Insecticide Resistance Evolution Negatively Affects the Fitness of Aphis gossypii Glover During Selection on Cotton Plants Under Laboratory Conditions
Abstract
1. Introduction
2. Results
2.1. Selection-Induced Insecticide Resistance Evolution
2.2. Impact of Insecticide Resistance on Different Developmental Stages of A. gossypii
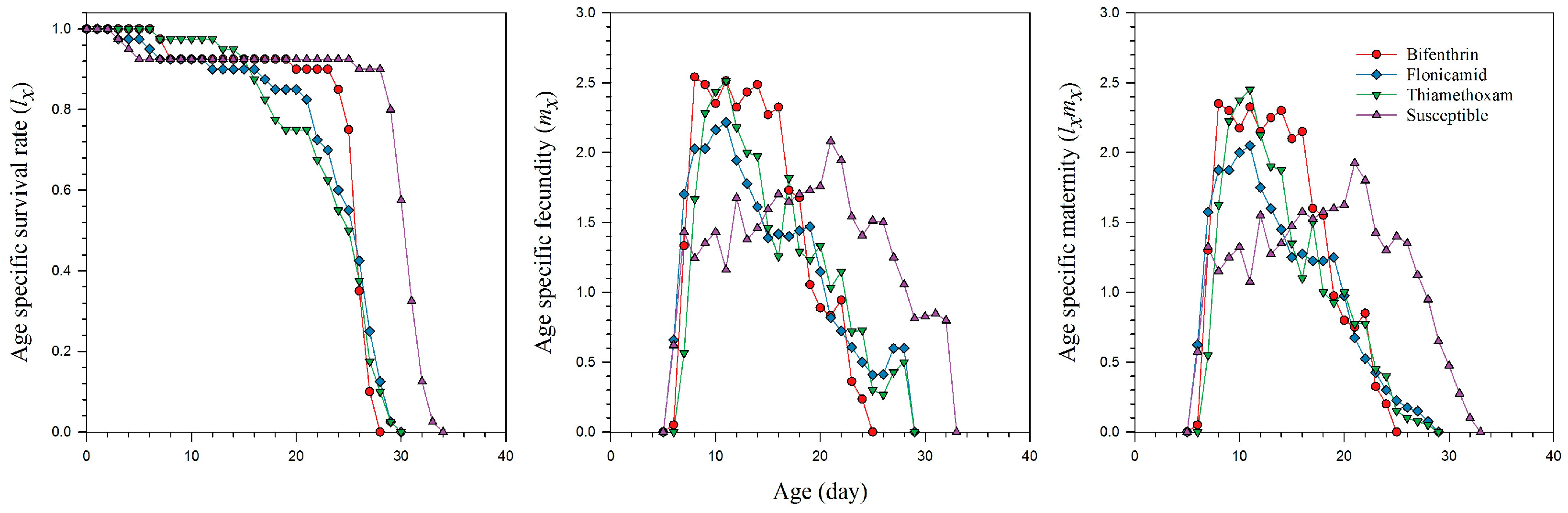
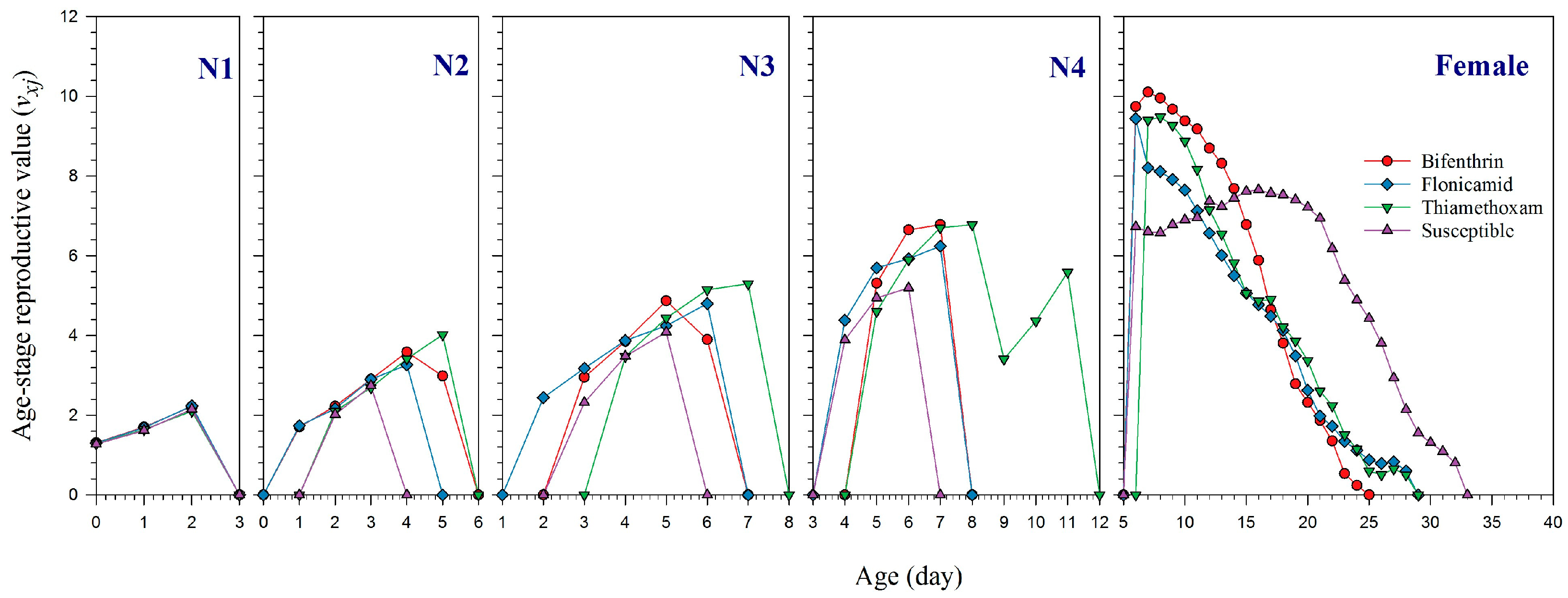
2.3. Reproduction and Life Table Parameters of the Insecticide-Resistant Strain of A. gossypii
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects and Insecticide
5.2. Toxicity Bioassays
5.3. Establishing the Resistant Strain
5.4. Fitness Comparisons
5.5. Life Table Data Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hullé, M.; Chaubet, B.; Turpeau, E.; Simon, J. Encyclop’Aphid: A website on aphids and their natural enemies. Entomol. Gen. 2020, 40, 97–101. [Google Scholar] [CrossRef]
- Abbas, A.; Ullah, F.; Hafeez, M.; Han, X.; Dara, M.Z.N.; Gul, H.; Zhao, C.R. Biological control of fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2704. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.-Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2023, 43, 21–30. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Guo, Q.-L.; Xia, X.-M.; Wang, H.-Y.; Liu, T.-X. Resistance of Aphis gossypii (Homoptera: Aphididae) to selected insecticides on cotton from five cotton production regions in Shandong, China. J. Pestic. Sci. 2007, 32, 372–378. [Google Scholar] [CrossRef]
- Koo, H.-N.; An, J.-J.; Park, S.-E.; Kim, J.-I.; Kim, G.-H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop Prot. 2014, 55, 91–97. [Google Scholar] [CrossRef]
- Amichot, M.; Brun-Barale, A.; Haddi, K.; Nauen, R.; Guedes, R.N.C.; Tarès, S. Current knowledge on the origin of insecticide resistance mechanisms: The tip of the iceberg? Entomol. Gen. 2023, 43, 501–503. [Google Scholar] [CrossRef]
- Mocchetti, A.; Dermauw, W.; Van Leeuwen, T. Incidence and molecular mechanisms of insecticide resistance in Frankliniella occidentalis, Thrips tabaci and other economically important thrips species. Entomol. Gen. 2023, 43, 587–604. [Google Scholar] [CrossRef]
- Haddi, K.; Nauen, R.; Benelli, G.; Guedes, R.N.C. Global perspectives on insecticide resistance in agriculture and public health. Entomol. Gen. 2023, 43, 495–500. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Nauen, R. The molecular mechanisms of insecticide resistance in aphid crop pests. Insect Biochem. Mol. Biol. 2023, 156, 103937. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.H.; Ahmad, M.; Crickmore, N. Fitness costs limit the development of resistance to indoxacarb and deltamethrin in Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Entomol. 2008, 101, 1927–1933. [Google Scholar] [CrossRef]
- Zang, X.; Gao, L.; Tian, X.; Qiao, H.; Zhu, C.; Chen, N.; Ren, B.; Wang, Y. Exposure to chlorantraniliprole alters the environmental adaptability of insecticide resistant insects. Entomol. Gen. 2023, 43, 1031–1040. [Google Scholar] [CrossRef]
- Carriere, Y.; Tabashnik, B. Reversing insect adaptation to transgenic insecticidal plants. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1475–1480. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef]
- Abbas, N.; Shah, R.M.; Shad, S.A.; Azher, F. Dominant fitness costs of resistance to fipronil in Musca domestica Linnaeus (Diptera: Muscidae). Vet. Parasitol. 2016, 226, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, Q.; Qiu, H.; Tang, L.; Zeng, D.; Liu, K.; Gao, Y. Resistance development, stability, cross-resistance potential, biological fitness and biochemical mechanisms of spinetoram resistance in Thrips hawaiiensis (Thysanoptera: Thripidae). Pest Manag. Sci. 2018, 74, 1564–1574. [Google Scholar] [CrossRef]
- Steinbach, D.; Moritz, G.; Nauen, R. Fitness costs and life table parameters of highly insecticide-resistant strains of Plutella xylostella (L.) (Lepidoptera: Plutellidae) at different temperatures. Pest Manag. Sci. 2017, 73, 1789–1797. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, K.; Liao, X.; He, B.; Jin, R.; Tang, T.; Wan, H.; Li, J. Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J. Pest Sci. 2018, 91, 1145–1151. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Acetamiprid resistance and fitness costs of melon aphid, Aphis gossypii: An age-stage, two-sex life table study. Pestic. Biochem. Physiol. 2021, 171, 104729. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Xia, J.; Lv, N.; Gao, X. Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 158, 40–46. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Gong, Y.-J.; Chen, J.-C.; Su, X.-C.; Cao, L.-J.; Hoffmann, A.A.; Wei, S.-J. Laboratory selection for resistance to sulfoxaflor and fitness costs in the green peach aphid Myzus persicae. J. Asia-Pac. Entomol. 2018, 21, 408–412. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Said, F.; Gao, X.; Song, D. Fitness costs in chlorfenapyr-resistant populations of the chive maggot, Bradysia odoriphaga. Ecotoxicology 2020, 29, 407–416. [Google Scholar] [CrossRef]
- Chi, H.; Kara, H.; Özgökçe, M.S.; Atlihan, R.; Güncan, A.; Rişvanlı, M.R. Innovative application of set theory, Cartesian product, and multinomial theorem in demographic research. Entomol. Gen. 2022, 42, 863–874. [Google Scholar] [CrossRef]
- Chi, H.; You, M.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.-J.; Fu, J.-W.; Xu, Y.-Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 102–123. [Google Scholar] [CrossRef]
- Ullah, F.; Guru-Pirasanna-Pandi, G.; Murtaza, G.; Sarangi, S.; Gul, H.; Li, X.; Chavarín-Gómez, L.E.; Ramírez-Romero, R.; Guedes, R.N.C.; Desneux, N. Evolving strategies in agroecosystem pest control: Transitioning from chemical to green management. J. Pest Sci 2025. [Google Scholar] [CrossRef]
- Gassmann, A.J. Fitness costs of resistance and their potential application for insect resistance management. In Insect Resistance Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 465–491. [Google Scholar]
- Cao, G.; Feng, H.; Guo, F.; Wu, K.; Li, X.; Liang, G.; Desneux, N. Quantitative Analysis of Fitness Costs Associated with the Development of Resistance to the Bt Toxin Cry1Ac in Helicoverpa armigera. Sci. Rep. 2014, 4, 5629. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Fitness costs in clothianidin-resistant population of the melon aphid, Aphis gossypii. PLoS ONE 2020, 15, e0238707. [Google Scholar] [CrossRef]
- Gul, H.; Haq, I.u.; Güncan, A.; Ullah, F.; Desneux, N.; Liu, X. Laboratory-Induced Bifenthrin, Flonicamid, and Thiamethoxam Resistance and Fitness Costs in Rhopalosiphum padi. Toxics 2023, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Georghiou, G.P. Rapid development of high-level permethrin resistance in a field-collected strain of the house fly (Diptera: Muscidae) under laboratory selection. J. Econ. Entomol. 1985, 78, 316–319. [Google Scholar] [CrossRef]
- Zewen, L.; Zhaojun, H.; Yinchang, W.; Lingchun, Z.; Hongwei, Z.; Chengjun, L. Selection for imidacloprid resistance in Nilaparvata lugens: Cross-resistance patterns and possible mechanisms. Pest Manag. Sci. Former. Pestic. Sci. 2003, 59, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, Z. Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 279–282. [Google Scholar]
- Ejaz, M.; Afzal, M.B.S.; Shabbir, G.; Serrão, J.E.; Shad, S.A.; Muhammad, W. Laboratory selection of chlorpyrifos resistance in an Invasive Pest, Phenacoccus solenopsis (Homoptera: Pseudococcidae): Cross-resistance, stability and fitness cost. Pestic. Biochem. Physiol. 2017, 137, 8–14. [Google Scholar] [CrossRef]
- Gul, H.; Gadratagi, B.G.; Güncan, A.; Tyagi, S.; Ullah, F.; Desneux, N.; Liu, X. Fitness costs of resistance to insecticides in insects. Front. Physiol. 2023, 14, 1238111. [Google Scholar] [CrossRef]
- Gul, H.; Ullah, F.; Güncan, A.; Desneux, N.; Liu, X. Thiamethoxam, bifenthrin, and flonicamid resistance in Schizaphis graminum and associated fitness costs. Entomol. Gen. 2023, 43, 575. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Haddi, K.; Carvalho, G.A.; de Paulo, P.D.; Hirose, E.; Guedes, R.N.C.; Smagghe, G.; Oliveira, E.E. Imidacloprid resistance in the Neotropical brown stink bug Euschistus heros: Selection and fitness costs. J. Pest Sci. 2019, 92, 847–860. [Google Scholar] [CrossRef]
- Ullah, F.; Xu, X.; Gul, H.; Güncan, A.; Hafeez, M.; Gao, X.; Song, D. Impact of Imidacloprid Resistance on the Demographic Traits and Expressions of Associated Genes in Aphis gossypii Glover. Toxics 2022, 10, 658. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, A.; Shan, T.; Dong, W.; Shi, X.; Gao, X. Cross-resistance and fitness cost analysis of resistance to thiamethoxam in melon and cotton aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 1946–1954. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.; Shad, S.A. Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitol. Res. 2015, 114, 247–255. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Q.; Qi, H.; Wang, Q.; Yuan, H.; Rui, C. Resistance selection of indoxacarb in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae): Cross-resistance, biochemical mechanisms and associated fitness costs. Pest Manag. Sci. 2018, 74, 2636–2644. [Google Scholar] [CrossRef]
- Tariq, K.; Noor, M.; Saeed, S.; Zhang, H. The effect of ultraviolet-A radiation exposure on the reproductive ability, longevity, and development of the Dialeurodes citri (Homoptera: Aleyrodidae) F1 generation. Environ. Entomol. 2015, 44, 1614–1618. [Google Scholar] [CrossRef]
- Tariq, K.; Noor, M.; Backus, E.A.; Hussain, A.; Ali, A.; Peng, W.; Zhang, H. The toxicity of flonicamid to cotton leafhopper, Amrasca biguttula (Ishida), is by disruption of ingestion: An electropenetrography study. Pest Manag. Sci. 2017, 73, 1661–1669. [Google Scholar] [CrossRef]
- De Loof, A. Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J. Insect Physiol. 2011, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Güncan, A.; Kavousi, A.; Gharakhani, G.; Atlihan, R.; Özgökçe, M.S.; Shirazi, J.; Amir-Maafi, M.; Maroufpoor, M.; Taghizadeh, R. TWOSEX-MSChart: The key tool for life table research and education. Entomol. Gen. 2022, 42, 845–849. [Google Scholar] [CrossRef]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-M.; Chi, H.; Wang, R.-C.; Wang, Y.-P.; Xu, Y.-Y.; Li, X.-D.; Yin, P.; Zheng, F.-Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead)(Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer)(Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]


| Gen. | LC50 (95%CI) a mg L−1 | Slope ± SE b | χ2 | p-Value | RR c |
|---|---|---|---|---|---|
| F0 | 0.31 (0.26–0.37) | 2.223 ± 0.206 | 9.416 | 0.895 | - |
| F1 | 0.47 (0.38–0.59) | 1.674 ± 0.171 | 4.678 | 0.997 | 1.51 |
| F2 | 0.87 (0.72–1.10) | 1.898 ± 0.186 | 10.136 | 0.859 | 2.77 |
| F3 | 1.92 (1.52–2.56) | 1.637 ± 0.190 | 4.588 | 0.997 | 6.12 |
| F4 | 3.46 (2.83–4.38) | 1.893 ± 0.190 | 10.477 | 0.841 | 11.05 |
| F5 | 6.65 (5.54–8.14) | 2.217 ± 0.260 | 7.315 | 0.967 | 21.24 |
| F6 | 12.0 (9.29–17.1) | 1.766 ± 0.230 | 4.935 | 0.996 | 38.32 |
| F7 | 20.6 (17.5–24.3) | 2.732 ± 0.293 | 5.110 | 0.995 | 65.97 |
| F8 | 29.0 (24.3–35.3) | 2.363 ± 0.282 | 4.204 | 0.999 | 92.67 |
| F9 | 38.1 (32.0–45.9) | 2.120 ± 0.194 | 13.017 | 0.672 | 121.60 |
| F10 | 49.6 (42.5–58.1) | 2.461 ± 0.212 | 6.542 | 0.981 | 158.60 |
| Gen. | LC50 (95%CI) a mg L−1 | Slope ± SE b | χ2 | p-Value | RR c |
|---|---|---|---|---|---|
| F0 | 1.82 (1.52–2.20) | 1.951 ± 0.178 | 13.303 | 0.650 | - |
| F1 | 2.52 (2.08–3.12) | 1.857 ± 0.178 | 4.686 | 0.997 | 1.38 |
| F2 | 5.23 (3.90–7.81) | 1.387 ± 0.170 | 3.994 | 0.999 | 2.87 |
| F3 | 9.59 (7.83–12.4) | 2.079 ± 0.228 | 8.421 | 0.935 | 5.28 |
| F4 | 15.5 (11.1–22.5) | 2.027 ± 0.271 | 7.623 | 0.959 | 8.51 |
| F5 | 26.2 (20.7–36.8) | 2.074 ± 0.308 | 5.11 | 0.995 | 14.44 |
| F6 | 49.3 (38.5–69.0) | 1.836 ± 0.223 | 7.163 | 0.970 | 27.13 |
| F7 | 87.7 (70.1–117.8) | 1.987 ± 0.250 | 5.475 | 0.993 | 48.28 |
| F8 | 131 (112–154) | 3.104 ± 0.413 | 12.974 | 0.675 | 72.37 |
| F9 | 183 (151–237) | 2.457 ± 0.384 | 6.629 | 0.980 | 100.56 |
| F10 | 235 (194–293) | 2.052 ± 0.207 | 7.195 | 0.969 | 129.18 |
| Gen. | LC50 (95%CI) a mg L−1 | Slope ± SE b | χ2 | p-Value | RR c |
|---|---|---|---|---|---|
| F0 | 0.37 (0.32–0.44) | 2.403 ± 0.205 | 8.826 | 0.920 | - |
| F1 | 0.41 (0.34–0.48) | 2.202 ± 0.189 | 6.296 | 0.985 | 1.09 |
| F2 | 0.52 (0.43–0.63) | 1.945 ± 0.177 | 4.282 | 0.998 | 1.38 |
| F3 | 0.73 (0.60–0.92) | 1.732 ± 0.175 | 6.038 | 0.988 | 1.96 |
| F4 | 1.30 (1.02–1.81) | 1.717 ± 0.218 | 6.329 | 0.984 | 3.50 |
| F5 | 3.85 (3.21–4.73) | 2.312 ± 0.271 | 9.019 | 0.913 | 10.33 |
| F6 | 7.08 (5.40–10.65) | 1.861 ± 0.283 | 9.92 | 0.871 | 19.03 |
| F7 | 11.97 (9.18–17.26) | 1.653 ± 0.202 | 7.741 | 0.956 | 32.19 |
| F8 | 19.9 (16.6–25.1) | 2.391 ± 0.312 | 4.635 | 0.997 | 53.62 |
| F9 | 29.3 (25.7–33.3) | 4.183 ± 0.514 | 5.071 | 0.995 | 78.88 |
| F10 | 39.0 (33.3–46.7) | 3.050 ± 0.406 | 5.873 | 0.989 | 104.75 |
| Stage | Susceptible | Bifenthrin | Flonicamid | Thiamethoxam | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| First-instar | 40 | 2.30 ± 0.07 a | 40 | 2.43 ± 0.11 a | 40 | 2.33 ± 0.10 a | 40 | 2.45 ± 0.08 a |
| Second-instar | 39 | 1.56 ± 0.08 b | 40 | 1.80 ± 0.11 b | 39 | 1.69 ± 0.09 b | 40 | 2.13 ± 0.06 a |
| Third-instar | 37 | 1.49 ± 0.08 b | 39 | 1.62 ± 0.09 ab | 38 | 1.50 ± 0.10 ab | 40 | 1.73 ± 0.07 a |
| Fourth-instar | 37 | 1.22 ± 0.07 c | 37 | 1.68 ± 0.09 a | 37 | 1.43 ± 0.08 b | 39 | 1.74 ± 0.09 a |
| Pre-adult | 37 | 6.59 ± 0.08 d | 37 | 7.41 ± 0.09 b | 37 | 6.92 ± 0.10 c | 39 | 8.05 ± 0.15 a |
| Adult | 37 | 24.32 ± 0.22 a | 37 | 18.70 ± 0.21 b | 37 | 18.22 ± 0.63 b | 39 | 15.79 ± 0.74 c |
| Total longevity | 37 | 30.92 ± 0.25 a | 37 | 26.11 ± 0.23 b | 37 | 25.14 ± 0.61 bc | 39 | 23.85 ± 0.72 c |
| Parameters a | Susceptible | Bifenthrin | Flonicamid | Thiamethoxam |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |
| R0 | 33.03 ± 1.55 a | 28.50 ± 1.35 b | 24.55 ± 1.48 c | 24.78 ± 1.43 bc |
| r | 0.2403 ± 0.0050 c | 0.2663 ± 0.0047 a | 0.2624 ± 0.0065 ab | 0.2475 ± 0.0056 bc |
| λ | 1.2716 ± 0.0063 c | 1.3052 ± 0.0062 a | 1.3000 ± 0.0085 ab | 1.2808 ± 0.0071 bc |
| T | 14.55 ± 0.18 a | 12.58 ± 0.11 bc | 12.20 ± 0.21 c | 12.97 ± 0.22 b |
| F | 35.70 ± 0.47 a | 30.81 ± 0.44 b | 26.54 ± 1.06 c | 25.41 ± 1.32 c |
| RPd | 22.57 ± 0.27 a | 13.73 ± 0.25 b | 12.84 ± 0.54 bc | 11.36 ± 0.53 c |
| APRP | 0.14 ± 0.06 b | 0.05 ± 0.04 b | 0.38 ± 0.10 a | 0.41 ± 0.09 a |
| TPRP | 6.73 ± 0.11 c | 7.46 ± 0.10 b | 7.30 ± 0.17 b | 8.46 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, H.; Güncan, A.; Abbas, A.; Ullah, Z.; Yuqing, X.; Ullah, F.; Desneux, N.; Liu, X. Insecticide Resistance Evolution Negatively Affects the Fitness of Aphis gossypii Glover During Selection on Cotton Plants Under Laboratory Conditions. Plants 2025, 14, 2527. https://doi.org/10.3390/plants14162527
Gul H, Güncan A, Abbas A, Ullah Z, Yuqing X, Ullah F, Desneux N, Liu X. Insecticide Resistance Evolution Negatively Affects the Fitness of Aphis gossypii Glover During Selection on Cotton Plants Under Laboratory Conditions. Plants. 2025; 14(16):2527. https://doi.org/10.3390/plants14162527
Chicago/Turabian StyleGul, Hina, Ali Güncan, Arzlan Abbas, Zeeshan Ullah, Xie Yuqing, Farman Ullah, Nicolas Desneux, and Xiaoxia Liu. 2025. "Insecticide Resistance Evolution Negatively Affects the Fitness of Aphis gossypii Glover During Selection on Cotton Plants Under Laboratory Conditions" Plants 14, no. 16: 2527. https://doi.org/10.3390/plants14162527
APA StyleGul, H., Güncan, A., Abbas, A., Ullah, Z., Yuqing, X., Ullah, F., Desneux, N., & Liu, X. (2025). Insecticide Resistance Evolution Negatively Affects the Fitness of Aphis gossypii Glover During Selection on Cotton Plants Under Laboratory Conditions. Plants, 14(16), 2527. https://doi.org/10.3390/plants14162527








