Gut–Liver Axis-Mediated Anti-Obesity Effects and Viscosity Characterization of a Homogenized Viscous Vegetable Mixture in Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Results
2.1. Nutrient Composition and Viscosity Analysis
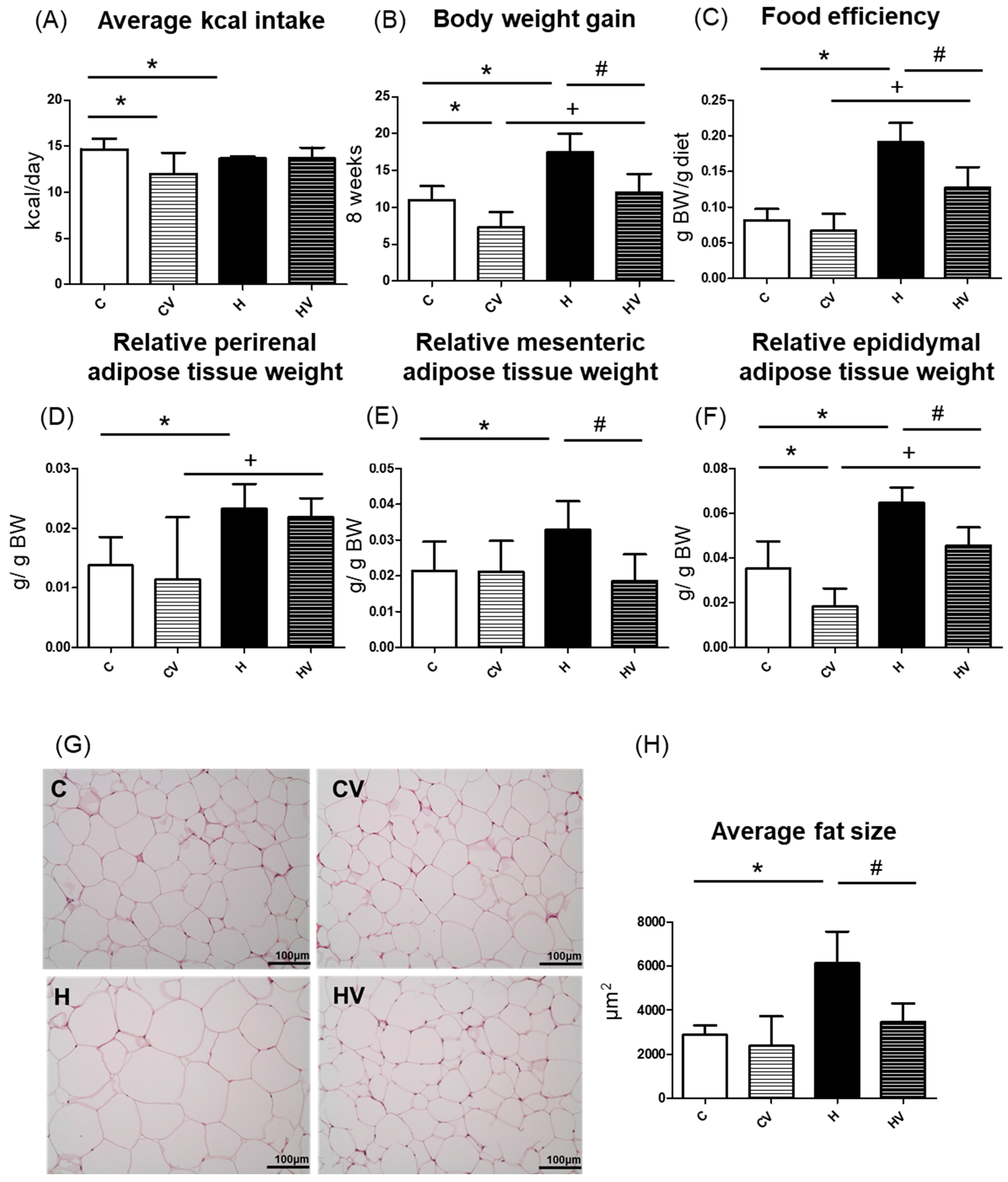
2.2. Obesity-Related Indicators
2.2.1. Food Intake and Body Weight (BW) Gain
2.2.2. Adipose Tissue Weights
2.2.3. Adipocyte Size
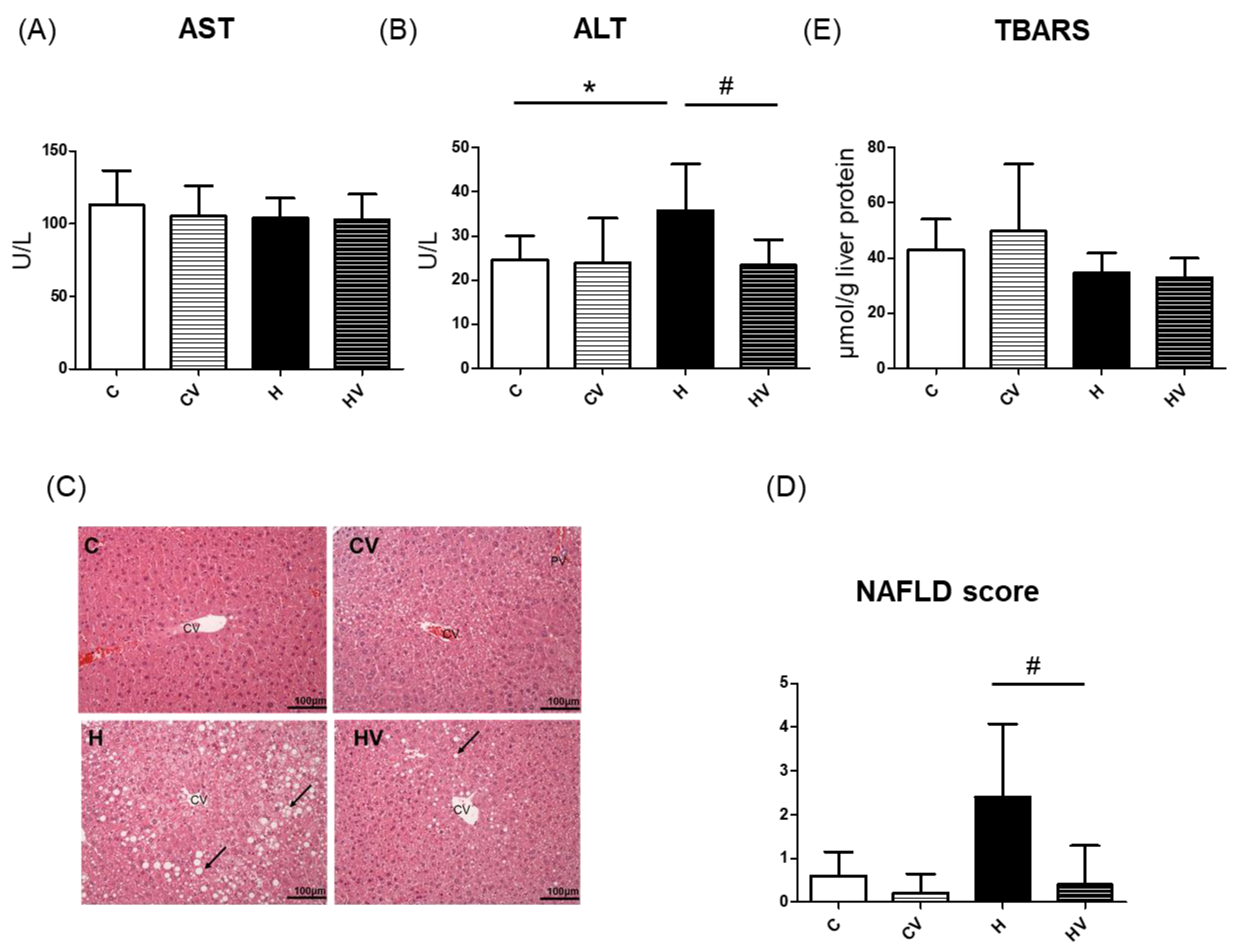
2.3. Hepatic Damage
2.3.1. Liver Function Index
2.3.2. Histopathological Examinations and Lipid Peroxidation
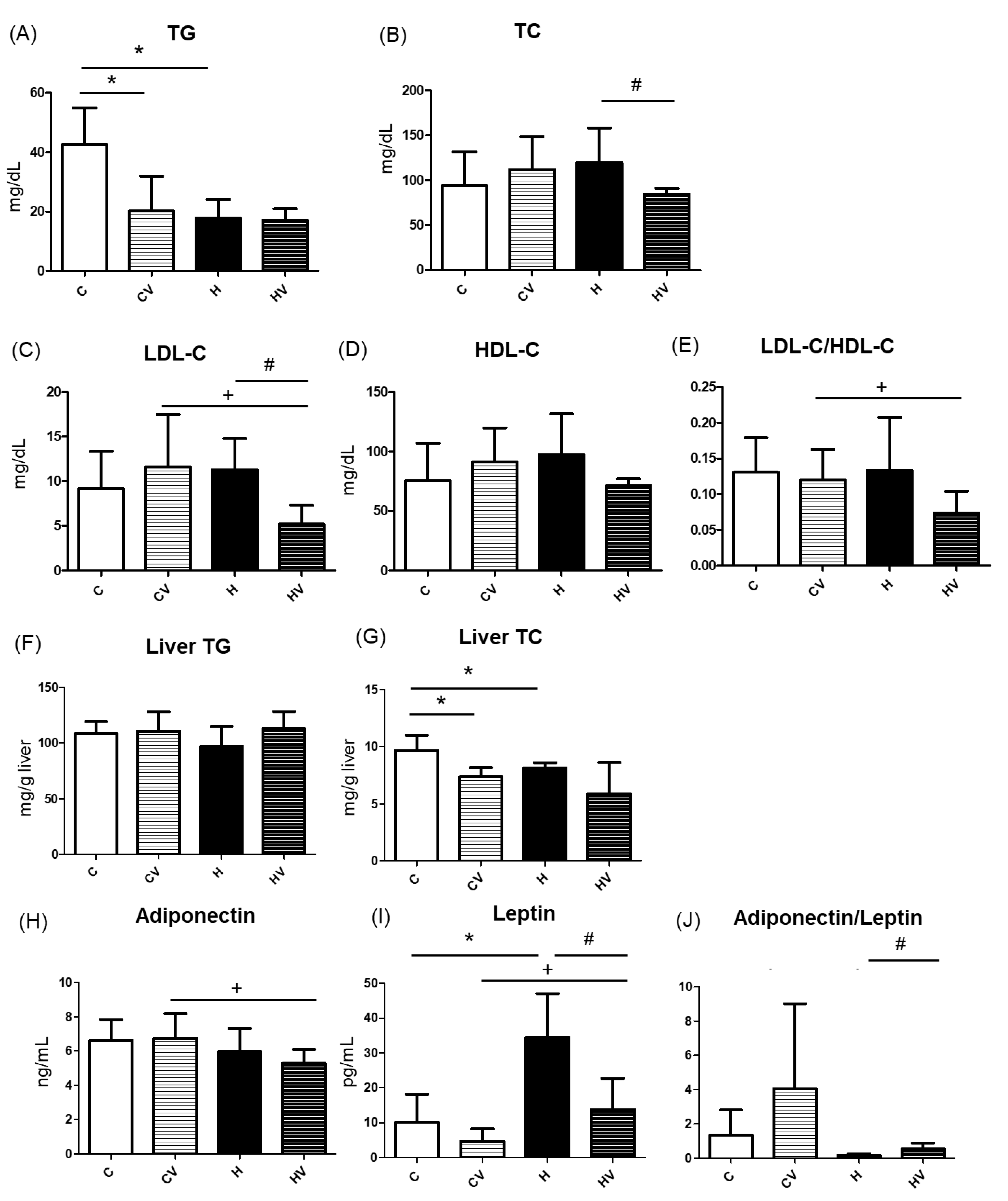
2.4. Lipid Metabolism-Related Factors
2.4.1. Serum Lipid Profiles
2.4.2. Hepatic TC and TG Concentrations
2.4.3. Plasma Adipokine Levels
2.4.4. Lipid Metabolism-Related mRNA Levels
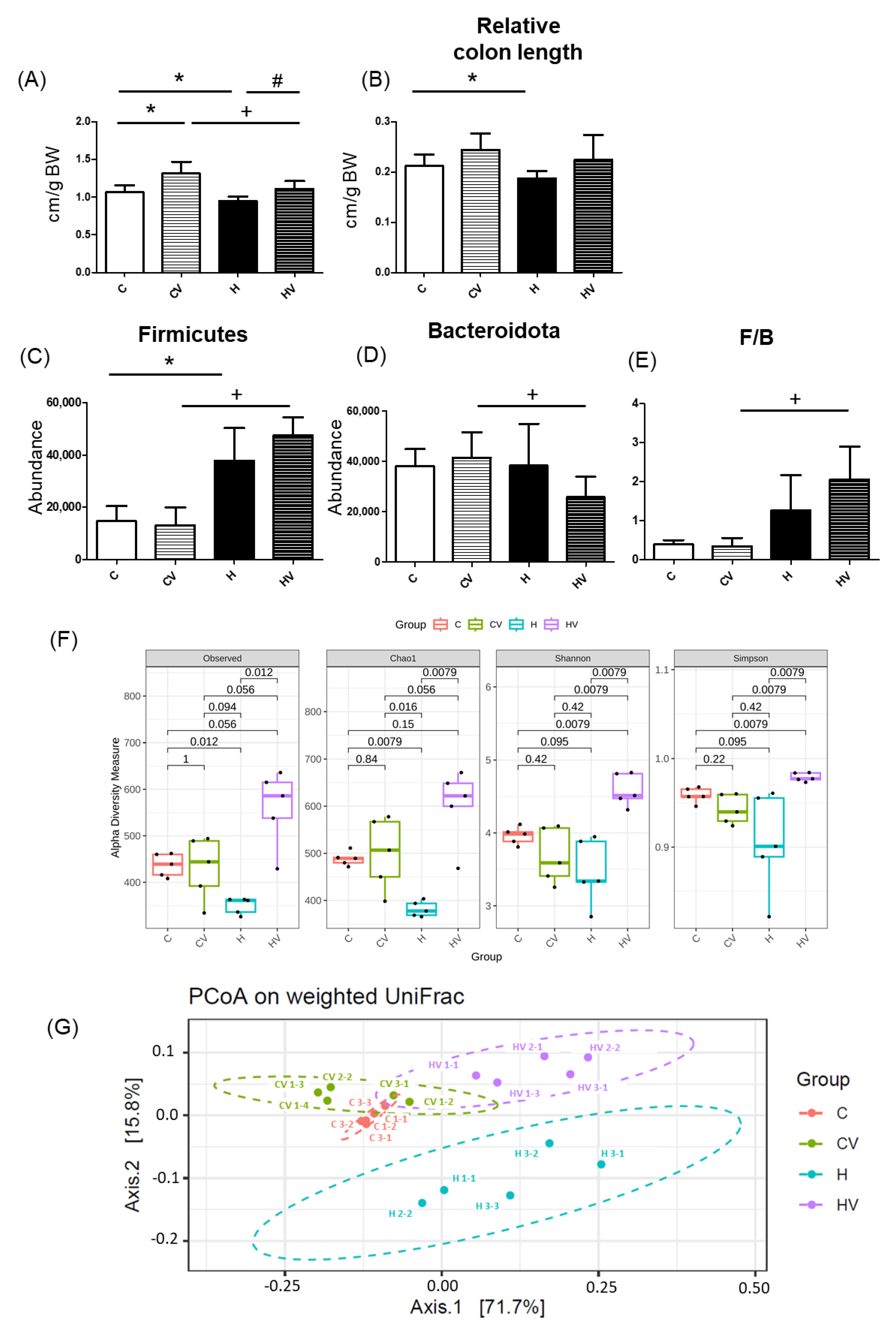
2.5. Intestinal Damage
2.5.1. Relative Intestine Length
2.5.2. Fecal Microbiotic Analysis
3. Discussion
3.1. Anti-Obesity Effects of the Homogenized VV Mixture
3.1.1. Viscosity Analysis
3.1.2. BW, and Body Fat and Calorie Intake
3.1.3. Daily Dosage
3.2. Hepatic Protective Effects of the Homogenized VV Mixture
3.3. Lipid Metabolism Regulation by the Homogenized VV Mixture
3.3.1. Serum and Hepatic Lipid Profiles
3.3.2. Hepatic Lipid Metabolism-Related Factors
3.4. Regulation of the Fecal Microbiotic Composition by a Homogenized VV Mixture
3.5. Research Applications and Limitations
4. Materials and Methods
4.1. Preparation of a Homogenized Viscous Vegetable (VV) Mixture
4.2. Viscosity Analysis
4.3. Animals and Diets
4.4. Obesity-Related Indicators
4.5. Liver Damage
4.5.1. Liver Function Index
4.5.2. Histopathological Examinations
4.5.3. Lipid Peroxidation Content
4.6. Lipid Metabolism-Related Factors
4.6.1. Serum Lipid Profiles
4.6.2. Hepatic TC and TG Concentrations
4.6.3. Plasma Adipokines Levels
4.6.4. Hepatic Fatty Acid and Cholesterol Metabolism-Related Gene Messenger (m)RNA Levels
4.7. Intestinal Damage
4.7.1. Relative Intestine Length
4.7.2. Fecal Microbiotic Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Barber, T.M.; Hanson, P.; Weickert, M.O. Metabolic-Associated Fatty Liver Disease and the Gut Microbiota. Endocrinol. Metab. Clin. N. Am. 2023, 52, 485–496. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 30 June 2025).
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Fan, S.; Chen, S.; Lin, L. Research progress of gut microbiota and obesity caused by high-fat diet. Front. Cell Infect. Microbiol. 2023, 13, 1139800. [Google Scholar] [CrossRef]
- Guo, G.J.; Yao, F.; Lu, W.P.; Xu, H.M. Gut microbiome and metabolic-associated fatty liver disease: Current status and potential applications. World J. Hepatol. 2023, 15, 867–882. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Singh, U.P. Impact of a High-Fat Diet on the Gut Microbiome: A Comprehensive Study of Microbial and Metabolite Shifts During Obesity. Cells 2025, 14, 463. [Google Scholar] [CrossRef]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. Jama 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Long, Q.; Luo, F.; Li, B.; Li, Z.; Guo, Z.; Chen, Z.; Wu, W.; Hu, M. Gut microbiota and metabolic biomarkers in metabolic dysfunction-associated steatotic liver disease. Hepatol. Commun. 2024, 8, e0310. [Google Scholar] [CrossRef]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef]
- Goksen, G.; Demir, D.; Dhama, K.; Kumar, M.; Shao, P.; Xie, F.; Echegaray, N.; Lorenzo, J.M. Mucilage polysaccharide as a plant secretion: Potential trends in food and biomedical applications. Int. J. Biol. Macromol. 2023, 230, 123146. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Taleb, M.; El-Shazly, M.; Zhao, C.; Farag, M.A. Polysaccharides (pectin, mucilage, and fructan inulin) and their fermented products: A critical analysis of their biochemical, gut interactions, and biological functions as antidiabetic agents. Phytother. Res. 2024, 38, 662–693. [Google Scholar] [CrossRef]
- Kassem, I.A.A.; Joshua Ashaolu, T.; Kamel, R.; Elkasabgy, N.A.; Afifi, S.M.; Farag, M.A. Mucilage as a functional food hydrocolloid: Ongoing and potential applications in prebiotics and nutraceuticals. Food Funct. 2021, 12, 4738–4748. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Y.; Chen, X.; Wang, C.; Chen, X.; Yuan, X.; Liu, L.; Yang, J.; Zhou, X. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 1. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chiu, K.C.; Yang, L.C. Amelioration of Obesity in Mice Fed a High-Fat Diet with Uronic Acid-Rich Polysaccharides Derived from Tremella fuciformis. Polymers 2022, 14, 1514. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Jin, H.; Oh, H.J.; Cho, S.; Lee, O.H.; Lee, B.Y. Okra (Abelmoschus esculentus L. Moench) prevents obesity by reducing lipid accumulation and increasing white adipose browning in high-fat diet-fed mice. Food Funct. 2022, 13, 11840–11852. [Google Scholar] [CrossRef]
- Zhao, R.; Ji, Y.; Chen, X.; Hu, Q.; Zhao, L. Polysaccharide from Flammulina velutipes attenuates markers of metabolic syndrome by modulating the gut microbiota and lipid metabolism in high fat diet-fed mice. Food Funct. 2021, 12, 6964–6980. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Izumo, N.; Hashimoto, M.; Tawara, S.; Mori, H.; Kuwahata, K. Anti-Obesity Effects of Sticky Japanese Diet (SJD) Assessed by Regulations of Leptin and Adiponectin. J. Nutr. Health Food Sci. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Yuan, J.; Liu, Z.; Ye, C.; Qin, S. Undaria pinnatifida improves obesity-related outcomes in association with gut microbiota and metabolomics modulation in high-fat diet-fed mice. Appl. Microbiol. Biotechnol. 2020, 104, 10217–10231. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Ren, D.; Yang, X. Effect of okra fruit powder supplementation on metabolic syndrome and gut microbiota diversity in high fat diet-induced obese mice. Food Res. Int. 2020, 130, 108929. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.Y.; Guo, W.L.; Cao, Y.J.; Lin, Y.C.; Cheng, W.J.; Chen, L.J.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanisms of macroalgae Laminaria japonica consumption against lipid metabolism disorders in high-fat diet-induced hyperlipidemic rats. Food Funct. 2020, 11, 3256–3270. [Google Scholar] [CrossRef]
- Cichero, J.A.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of International Terminology and Definitions for Texture-Modified Foods and Thickened Fluids Used in Dysphagia Management: The IDDSI Framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef]
- Kang, A.J.; Kim, D.K.; Kang, S.H.; Seo, K.M.; Park, H.S.; Park, K.H. EMG Activity of Masseter Muscles in the Elderly According to Rheological Properties of Solid Food. Ann. Rehabil. Med. 2016, 40, 447–456. [Google Scholar] [CrossRef]
- Lund, A.M.; Garcia, J.M.; Chambers, E.T. Line spread as a visual clinical tool for thickened liquids. Am. J. Speech Lang. Pathol. 2013, 22, 566–571. [Google Scholar] [CrossRef]
- Yamagata, Y.; Itadani, R.; Ikarashi, S.; Kayashita, A.; Niu, K.; Hikino, Y.; Kawashima, K.; Ooba, K.; Kayashita, J. Evaluation of classification of universal design foods corresponding to the code of japanese dysphagia diet 2013 by the JSDR dysphagia diet committee. Jpn. J. Dysphagia Rehabil. 2021, 25, 81–89. [Google Scholar]
- Wong, M.C.; Chan, K.M.K.; Wong, T.T.; Tang, H.W.; Chung, H.Y.; Kwan, H.S. Quantitative Textural and Rheological Data on Different Levels of Texture-Modified Food and Thickened Liquids Classified Using the International Dysphagia Diet Standardisation Initiative (IDDSI) Guideline. Foods 2023, 12, 3765. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
- Fan, S.; Guo, L.; Zhang, Y.; Sun, Q.; Yang, B.; Huang, C. Okra polysaccharide improves metabolic disorders in high-fat diet-induced obese C57BL/6 mice. Mol. Nutr. Food Res. 2013, 57, 2075–2078. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Zhang, X. Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders. Foods 2022, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Chen, T.; Huang, L.; Zhang, Y.; Feng, Y.; Qu, S.; Yin, X.; Liang, L.; Yan, J.; Liu, W. Tremella fuciformis polysaccharide reduces obesity in high-fat diet-fed mice by modulation of gut microbiota. Front. Microbiol. 2022, 13, 1073350. [Google Scholar] [CrossRef] [PubMed]
- Łagowska, K.; Jurgoński, A.; Mori, M.; Yamori, Y.; Murakami, S.; Ito, T.; Toda, T.; Pieczyńska-Zając, J.M.; Bajerska, J. Effects of dietary seaweed on obesity-related metabolic status: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2025, 83, e116–e130. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO scientific update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61 (Suppl. S1), S132–S137. [Google Scholar] [CrossRef]
- Dietary reference values for food energy and nutrients for the United Kingdom. Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Rep. Health Soc. Subj. 1991, 41, 1–210.
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Khan, T.J.; Xu, X.; Xie, X.; Dai, X.; Sun, P.; Xie, Q.; Zhou, X. Tremella fuciformis Crude Polysaccharides Attenuates Steatosis and Suppresses Inflammation in Diet-Induced NAFLD Mice. Curr. Issues Mol. Biol. 2022, 44, 1224–1234. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F.; Mohammed, B.S.; Pietka, T.; Abumrad, N.A.; Patterson, B.W.; Okunade, A.; Klein, S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 15430–15435. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Eng, J.M.; Estall, J.L. Diet-Induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol. Cells 2021, 10, 1805. [Google Scholar] [CrossRef]
- Han, Y.; Guo, X.; Du, H.; Guo, Y.; Ding, Q.; Li, F.; Meng, Y.; Xiao, H. Modulation of Bile Acids and Farnesoid X Receptor by Dietary Polysaccharides: Critical Roles in Health and Disease. Trends Food Sci. Technol. 2025, 162, 105075. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Dahlhoff, C.; Keller, S.; Sailer, M.; Jahreis, G.; Daniel, H. C57Bl/6 N mice on a western diet display reduced intestinal and hepatic cholesterol levels despite a plasma hypercholesterolemia. BMC Genom. 2012, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ji, G.; Jin, G.; Yuan, Z. Different responsiveness to a high-fat/cholesterol diet in two inbred mice and underlying genetic factors: A whole genome microarray analysis. Nutr. Metab. 2009, 6, 43. [Google Scholar] [CrossRef]
- Németh, K.; Tóth, B.; Sarnyai, F.; Koncz, A.; Lenzinger, D.; Kereszturi, É.; Visnovitz, T.; Kestecher, B.M.; Osteikoetxea, X.; Csala, M.; et al. High fat diet and PCSK9 knockout modulates lipid profile of the liver and changes the expression of lipid homeostasis related genes. Nutr. Metab. 2023, 20, 19. [Google Scholar] [CrossRef]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia is required for the development of leptin resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Reddy, J.K.; Hashimoto, T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Jia, F.; Gao, Y.; Zhang, J.; Hou, F.; Shi, J.; Song, S.; Yang, S. Flammulina velutipes mycorrhizae dietary fiber attenuates the development of obesity via regulating lipid metabolism in high-fat diet-induced obese mice. Front. Nutr. 2025, 12, 1551987. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, Q.; Li, Y.; Bai, Y.; Zhang, J.; Xu, W.; Xu, J. Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice. Nutrients 2022, 14, 3830. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Soares, A.; Beraldi, E.J.; Ferreira, P.E.; Bazotte, R.B.; Buttow, N.C. Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice. BMC Gastroenterol. 2015, 15, 3. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Lecomte, V.; Kaakoush, N.O.; Maloney, C.A.; Raipuria, M.; Huinao, K.D.; Mitchell, H.M.; Morris, M.J. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE 2015, 10, e0126931. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Zhang, X.; You, Y.; Wang, L.; Ai, C.; Huang, L.; Wang, S.; Wang, Z.; Song, S.; Zhu, B. Anti-obesity effects of Laminaria japonica fucoidan in high-fat diet-fed mice vary with the gut microbiota structure. Food Funct. 2022, 13, 6259–6270. [Google Scholar] [CrossRef]
- Li, T.; Teng, H.; An, F.; Huang, Q.; Chen, L.; Song, H. The beneficial effects of purple yam (Dioscorea alata L.) resistant starch on hyperlipidemia in high-fat-fed hamsters. Food Funct. 2019, 10, 2642–2650. [Google Scholar] [CrossRef]
- Hu, R. Grifola frondosa may play an anti-obesity role by affecting intestinal microbiota to increase the production of short-chain fatty acids. Front. Endocrinol. 2022, 13, 1105073. [Google Scholar] [CrossRef]
- Kovynev, A.; Charchuta, M.M.; Begtašević, A.; Ducarmon, Q.R.; Rensen, P.C.N.; Schönke, M. Combination of dietary fiber and exercise training improves fat loss in mice but does not ameliorate MASLD more than exercise alone. Am. J. Physiol. Gastrointest. Liver Physiol. 2025, 328, G399–G410. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut Firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Guo, C.; Geng, X.; Yao, Y.; Guo, J.; Zhang, Y.; Zhang, J.; Mi, S. Effects of fruits and vegetables on gut microbiota in a mouse model of metabolic syndrome induced by high-fat diet. Food Sci. Nutr. 2022, 11, 794–805. [Google Scholar] [CrossRef]
- DeVries, J.W. Dietary fiber: The influence of definition on analysis and regulation. J. AOAC Int. 2004, 87, 682–706. [Google Scholar] [CrossRef]
- US FDA. Questions and Answers on Dietary Fiber. Available online: https://www.fda.gov/food/food-labeling-nutrition/questions-and-answers-dietary-fiber (accessed on 18 November 2022).
- Kim, Y.H.; Jeong, G.Y.; Yoo, B. Comparative study of IDDSI flow test and line-spread test of thickened water prepared with different dysphagia thickeners. J. Texture Stud. 2018, 49, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, M.A.; Robbins, J. The usefulness of the line spread test as a measure of liquid consistency. Dysphagia 2007, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Huang, W.C.; Ng, X.E.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Wu, H.H.; Yeh, C.L.; Shirakawa, H.; Budijanto, S.; et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients 2019, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef]


| Viscous Vegetable Mixture (100 g) | |
|---|---|
| Crude protein (g) | 17.7 |
| Crude fat (g) | 3.8 |
| Saturated fat (g) | 1.25 |
| Trans fat (g) | - |
| Carbohydrates (g) | 62.5 |
| Soluble dietary fiber (g) | 16.0 |
| Insoluble dietary fiber (g) | 26.1 |
| Sugar (g) | 2.7 |
| Ash (g) | 7.5 |
| Sodium (mg) | 773.7 |
| Moisture (g) | 8.5 |
| Calories (kcal) | 270.8 |
| Total Polysaccharides (mg/g) | Total Polyphenols (mg GAE/g) | |
|---|---|---|
| Seaweed (Laminariaceae) | 13.7 | 1.2 |
| Kelp (Undaria pinnatifida) | 4.5 | 0.4 |
| Agar (Gelidiaceae) | 1.1 | 0.03 |
| White tremella (Tremella fuciformis) | 1.7 | 0.08 |
| Shiitake mushroom (Lentinula edodes) | 18.3 | 1.2 |
| Yellow strain Flammulina velutipes | 25.6 | 1.8 |
| Okra (Abelmoschus esculentus) | 122.7 | 3.4 |
| Laver (root of Pyropia) | 2.6 | 2.2 |
| Purple yam (Dioscorea alata) | 7.9 | 0.4 |
| Brown shimeji mushroom (Hypsizygus tessellatus) | 13.9 | 1.3 |
| Total | 212 | 12 |
| Ratio 2 | Quadrant 1 | Quadrant 2 | Quadrant 3 | Quadrant 4 | Quadrant 5 | Quadrant 6 | LST Value | Classification 3 |
|---|---|---|---|---|---|---|---|---|
| 1:9 | 35 | 36.5 | 32.5 | 30.5 | 33.5 | 31 | 33.2 | Grade 2 |
| 1:12 | 44 | 43 | 47.5 | 49.5 | 51 | 43.5 | 46.4 | Below threshold |
| 1:14 | 42 | 41.5 | 44 | 51 | 53.5 | 49 | 46.8 | Below threshold |
| 1:17 | 45.5 | 47 | 44.5 | 50 | 50 | 47 | 47.3 | Below threshold |
| 1:19 | 52 | 51 | 52.5 | 56 | 58 | 57.5 | 54.5 | Below threshold |
| Ratio 2 | Volume Remaining in Syringe After 10 s (mL) | Classification 3 |
|---|---|---|
| 1:9 | 10 | Level 4 |
| 1:12 | 9 | Level 3 |
| 1:14 | 9 | Level 3 |
| 1:17 | 8 | Level 3 |
| 1:19 | 8 | Level 3 |
| C | CV | H | HV | |
|---|---|---|---|---|
| Protein (kcal%) | 14.7% | 15.5% | 19.9% | 20.2% |
| Carbohydrates (kcal%) | 75.6% | 72.3% | 19.3% | 19.9% |
| Fat (kcal%) | 9.5% | 9.7% | 60.5% | 57.9% |
| kcal/g | 3.81 | 3.70 | 5.25 | 5.00 |
| Ingredients (g/kg) | ||||
| Cornstarch 1 | 465 | 418.5 | 0 | 0 |
| Maltodextrin 2 | 155 | 139.5 | 163.4 | 147.06 |
| Sucrose 3 | 100 | 90 | 90 | 81 |
| Casein 4 | 140 | 126 | 261.5 | 235.35 |
| L-cysteine 5 | 2 | 1.8 | 3.9 | 3.51 |
| Soybean oil 6 | 40 | 36 | 32.7 | 29.43 |
| Lard 7 | 0 | 0 | 320.4 | 288.36 |
| Cellulose 8 | 50 | 45 | 65.4 | 58.86 |
| Mineral mixture (AIN-93M-MIX) 9 | 35 | 31.5 | 45.8 | 41.22 |
| Vitamin mixture (AIN-93M-MIX) 10 | 10 | 9 | 13.1 | 11.79 |
| Choline bitarate 11 | 3 | 2.7 | 3.9 | 3.51 |
| Tert-butylhydroquinone 12 | 0.008 | 0.0072 | 0.01 | 0.009 |
| Homogenized viscous vegetable mixture 13 | 0 | 100 | 0 | 100 |
| Forward 5′ → 3′ | Reverse 5′ → 3′ | |
|---|---|---|
| SREBP1c | AGATCCAGGTTTAGGTGGG | ATCGCAAACAAGCTGACCTG |
| AMPKα | TGATGTGAGGGTGCCTGAAC | GAAAGTGAAGGTGGGCAAGC |
| ACC1 | GGACCACTGCATGGAATGTTAA | TGAGTGACTGCCGAAACATCTC |
| FAS | AACCTGATGGATGAGCACC | CTGTGCCCGTCGTCTATACC |
| SCD1 | CCTCCTGCAAGCTCTACACC | CTGCCTTGGGTCAGAGGGTA |
| PPARα | TTGCAGCTTCGATCACACTTGTCG | TACCACTATGGAGTCCACGCATGT |
| PPARγ | ACCTGATGGCATTGTGAGACA | ATTGAGTGCCGAGTCTGTGG |
| PGC1 | GGAATATGGTGATCGGGAACA | AAAGGATGCGCTCTCGTTCA |
| SIRT1 | TTGACCGATGGACTCCTCACT | ATTGTTCGAGGATCGGTGCC |
| AdipoR2 | AGAATCCGTGGAGCTCAGCA | TGTCCAAATGTTGCCCGTCT |
| MCAD | AACTAAACATGGGCCAGCGA | GAAACCTGCTCCTTCACCGA |
| ACO1 | TTTGTGGAACCTGTTGGCCT | AAAATCTGGGGCTCTGGCTC |
| CPT1 | ACTCCGCTCGCTCATTCCG | GAGATCGATGCCATCAGGGG |
| SREBP2 | TGAGTACATGTGGGGAGCTT | TCAAACCCCACGGCAACAA |
| CYP7A1 | GGGCAGGCTTGGGAATTTTG | ACAGCTACTAGGGGGCTTCA |
| β actin | CTGAGCTGCGTTTTACACCC | TTTGGGGGATGTTTGCTCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.-A.; Chen, Y.-H.; Fu, L.-C.; Yeh, C.-L.; Lin, S.-H.; Huang, Y.-T.; Watanabe, Y.; Yang, S.-C. Gut–Liver Axis-Mediated Anti-Obesity Effects and Viscosity Characterization of a Homogenized Viscous Vegetable Mixture in Mice Fed a High-Fat Diet. Plants 2025, 14, 2510. https://doi.org/10.3390/plants14162510
Wei Y-A, Chen Y-H, Fu L-C, Yeh C-L, Lin S-H, Huang Y-T, Watanabe Y, Yang S-C. Gut–Liver Axis-Mediated Anti-Obesity Effects and Viscosity Characterization of a Homogenized Viscous Vegetable Mixture in Mice Fed a High-Fat Diet. Plants. 2025; 14(16):2510. https://doi.org/10.3390/plants14162510
Chicago/Turabian StyleWei, Yu-An, Yi-Hsiu Chen, Lu-Chi Fu, Chiu-Li Yeh, Shyh-Hsiang Lin, Yuh-Ting Huang, Yasuo Watanabe, and Suh-Ching Yang. 2025. "Gut–Liver Axis-Mediated Anti-Obesity Effects and Viscosity Characterization of a Homogenized Viscous Vegetable Mixture in Mice Fed a High-Fat Diet" Plants 14, no. 16: 2510. https://doi.org/10.3390/plants14162510
APA StyleWei, Y.-A., Chen, Y.-H., Fu, L.-C., Yeh, C.-L., Lin, S.-H., Huang, Y.-T., Watanabe, Y., & Yang, S.-C. (2025). Gut–Liver Axis-Mediated Anti-Obesity Effects and Viscosity Characterization of a Homogenized Viscous Vegetable Mixture in Mice Fed a High-Fat Diet. Plants, 14(16), 2510. https://doi.org/10.3390/plants14162510








