The Effect of Far-Red Light on the Growth of Tobacco Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Determination of Leaf Morphological Parameters and Biomass
2.3. Anatomy of the Leaf Blade
2.4. Photosynthetic Characterization
- —maximum fluorescence intensity;
- —steady-state fluorescence intensity;
- —initial fluorescence intensity.
- — a;
- b b;
- V—volume of extract (mL);
- W—mass (g);
2.5. Extraction of the Total RNA and Analysis Using Real-Time qPCR
2.6. Hormone Analysis
2.7. Statistical Analysis
3. Results
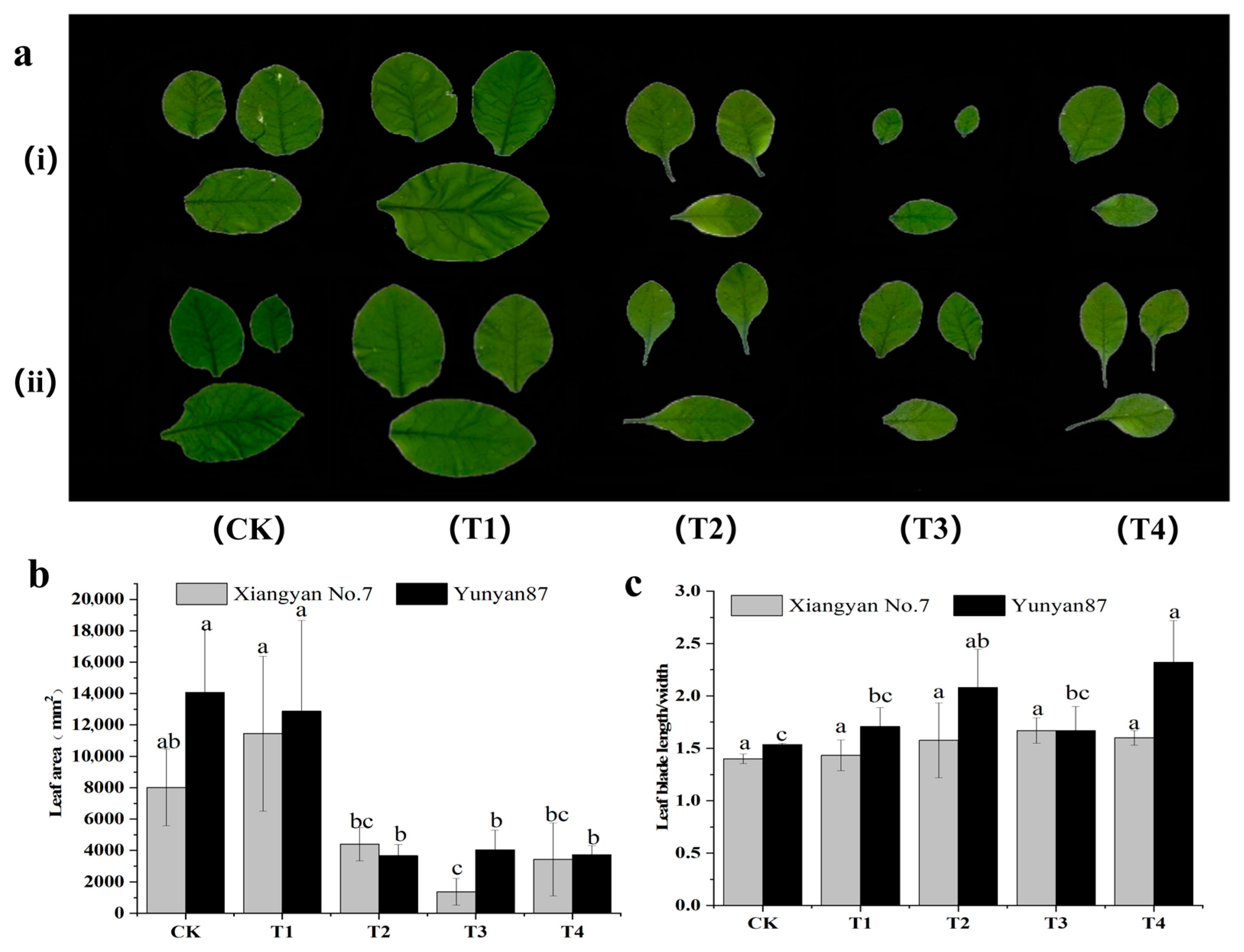
3.1. Changes in Leaf Morphology of Tobacco Seedlings Under Different Light Conditions
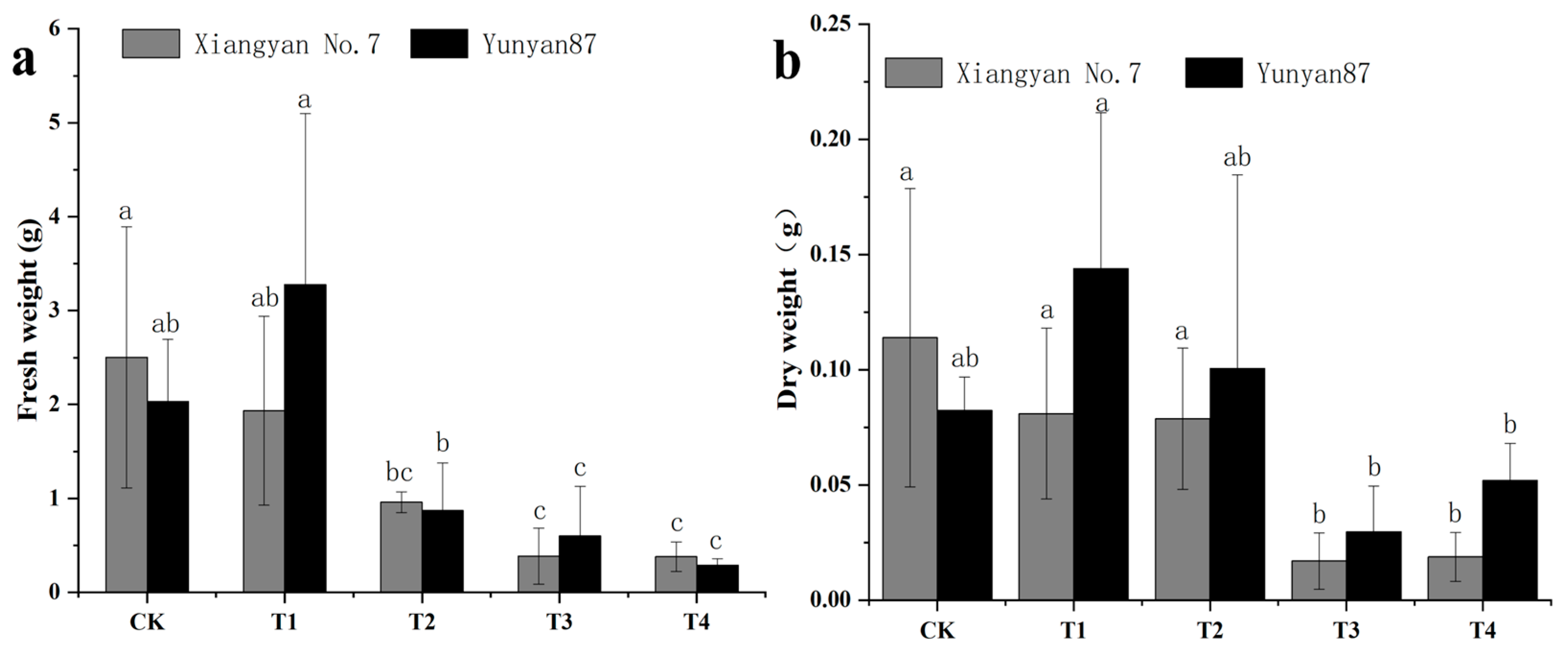
3.2. Changes in Tobacco Seedling Biomass Under Different Light Conditions
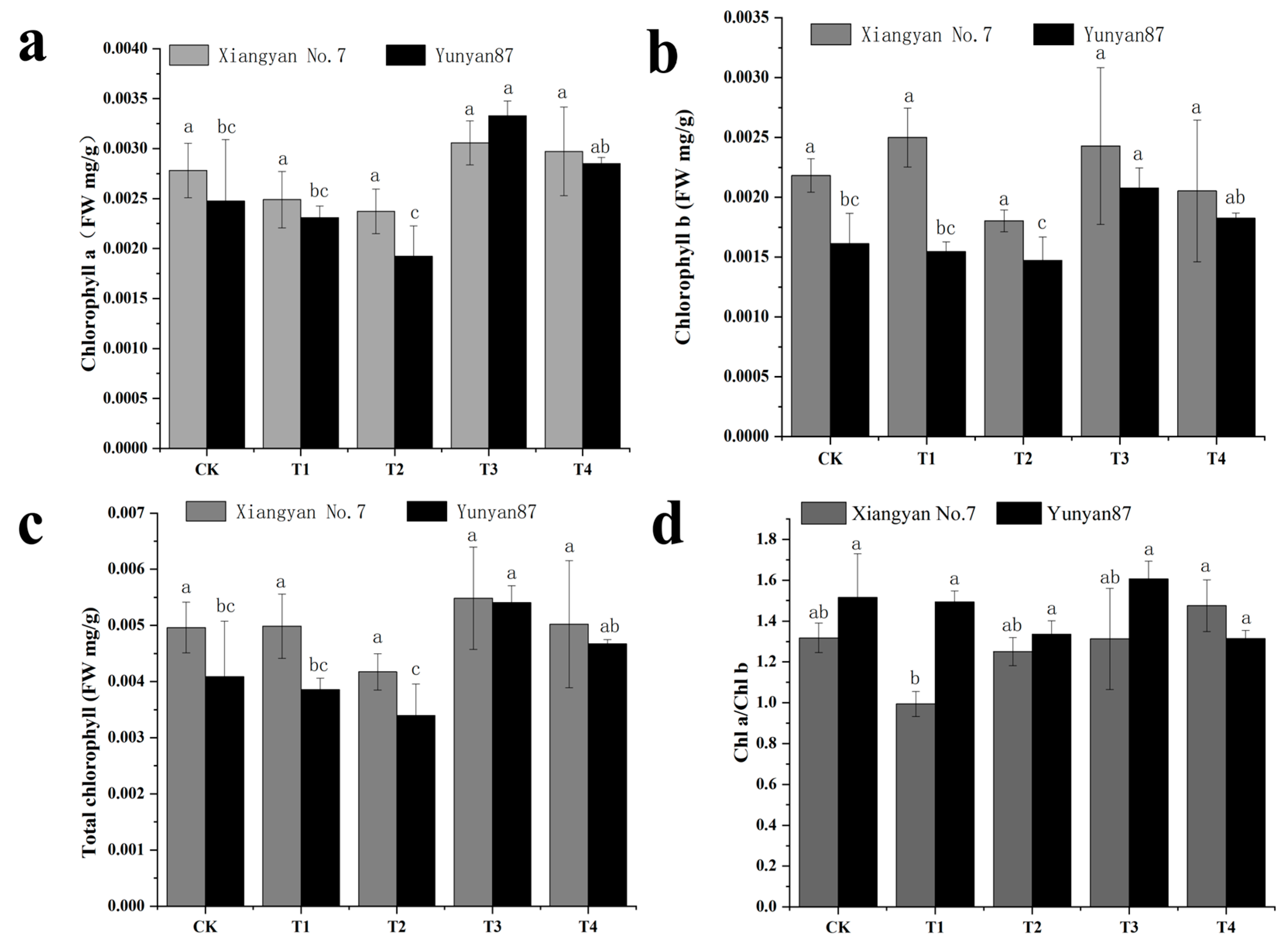
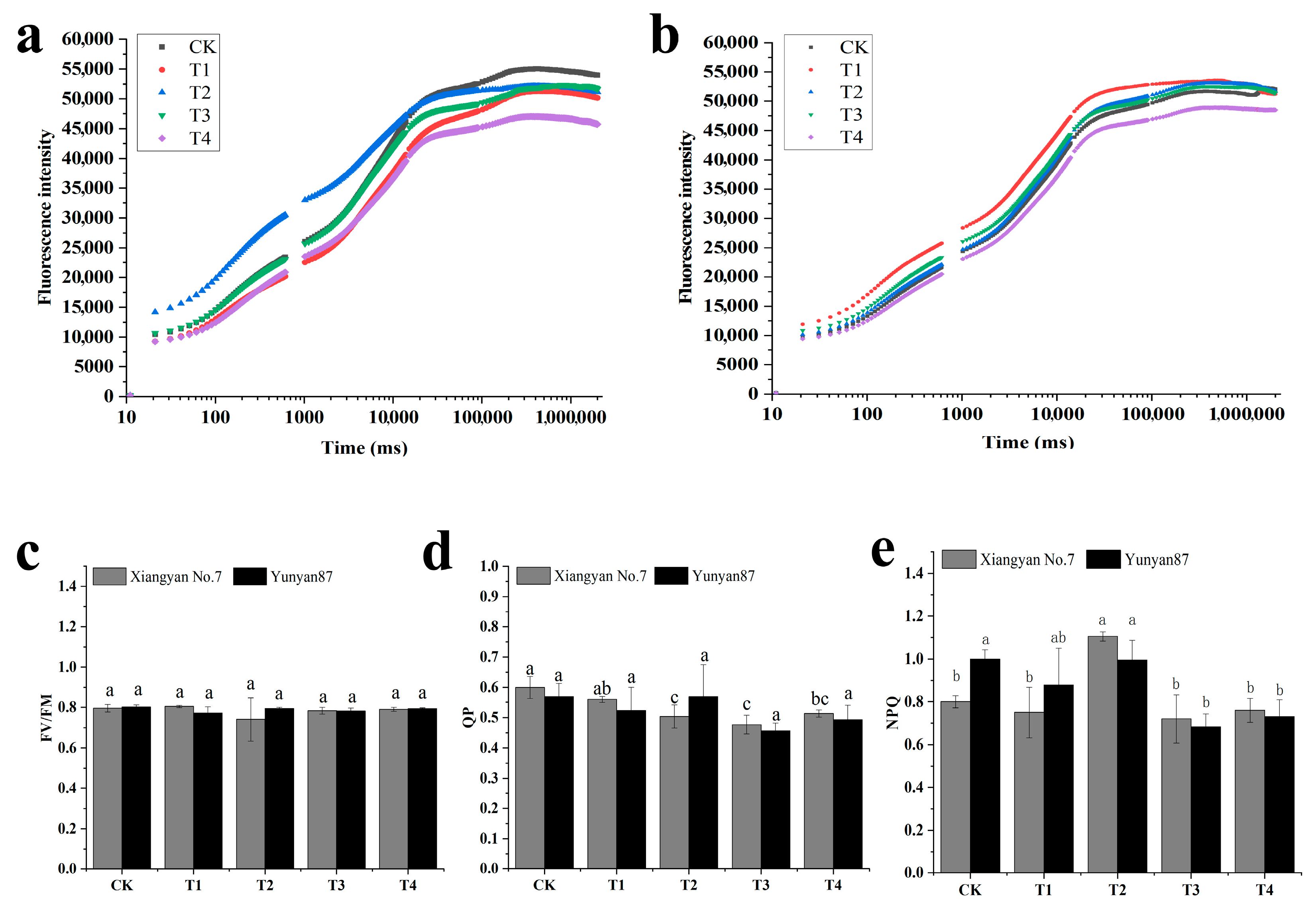
3.3. Changes in Tobacco Seedling Photosynthetic Characteristics Under Different Light Conditions
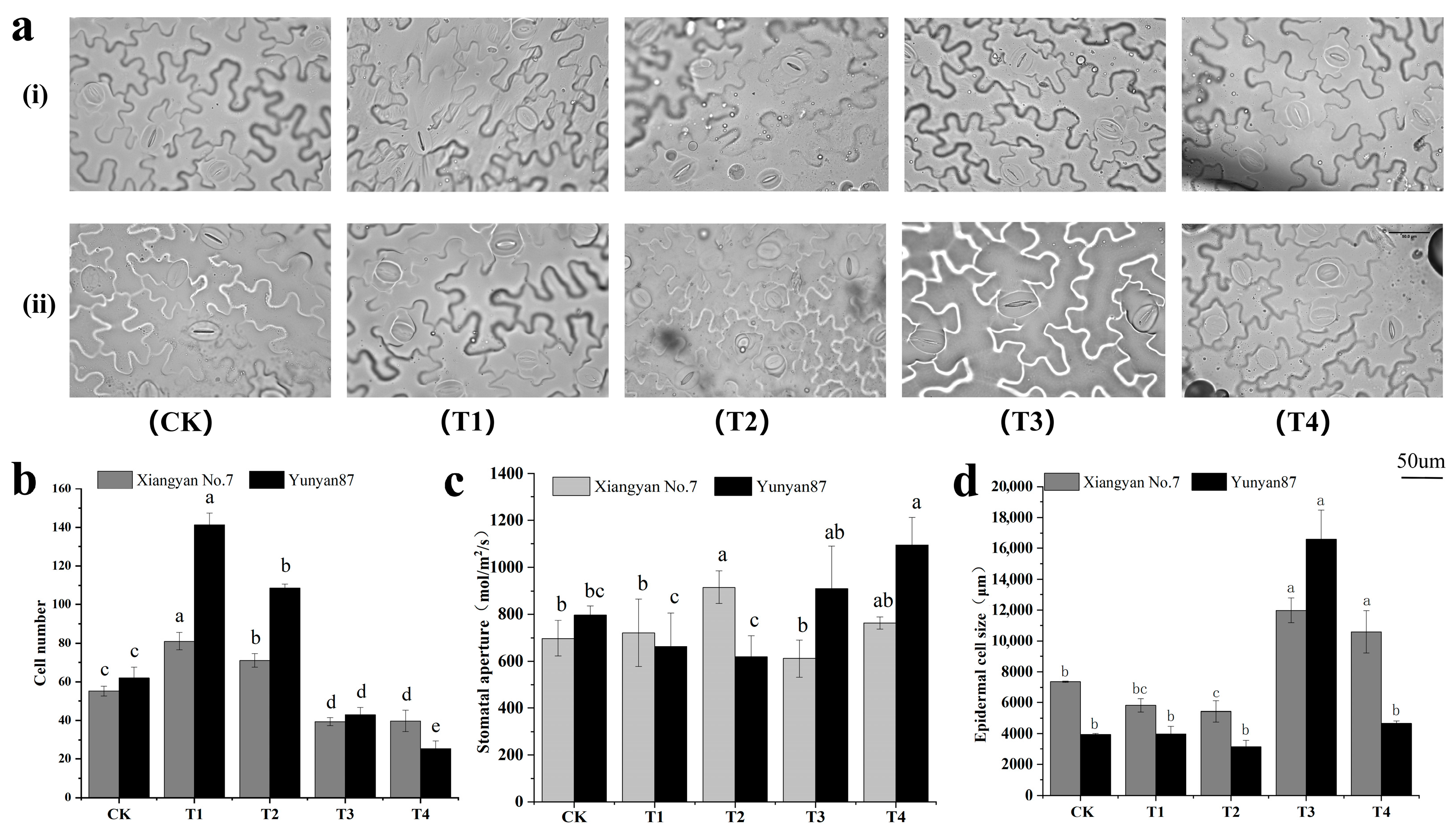
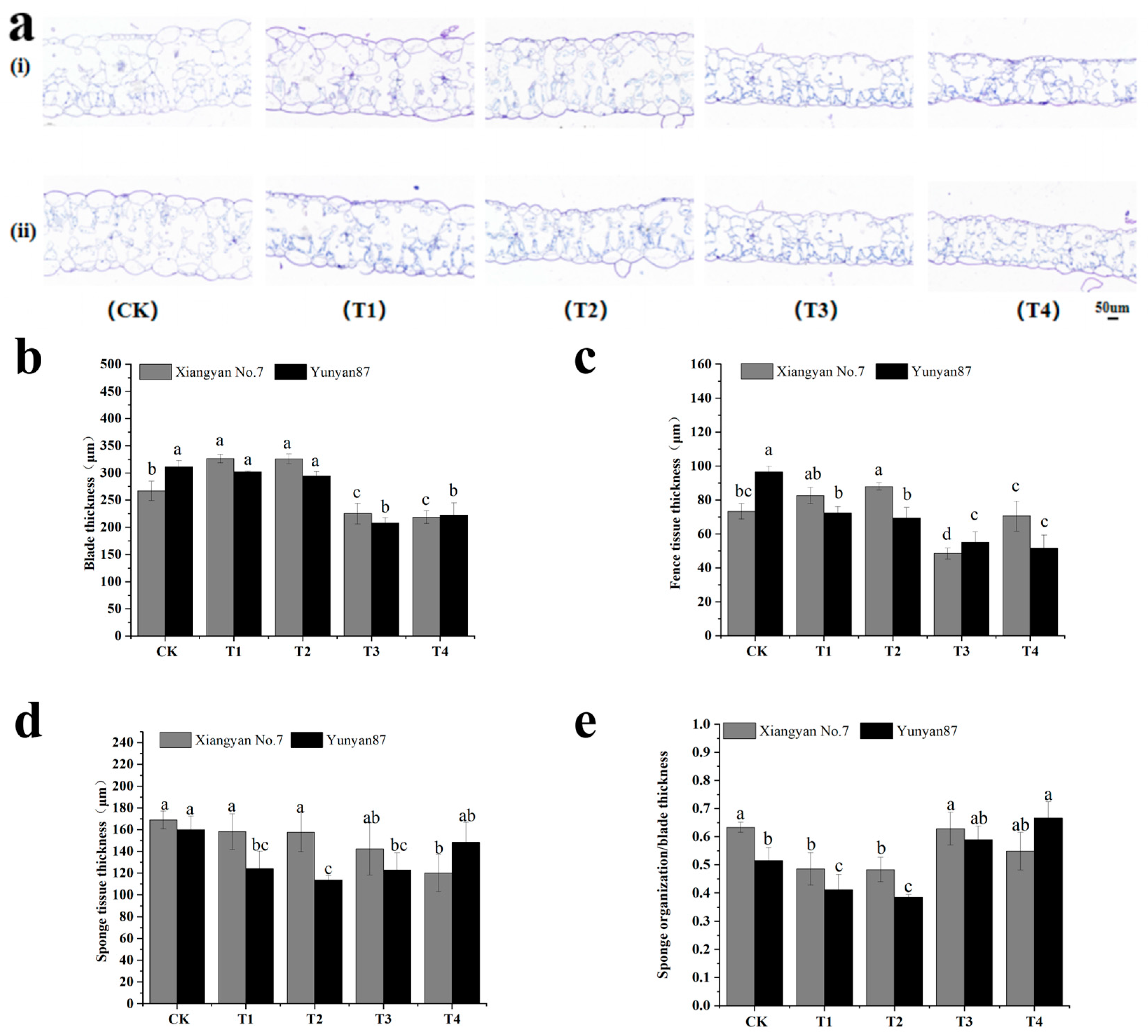
3.4. Anatomical Analysis of Tobacco Seedlings Under Different Light Conditions
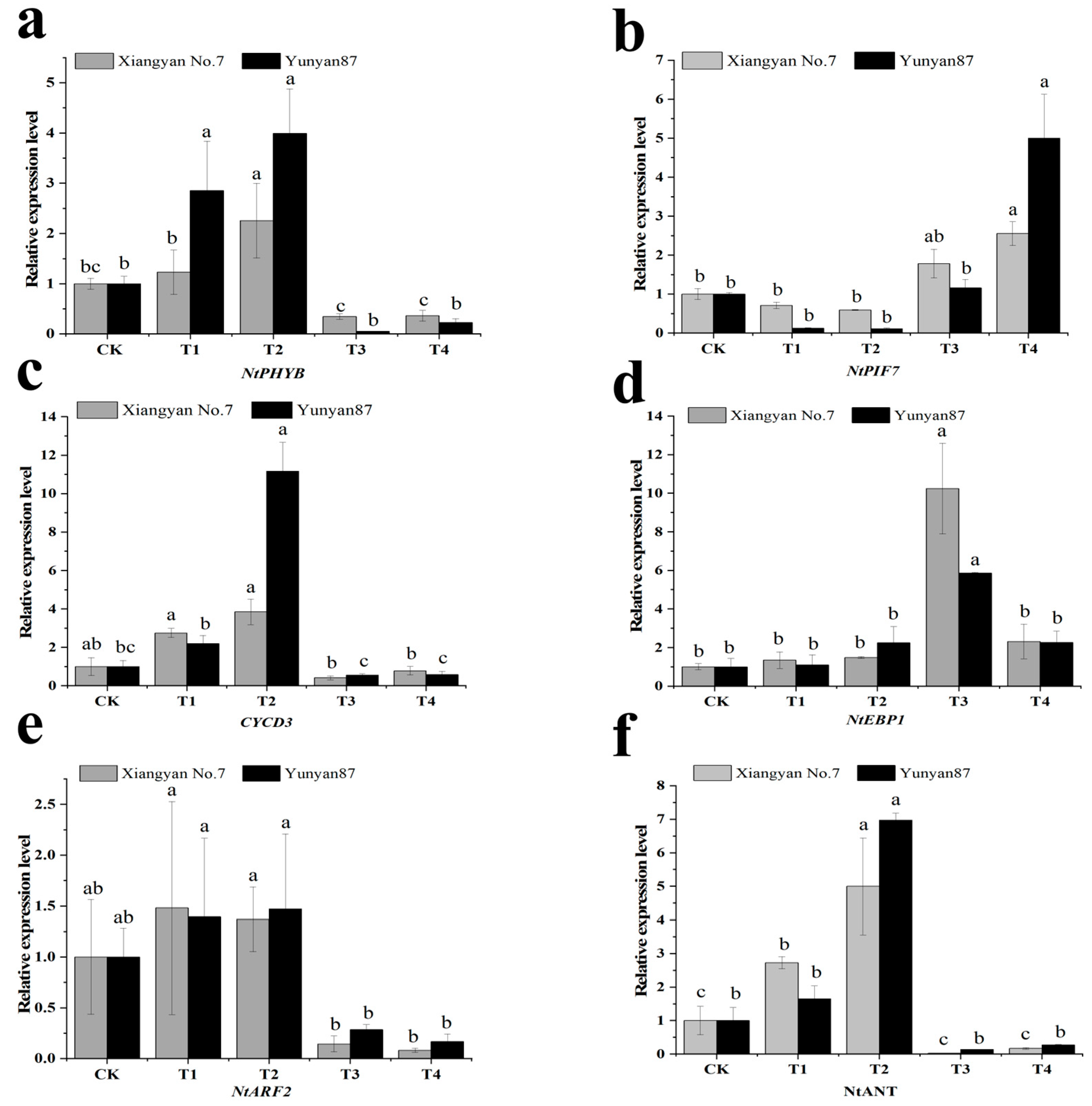
3.5. Gene Expression Analysis Related to Cell Proliferation and Expansion
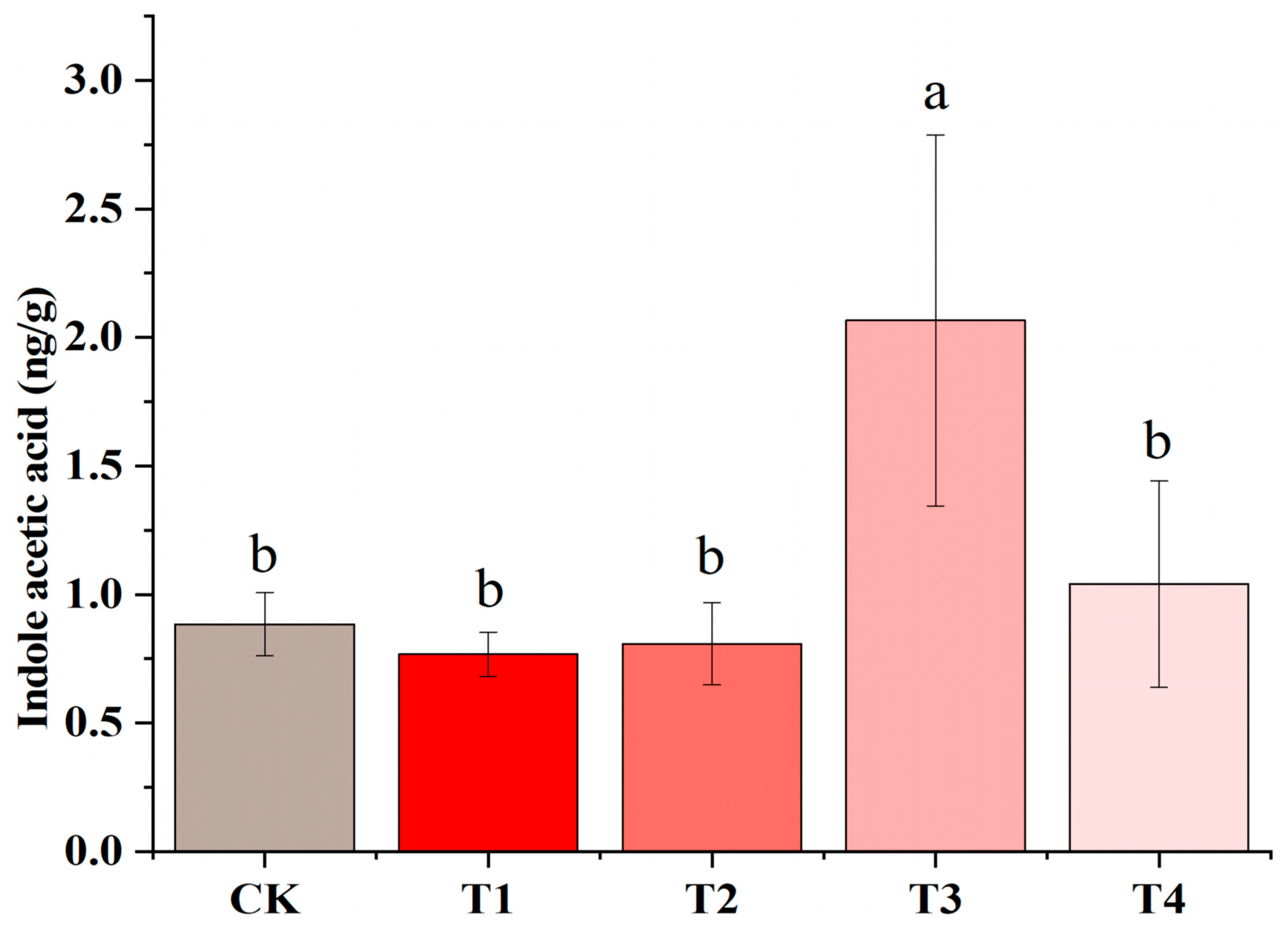
3.6. IAA Changes in Tobacco Seedlings Under Different Light Conditions
4. Discussion
4.1. Leaf Area of Tobacco Seedlings Under Far-Red Light Was Reduced, and Thus, Inhibited Leaf Growth
4.2. Significant Changes in Photosynthetic Characteristics of Tobacco Seedlings Under Far-Red Light
4.3. Anatomical Analysis of Tobacco Seedlings Under Far-Red Light and Validation of Related Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAS | Blindfold syndrome. |
| OJIP curve | Fast chlorophyll fluorescence induction curve. |
| FV/FM | Maximum fluorescence ratio. |
| NPQ | Non-photochemical quenching of chlorophyll fluorescence. |
| QP | Quantum yield of chlorophyll fluorescence. |
| PSII | Optical system II. |
References
- He, L.; Xu, M.; Wang, W.; Liu, C.; Yu, L.; Liu, W.; Yang, W. The Interaction between Strigolactone and Auxin Results in the Negative Effect of Shading on Soybean Branching Development. Agronomy 2023, 13, 2383. [Google Scholar] [CrossRef]
- Islam, N.S. Maize ZmWRKY28: A target to regulate shade avoidance response under high planting density. J. Exp. Bot. 2023, 74, 2937–2939. [Google Scholar] [CrossRef]
- Pierik, R.; BallarÉ, C.L.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2014, 37, 1845–1853. [Google Scholar] [CrossRef]
- Pierik, R.; de Wit, M. Shade avoidance: Phytochrome signalling and other aboveground neighbour detection cues. J. Exp. Bot. 2014, 65, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ocampo, G.; Cascales, J.; Medina-Fraga, A.L.; Ploschuk, E.L.; Mantese, A.I.; Crocco, C.D.; Matsusaka, D.; Sánchez, D.H.; Botto, J.F. Transcriptomic and physiological shade avoidance responses in potato (Solanum tuberosum) plants. Physiol. Plant. 2023, 175, e13991. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Mu, R.; Liu, B. Shade avoidance syndrome in soybean and ideotype toward shade tolerance. Mol. Breed. 2023, 43, 31. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Zhen, S.; van Iersel, M.; Bugbee, B. Why Far-Red Photons Should Be Included in the Definition of Photosynthetic Photons and the Measurement of Horticultural Fixture Efficacy. Front. Plant Sci. 2021, 12, 693445. [Google Scholar] [CrossRef]
- Elias, E.; Oliver, T.J.; Croce, R. Oxygenic Photosynthesis in Far-Red Light: Strategies and Mechanisms. Annu. Rev. Phys. Chem. 2024, 75, 231–256. [Google Scholar] [CrossRef]
- Kong, J.; Zhao, Y.; Fan, P.; Wang, Y.; Xu, X.; Wang, L.; Li, S.; Duan, W.; Liang, Z.; Dai, Z. Far-red light modulates grapevine growth by increasing leaf photosynthesis efficiency and triggering organ-specific transcriptome remodelling. BMC Plant Biol. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.W. Far-red light is needed for efficient photochemistry and photosynthesis. J. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2018, 167, 21–33. [Google Scholar] [CrossRef]
- Huber, M.; de Boer, H.J.; Romanowski, A.; van Veen, H.; Buti, S.; Kahlon, P.S.; van der Meijden, J.; Koch, J.; Pierik, R. Far-red light enrichment affects gene expression and architecture as well as growth and photosynthesis in rice. Plant Cell Environ. 2024, 47, 2936–2953. [Google Scholar] [CrossRef]
- Lee, D.W.; Oberbauer, S.F.; Johnson, P.; Krishnapilay, B.; Mansor, M.; Mohamad, H.; Yap, S.K. Effects of irradiance and spectral quality on leaf structure and function in seedlings of two Southeast Asian Hopea (Dipterocarpaceae) species. Am. J. Bot. 2000, 87, 447–455. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Sims, D.A.; Pearcy, R.W. Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am. J. Bot. 1992, 79, 449–455. [Google Scholar] [CrossRef]
- Han, R.; Ma, L.; Lv, Y.; Qi, L.; Peng, J.; Li, H.; Zhou, Y.; Song, P.; Duan, J.; Li, J.; et al. SALT OVERLY SENSITIVE2 stabilizes phytochrome-interacting factors PIF4 and PIF5 to promote Arabidopsis shade avoidance. Plant Cell 2023, 35, 2972–2996. [Google Scholar] [CrossRef]
- Zhang, Y.-t.; Zhang, Y.-q.; Yang, Q.-c.; Li, T. Overhead supplemental far-red light stimulates tomato growth under intra-canopy lighting with LEDs. J. Integr. Agric. 2019, 18, 62–69. [Google Scholar] [CrossRef]
- Romanowski, A.; Furniss, J.J.; Hussain, E.; Halliday, K.J. Phytochrome regulates cellular response plasticity and the basic molecular machinery of leaf development. Plant Physiol. 2021, 186, 1220–1239. [Google Scholar] [CrossRef] [PubMed]
- Uzair, M.; Long, H.; Zafar, S.A.; Patil, S.B.; Chun, Y.; Li, L.; Fang, J.; Zhao, J.; Peng, L.; Yuan, S.; et al. Narrow Leaf21, encoding ribosomal protein RPS3A, controls leaf development in rice. Plant Physiol. 2021, 186, 497–518. [Google Scholar] [CrossRef]
- Horváth, B.M.; Magyar, Z.; Zhang, Y.; Hamburger, A.W.; Bakó, L.; Visser, R.G.F.; Bachem, C.W.B.; Bögre, L. EBP1 regulates organ size through cell growth and proliferation in plants. EMBO J. 2006, 25, 4909–4920. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, Y.; Liu, X.; Zhang, X.S.; Su, Y.H. The Arabidopsis MATERNAL EFFECT EMBRYO ARREST45 protein modulates maternal auxin biosynthesis and controls seed size by inducing AINTEGUMENTA. Plant Cell 2021, 33, 1907–1926. [Google Scholar] [CrossRef]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef]
- Kim, C.; Kwon, Y.; Jeong, J.; Kang, M.; Lee, G.S.; Moon, J.H.; Lee, H.-J.; Park, Y.-I.; Choi, G. Phytochrome B photobodies are comprised of phytochrome B and its primary and secondary interacting proteins. Nat. Commun. 2023, 14, 1708. [Google Scholar] [CrossRef]
- Hussain, E.; Romanowski, A.; Halliday, K.J. PIF7 controls leaf cell proliferation through an AN3 substitution repression mechanism. Proc. Natl. Acad. Sci. USA 2022, 119, e2115682119. [Google Scholar] [CrossRef]
- Burko, Y.; Willige, B.C.; Seluzicki, A.; Novák, O.; Ljung, K.; Chory, J. PIF7 is a master regulator of thermomorphogenesis in shade. Nat. Commun. 2022, 13, 4942. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, W.; Yang, W. Shade Inhibits Leaf Size by Controlling Cell Proliferation and Enlargement in Soybean. Sci. Rep. 2017, 7, 9259. [Google Scholar] [CrossRef]
- Zou, Y.; Li, R.; Baldwin, I.T. ZEITLUPE is required for shade avoidance in the wild tobacco Nicotiana attenuata. J. Integr. Plant Biol. 2020, 62, 1341–1351. [Google Scholar] [CrossRef]
- Guo, H.; Dong, Q.; Li, S.; Cha, X.; Sun, L.; Duan, H.; Li, S.; Jin, Y.; Zhang, M. Effects of exogenous calcium on growth, chlorophyll fluorescence characteristics and antioxidant system of Fraxinus malacophylla seedlings. Plant Physiol. Biochem. 2023, 201, 107860. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative Study on Several Determination Methods of Chlorophyll Content in Plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, S.; Gai, S.; Gao, P.; Xu, C.; Xia, M.; Tang, W.; Lu, X. Affordable phosphor-converted LEDs with specific light quality facilitate the tobacco seedling growth with low energy consumption in Industrial Seedling Raising. J. Photochem. Photobiol. B Biol. 2022, 235, 112564. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stone, W.L.; Dratz, E.A. Visual photoreceptors. Photochem. Photobiol. 1977, 26, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Song, Z.; Wang, S.; Sang, S.; Li, F.; Wang, Z.; Yang, J. Effects of period of nitrogen application and R/FR ratio on growth physiological traits and yield of maize. Chin. Agric. Sci. Bull. 2018, 34, 10–18. [Google Scholar]
- Kim, H.-J.; Lin, M.-Y.; Mitchell, C.A. Light spectral and thermal properties govern biomass allocation in tomato through morphological and physiological changes. Environ. Exp. Bot. 2019, 157, 228–240. [Google Scholar] [CrossRef]
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, Y.; Chen, J.; Wang, X.; Yong, T.; et al. Effect of interactions between light intensity and red-to- far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; He, S.; He, D.; Shang, W.; Song, Y. Effects of different light quality ratios of LED red/blue light on growth and antioxidant enzyme activities of Photinia fraseri plantlets in vitro. J. Northwest A F Univ. Nat. Sci. Ed. 2018, 46, 49–56. [Google Scholar]
- Garab, G.; Magyar, M.; Sipka, G.; Lambrev, P.H.; Dietz, K.-J. New foundations for the physical mechanism of variable chlorophyll a fluorescence. Quantum efficiency versus the light-adapted state of photosystem II. J. Exp. Bot. 2023, 74, 5458–5471. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Wang, F.; Blackburn, G.A.; Zhang, H.K.; Wang, X.; Wei, C.; Zhang, K.; Wei, C. An extended PROSPECT: Advance in the leaf optical properties model separating total chlorophylls into chlorophyll a and b. Sci. Rep. 2017, 7, 6429. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of Continuous or End-of-Day Far-Red Light on Tomato Plant Growth, Morphology, Light Absorption, and Fruit Production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef]
- Wientjes, E.; Philippi, J.; Borst, J.W.; van Amerongen, H. Imaging the Photosystem I/Photosystem II chlorophyll ratio inside the leaf. Biochim. Biophys. Acta BBA Bioenerg. 2017, 1858, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, B.; Li, F.; Eneji, A.E.; Du, M.; Tian, X. Correction: Growth, leaf anatomy, and photosynthesis of cotton (Gossypium hirsutum L.) seedlings in response to four light-emitting diodes and high pressure sodium lamp. J. Cotton Res. 2024, 7, 8. [Google Scholar] [CrossRef]
- Ritter, M.; Oetama, V.S.P.; Schulze, D.; Muetzlaff, K.; Meents, A.K.; Seidel, R.A.; Görls, H.; Westerhausen, M.; Boland, W.; Pohnert, G. Pyrrolic and Dipyrrolic Chlorophyll Degradation Products in Plants and Herbivores. Chemistry 2020, 26, 6205–6213. [Google Scholar] [CrossRef]
- Keller, B.; Vass, I.; Matsubara, S.; Paul, K.; Jedmowski, C.; Pieruschka, R.; Nedbal, L.; Rascher, U.; Muller, O. Maximum fluorescence and electron transport kinetics determined by light-induced fluorescence transients (LIFT) for photosynthesis phenotyping. Photosynth. Res. 2018, 140, 221–233. [Google Scholar] [CrossRef]
- Magney, T.S.; Bowling, D.R.; Logan, B.A.; Grossmann, K.; Stutz, J.; Blanken, P.D.; Burns, S.P.; Cheng, R.; Garcia, M.A.; Köhler, P.; et al. Mechanistic evidence for tracking the seasonality of photosynthesis with solar-induced fluorescence. Proc. Natl. Acad. Sci. USA 2019, 116, 11640–11645. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Zhu, X.; Weng, Y. NPQ regulation mechanism and optimization of light harvesting complex in higher plants. Chin. Bull. Life Sci. 2024, 36, 1149–1159. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Dwivedi, A.; Ranjan, A. High temperature restricts cell division and leaf size by coordination of PIF4 and TCP4 transcription factors. Plant Physiol. 2022, 190, 2380–2397. [Google Scholar] [CrossRef]
- Wang, P.; Abid, M.A.; Qanmber, G.; Askari, M.; Zhou, L.; Song, Y.; Liang, C.; Meng, Z.; Malik, W.; Wei, Y.; et al. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 2022, 194, 104704. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E.; Al-Sady, B.; Carle, C.; Storer, A.; Alonso, J.M.; Ecker, J.R.; Quail, P.H. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell 2008, 20, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, W.; Liang, W.; Ling, X.; Ma, J.; Yang, C.; Li, L. Phytochrome B inhibits the activity of phytochrome-interacting factor 7 involving phase separation. Cell Rep. 2023, 42, 113562. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial Auxin Signaling Controls Leaf Flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C.; Yu, X.; Tian, Y.; Wang, W.; Zhang, Y.; Bai, W.; Yang, N.; Zhang, T.; Zheng, H.; et al. Auxin regulates source-sink carbohydrate partitioning and reproductive organ development in rice. Dev. Biol. 2022, 119, e2121671119. [Google Scholar] [CrossRef] [PubMed]
- Schruff, M.C.; Spielman, M.; Tiwari, S.; Adams, S.; Fenby, N.; Scott, R.J. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 2006, 133, 251–261. [Google Scholar] [CrossRef]
- Krizek, B.A.; Blakley, I.C.; Ho, Y.Y.; Freese, N.; Loraine, A.E. The Arabidopsis transcription factor AINTEGUMENTA orchestrates patterning genes and auxin signaling in the establishment of floral growth and form. Plant J. 2020, 103, 752–768. [Google Scholar] [CrossRef]
- Krizek, B.A. Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Dev. Genet. 1999, 25, 224–236. [Google Scholar] [CrossRef]

 is negative regulation.
is negative regulation.
 is negative regulation.
is negative regulation.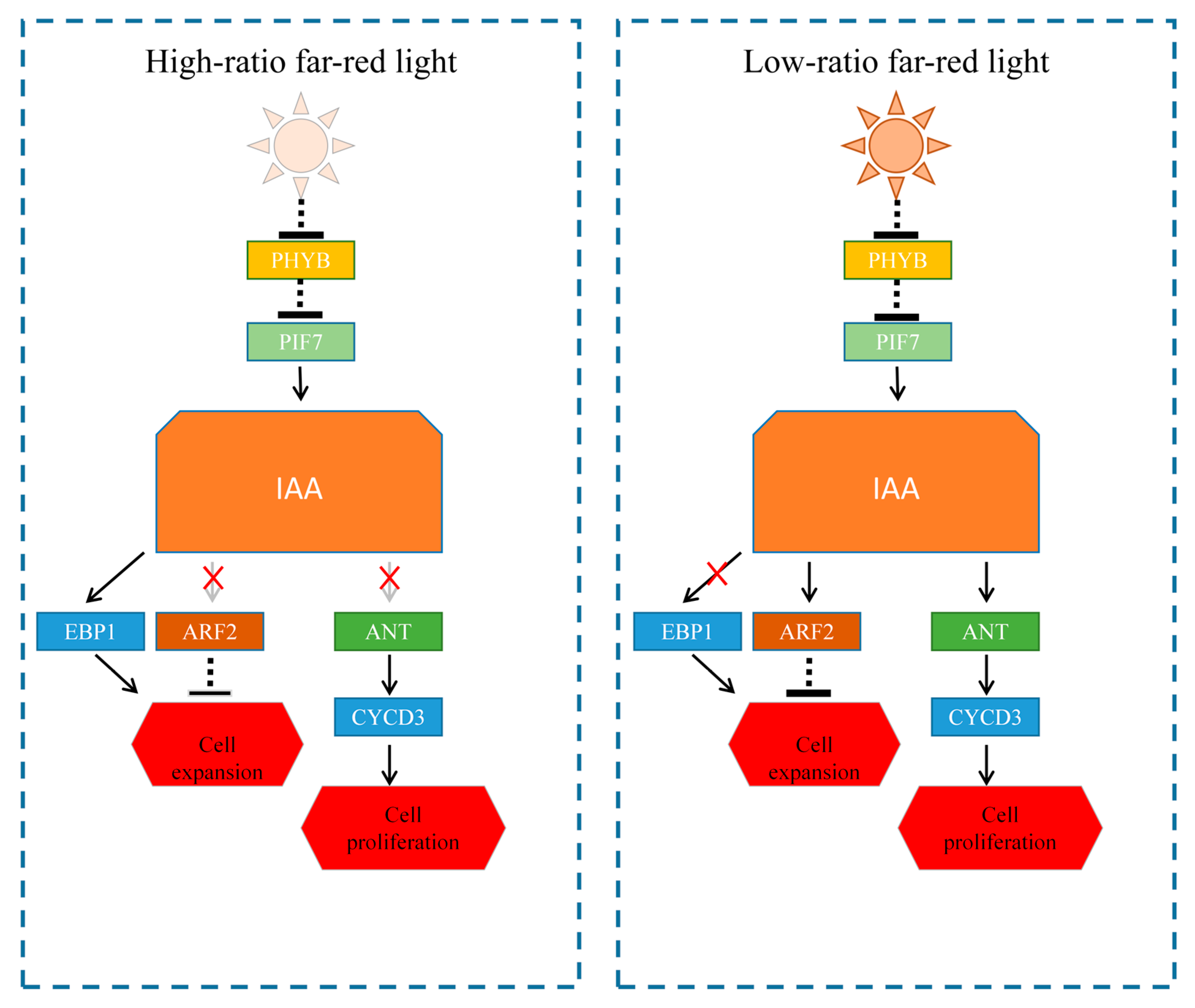
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Gai, S.; Liu, C.; Zeng, Z.; Tan, X.; Li, J.; Zhou, Z. The Effect of Far-Red Light on the Growth of Tobacco Leaves. Plants 2025, 14, 2520. https://doi.org/10.3390/plants14162520
Liu L, Gai S, Liu C, Zeng Z, Tan X, Li J, Zhou Z. The Effect of Far-Red Light on the Growth of Tobacco Leaves. Plants. 2025; 14(16):2520. https://doi.org/10.3390/plants14162520
Chicago/Turabian StyleLiu, Lei, Shujie Gai, Chuanke Liu, Zouguo Zeng, Xudong Tan, Jiawei Li, and Zhi Zhou. 2025. "The Effect of Far-Red Light on the Growth of Tobacco Leaves" Plants 14, no. 16: 2520. https://doi.org/10.3390/plants14162520
APA StyleLiu, L., Gai, S., Liu, C., Zeng, Z., Tan, X., Li, J., & Zhou, Z. (2025). The Effect of Far-Red Light on the Growth of Tobacco Leaves. Plants, 14(16), 2520. https://doi.org/10.3390/plants14162520





