATGGCAGCGACC | AGGGGATCATGTGCCGAAGGCC |
| RM5672 | CACCCTACAAGGAAACAAGC | TGCCCAATATAGAGGCAACC |
| R7M7 | ACCTTCCCTCCCCTTTTGAT | AACTTGGTCTTCCTGTTTTATTG |
| RM6776 | AGCCCGGACATGCAAAAC | GAAGCAGGCGAAATCTCCTC |
| RM1253 | CTGAACTTGCCTGAGAACTC | GACGACCTCTCCATGCTCG |
| RM3718 | AGCGCTCGAGAATTTCTAGG | ATGCTGACGTCACCCCAC |
| R7M20 | GTTTTGTGCATTCCTTTAC | TTTATGACATTTGACCG |
| Actin-qPCR | CCAAGGCCAATCGTGAGAAGA | AATCAGTGAGATCACGCCCAG |
| Nal25-qPCR | ATTCTATGAAGGTGGCTTTCAA | TCCTTTTGTCTTCCCTAACAAA |
| Agronomic Traits | Material | |
|---|---|---|
| ‘Huazhan’ | nal25 | |
| Plant height/cm | 108.00 ± 1.53 | 90.80 ± 2.48 *** |
| Flag leaf length/cm | 36.57 ± 1.05 | 41.80 ± 1.66 ** |
| Flag leaf width/cm | 1.73 ± 0.05 | 0.57 ± 0.02 ** |
| No. of effective panicles | 25 ± 0.82 | 52 ± 1.63 **** |
| Panicle length/cm | 25.63 ± 1.04 | 22.06 ± 1.33 **** |
| Total grain number of per spike | 270.20 ± 5.53 | 75.25 ± 3.49 **** |
| Seed setting rate/% | 94.25 ± 0.83 | 66.00 ± 2.92 *** |
| Grain length/cm | 9.32 ± 0.18 | 8.97 ± 0.09 * |
| Grain width/cm | 2.33 ± 0.05 | 2.09 ± 0.04 *** |
| 1000-grain weight/g | 19.78 ± 0.21 | 15.33 ± 1.52 * |
| Cross Combination | F1 | F2 | χ2 (χ20.05 = 3.84) | ||
|---|---|---|---|---|---|
| Total No. of Plants | No. of Wild-Type Plants | No. of Mutant-Type Plants | |||
| nal25/Shenhui 26 | normal | 1145 | 862 | 283 | 0.05 |
| nal25/Huazhan | normal | 1192 | 905 | 287 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, K.; Niu, F.; Hu, P.; Cheng, C.; Chu, H.; Zhou, J.; Sun, B.; Dai, Y.; Cao, L.; Zhang, A. Phenotypic Identification and Fine-Mapping of the Rice Narrow-Leaf Mutant nal25. Plants 2025, 14, 2528. https://doi.org/10.3390/plants14162528
Xie K, Niu F, Hu P, Cheng C, Chu H, Zhou J, Sun B, Dai Y, Cao L, Zhang A. Phenotypic Identification and Fine-Mapping of the Rice Narrow-Leaf Mutant nal25. Plants. 2025; 14(16):2528. https://doi.org/10.3390/plants14162528
Chicago/Turabian StyleXie, Kaizhen, Fuan Niu, Peng Hu, Can Cheng, Huangwei Chu, Jihua Zhou, Bin Sun, Yuting Dai, Liming Cao, and Anpeng Zhang. 2025. "Phenotypic Identification and Fine-Mapping of the Rice Narrow-Leaf Mutant nal25" Plants 14, no. 16: 2528. https://doi.org/10.3390/plants14162528
APA StyleXie, K., Niu, F., Hu, P., Cheng, C., Chu, H., Zhou, J., Sun, B., Dai, Y., Cao, L., & Zhang, A. (2025). Phenotypic Identification and Fine-Mapping of the Rice Narrow-Leaf Mutant nal25. Plants, 14(16), 2528. https://doi.org/10.3390/plants14162528






