Foliar Morphoanatomical and Phytochemical Variations Shape Resistance to Key Insect Herbivores and Leaf Quality in Cyclocarya paliurus
Abstract
1. Introduction
2. Results
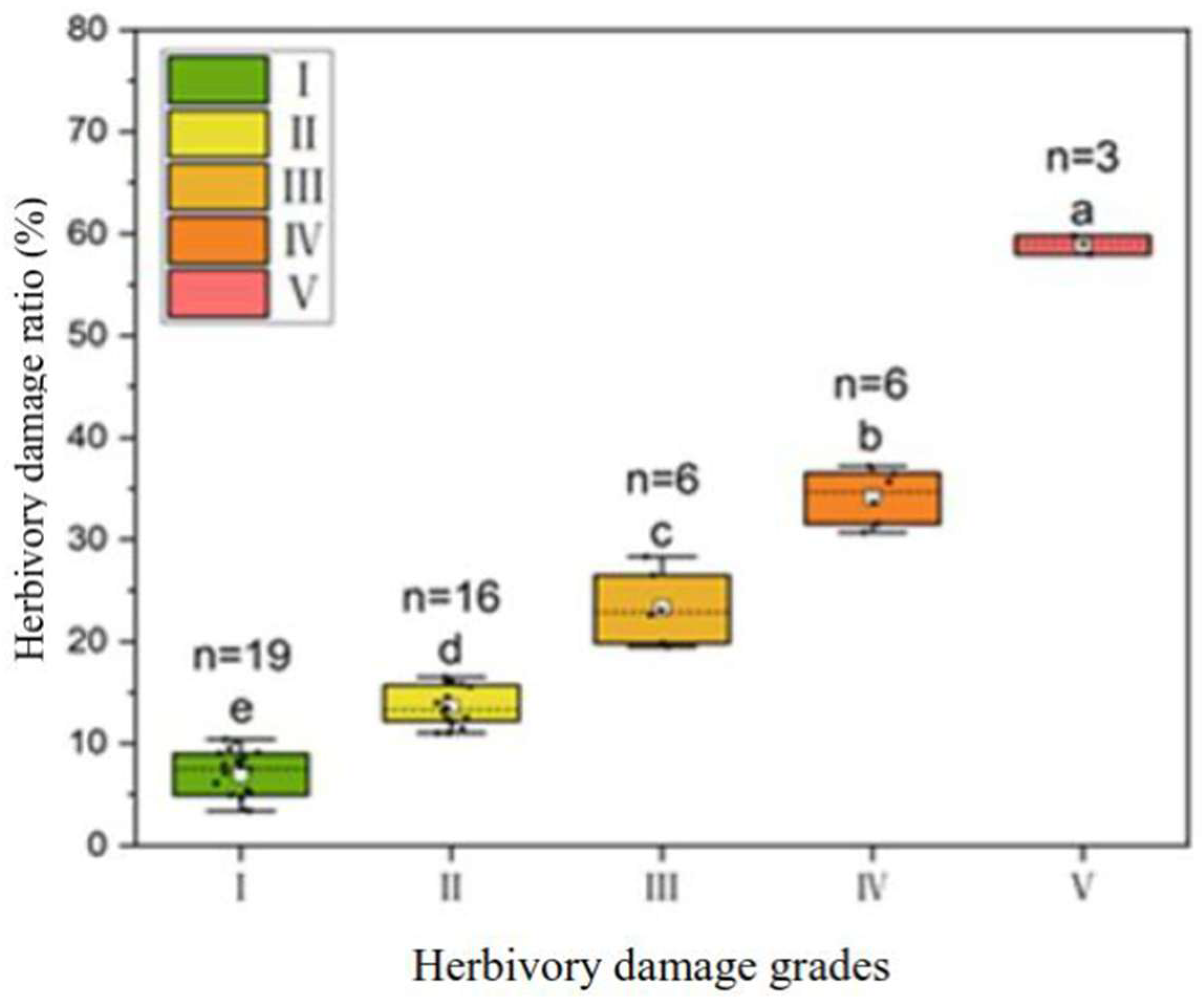
2.1. Variation in Leaf Herbivory Damage Ratio
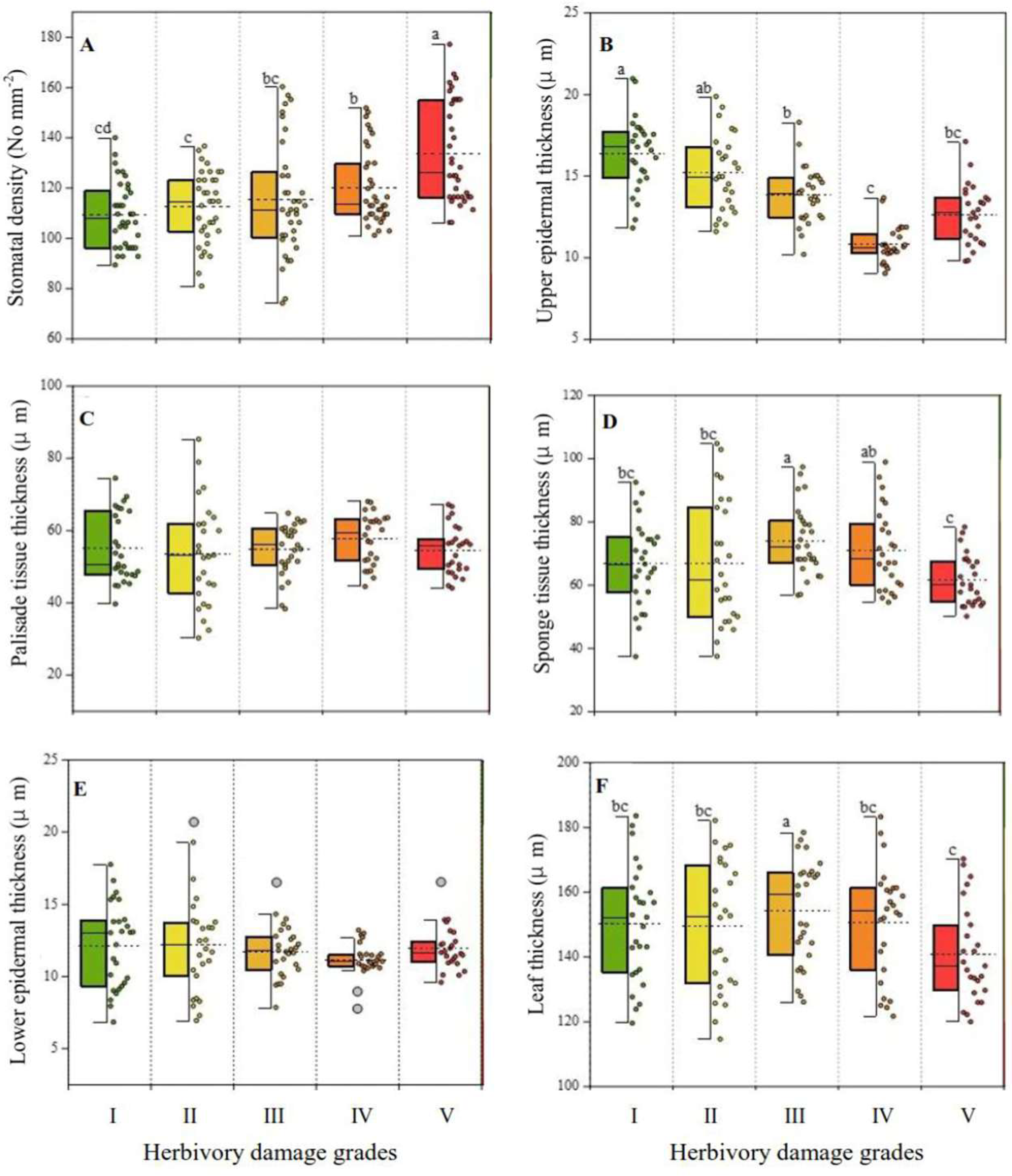
2.2. Variations in Leaf Morphoanatomical Characteristics
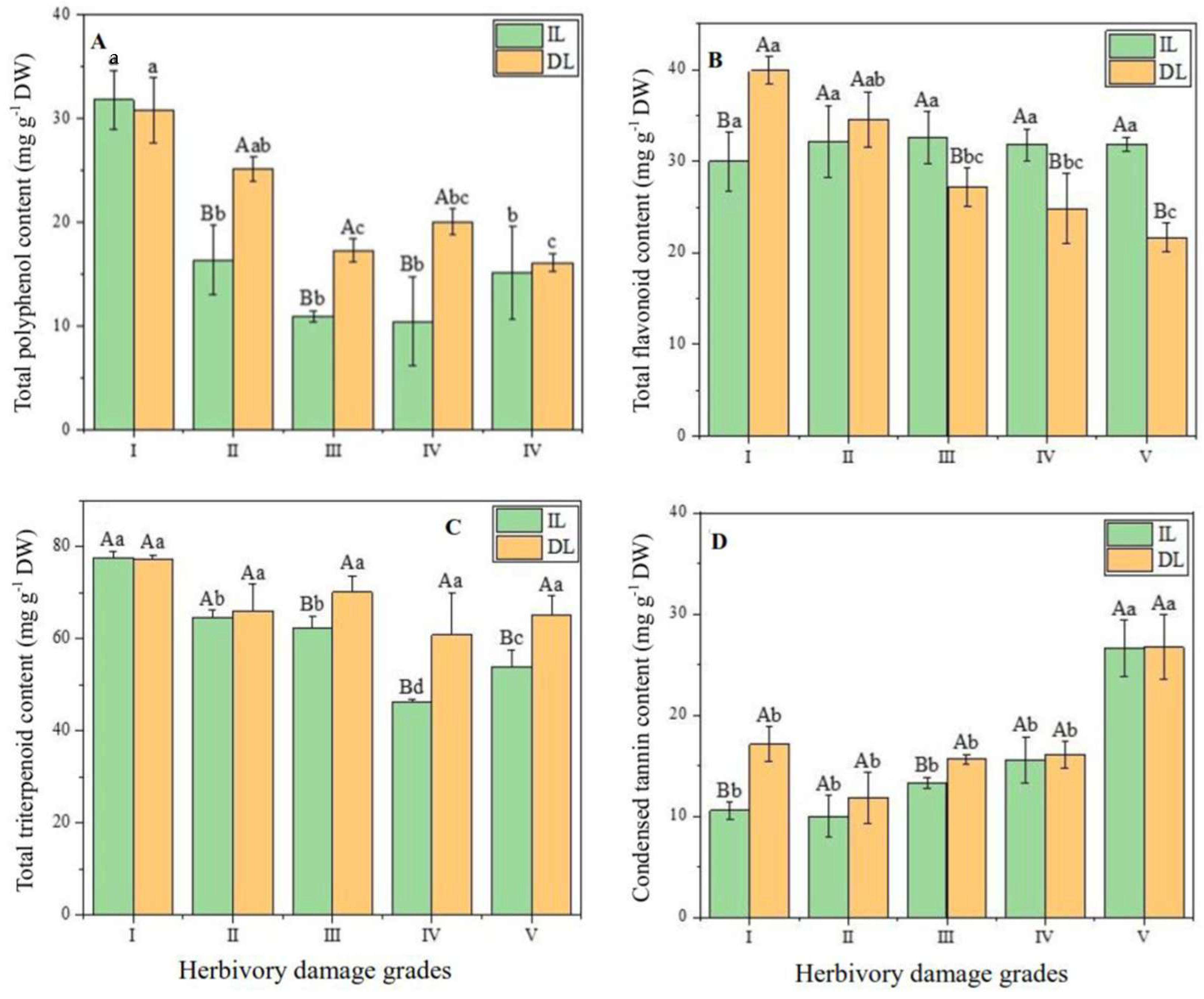
2.3. Variations in Leaf Secondary Metabolites
2.4. Variation in Leaf Quality per Plant
2.5. Relationships of Herbivory Insect Attack to the Measured Traits
3. Discussion
3.1. Responses of Leaf Secondary Metabolites to Herbivory Insect Attacks
3.2. Relationships Between Leaf Morphoanatomical Traits and Insect Damage
3.3. Effects of Herbivory Damage Ratio on Leaf Quality per Plant
4. Materials and Methods
4.1. Study Site and Plant Materials
4.2. Field Investigation on Insect Herbivores and Leaf Damage
4.3. Leaf Sampling Method
4.4. Measurements of Leaf Morphoanatomical Characteristics
4.5. Extraction and Analysis of Leaf Secondary Metabolites
4.6. Comprehensive Assessment of Leaf Quality
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HDR | Herbivory damage ratio |
| HDG | Herbivory damage grade |
| ILs | Intact leaflets |
| DLs | Damaged leaflets |
| SLA | Specific leaf area |
| TOPSIS | Technique for Order of Preference by Similarity to Ideal Solution |
References
- Lombarderoa, M.J.; Ayresb, M.P.; Bonelloc, P.; Cipollinid, D.; Daniel, A.H. Effects of defoliation and site quality on growth and defenses of Pinus pinaster and P. radiata. For. Ecol. Manag. 2016, 382, 39–50. [Google Scholar] [CrossRef]
- Cosmo, L.G.; Yamaguchi, L.F.; Felix, G.M.F.; Kato, M.J.; Cogni, R.; Pareja, M. From the leaf to the community: Distinct dimensions of phytochemical diversity shape insect–plant interactions within and among individual plants. J. Ecol. 2021, 109, 2475–2487. [Google Scholar] [CrossRef]
- Field, E.; Hector, A.; Barsoum, N.; Koricheva, J. Tree diversity reduces pathogen damage in temperate forests: A systematic review and meta-analysis. For. Ecol. Manag. 2025, 578, 122398. [Google Scholar] [CrossRef]
- Wetzel, W.C.; Kharouba, H.M.; Robinson, M.; Holyoak, M.; Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Lebbink, G.; Risch, A.C.; Schuetz, M.; Firn, J. How plant traits respond to and affect vertebrate and invertebrate herbivores—Are measurements comparable across herbivore types? Plant Cell Environ. 2024, 47, 5–23. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hua, J.; Zhang, J.; Luo, S. Negative interactions balance growth and defense in plants confronted with herbivores or pathogens. J. Agric. Food Chem. 2022, 70, 12723–12732. [Google Scholar] [CrossRef]
- Barker, H.L.; Holeski, L.M.; Lindroth, R.L. Independent and interactive effects of plant genotype and environment on plant traits and insect herbivore performance: A meta-analysis with Salicaceae. Funct. Ecol. 2018, 33, 422–435. [Google Scholar] [CrossRef]
- Eisenring, M.; Unsicker, S.B.; Lindroth, R.L. Spatial, genetic and biotic factors shape within-crown leaf trait variation and herbivore performance in a foundation tree species. Funct. Ecol. 2021, 35, 54–66. [Google Scholar] [CrossRef]
- Herrera, C.M. The ecology of subindividual variability in plants: Patterns, processes, and prospects. Web Ecol. 2017, 17, 51–64. [Google Scholar] [CrossRef]
- Wetzel, W.C.; Meek, M.H. Physical defenses and herbivory vary more within plants than among plants in the tropical understory shrub Piper polytrichum. Botany-Botanique 2019, 97, 113–121. [Google Scholar] [CrossRef]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011, 72, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Rubert-Nason, K.F.; Couture, J.J.; Major, I.T.; Constabel, C.P.; Lindroth, R.L. Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J. Chem. Ecol. 2015, 41, 651–661. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, Z.; Liu, X.; Zeng, L.; Cheng, S.; Li, J.; Tang, J.; Yang, Z. Effect of major tea insect attack on formation of quality-related nonvolatile specialized metabolites in tea (Camellia sinensis) leaves. J. Agric. Food Chem. 2019, 67, 6716–6724. [Google Scholar] [CrossRef]
- Vaca-Sanchez, M.S.; Espírito-Santo, M.M.; Maldonado-Lopez, Y.; Oyama, K.; P´erez-Solache, A.; de Faria, M.L.; Borges, M.A.Z.; Fernandes, G.W.; Cuevas-Reyes, P. Functional leaf-trait variability and herbivory in oaks along a Mexican avocado agrosystem mosaic. Flora 2024, 310, 152437. [Google Scholar] [CrossRef]
- Ruiz-Carbayo, H.; Pino, J.; Bonal, R.; James, P.; Hampe, A.; Molowny-Horas, R.; Espelta, J.M. Insect herbivory in novel Quercus ilex L. forests: The role of landscape attributes, forest composition and host traits. Ann. For. Sci. 2020, 77, 32. [Google Scholar] [CrossRef]
- Liao, K.Y.; Wang, R.Q.; Li, H.; Liu, J.H.; Chen, S.L.; Wei, S.Y.; Zhao, J.J.; Yang, P.; Deng, X.; Wang, Y.K.; et al. Insect resistance in Quercus robur: A comparative metabolomic and transcriptomic analysis of normal and curly leaf varieties. J. Agric. Food Chem. 2025, 73, 9112–9127. [Google Scholar] [CrossRef] [PubMed]
- Manchester, S.R.; Chen, Z.; Lu, A.; Uemura, K. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol. 2009, 47, 1–42. [Google Scholar] [CrossRef]
- Fang, S.; Wang, J.; Wei, Z.; Zhu, Z. Methods to break seed dormancy in Cyclocarya paliurus (Batal)Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Qin, J.; Yue, X.; Fang, S.; Qian, M.; Zhou, S.; Shang, X.; Yang, W. Responses of nitrogen metabolism, photosynthetic parameter and growth to nitrogen fertilization in Cyclocarya paliurus. For. Ecol. Manag. 2021, 502, 119715. [Google Scholar] [CrossRef]
- Fang, S. A review on the development history and the resource silviculture of Cyclocarya paliurus industry. J. Nanjing Norm. Univ. (Nat. Sci. Ed.) 2022, 46, 115–126. (In Chinese) [Google Scholar]
- Deng, B.; Fang, S.; Shang, X.; Fu, X.; Li, Y. Influence of provenance and shade on biomass production and triterpenoid accumulation in Cyclocarya paliurus. Agrofor. Syst. 2019, 93, 483–492. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, W.; Wan, S.; Fang, S. Insights into the hormone-regulating mechanism of adventitious root formation in softwood cuttings of Cyclocarya paliurus and optimization of the hormone-based formula for promoting rooting. Int. J. Mol. Sci. 2024, 25, 1343. [Google Scholar] [CrossRef]
- Deng, B.; Cao, Y.; Fang, S.; Shang, X.; Yang, W.; Qian, C. Variation and stability of growth and leaf flavonoid content in Cyclocarya paliurus across environments. Ind. Crops Prod. 2015, 76, 386–393. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Fang, J.; Zhang, L.; Jin, H.Y.; Fang, S.Z. Genome-wide identification of bHLH transcription factors and their response to salt stress in Cyclocarya paliurus. Front. Plant Sci. 2023, 14, 1117246. [Google Scholar] [CrossRef]
- Li, C.; Wan, Y.; Shang, X.; Fang, S. Integration of transcriptomic and metabolomic analysis unveils the response mechanism of sugar metabolism in Cyclocarya paliurus seedlings subjected to PEG-induced drought stress. Plant Physiol. Biochem. 2023, 201, 107856. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, M.; Shang, X.; Fang, S.; Chen, F. Etiology of Cyclocarya paliurus anthracnose in Jiangsu Province, China. Front. Plant Sci. 2021, 11, 613499. [Google Scholar] [CrossRef]
- Afroz, M.; Rahman, M.; Amin, R. Insect plant interaction with reference to secondary metabolites: A review. Agric. Rev. 2021, 42, 427–433. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.K.; Tiwari, K.N. Role of secondary metabolites in plant defense mechanisms: A molecular and biotechnological insights. Phytochem. Rev. 2025, 24, 953–983. [Google Scholar] [CrossRef]
- Mei, X.; Liu, X.Y.; Zhou, Y.; Wang, X.Q.; Zeng, L.T.; Fu, X.M.; Li, J.L.; Tang, J.C.; Dong, F.; Yang, Z.Y. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda). Food Chem. 2017, 237, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.A.; Rodriguez-Cabal, M.A.; Wu, E.T.; Julkunen-Tiitto, R.; Ritland, C.E.; Miscampbell, A.E.; Jules, E.S.; Crutsinger, G.M. Multiple plant traits shape the genetic basis of herbivore community assembly. Funct. Ecol. 2015, 29, 995–1006. [Google Scholar] [CrossRef]
- Ruhnke, H.; Scha¨dler, M.; Klotz, S.; Matthies, D.; Brandl, R. Variability in leaf traits, insect herbivory and herbivore performance within and among individuals of four broad-leaved tree species. Basic Appl. Ecol. 2009, 10, 726–736. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Jactel, H. Unraveling plant–animal diversity relationships: A meta-regression analysis. Ecology 2012, 93, 2115–2124. [Google Scholar] [CrossRef]
- Tateda, C.; Obara, K.; Abe, Y.; Sekine, R.; Nekoduka, S.; Hikage, T.; Nishihara, M.; Sekine, K.T.; Fujisaki, K. The Host Stomatal Density Determines Resistance to Septoria gentianae in Japanese Gentian. Mol. Plant-Microbe Interact. 2019, 32, 428–436. [Google Scholar] [CrossRef]
- Hou, S.; Rodrigues, O.; Liu, Z.; Shan, L.; He, P. Small holes, big impact: Stomata in plant–pathogen–climate epic trifecta. Mol. Plant 2024, 17, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Moura de Melo, L.F.; de Queiroz Aquino-Martins, V.G.; da Silva, A.P.; Rocha, H.A.O.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Y.; Tan, H.B.; Huang, H.T.; Yu, J.Z.; Zeng, L.T.; Liao, Y.Y.; Wu, P.; Yang, Z.Y. Light synergistically promotes the tea green leafhopper infestation-induced accumulation of linalool oxides and their glucosides in tea (Camellia sinensis). Food Chem. 2022, 394, 133460. [Google Scholar] [CrossRef]
- Hazarika, L.K.; Bhuyan, M.; Hazarika, B.N. Insect pests of tea and their management. Annu. Rev. Entomol. 2009, 54, 267–284. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Y.; Song, F.; Liu, F. Differential responses of stomatal morphology to partial root-zone drying and deficit irrigation in potato leaves under varied nitrogen rates. Sci. Hortic. 2012, 145, 76–83. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, H.; Fang, F.; Wu, N.; Zhang, Y.; Tang, M. Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus × canadensis ‘Neva’. J. Plant Growth Regul. 2017, 36, 824–835. [Google Scholar] [CrossRef]
- Cao, Y.N.; Fang, S.Z.; Yin, Z.Q.; Fu, X.X.; Shang, X.L.; Yang, W.X.; Yang, H.M. Chemical fingerprint and multicomponent quantitative analysis for the quality evaluation of Cyclocarya paliurus leaves by HPLC-Q-TOF-MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Phenolic content and antioxidant capacity of selected cucurbit fruits extracted with different solvents. Food Chem. 2019, 115, 785–788. [Google Scholar] [CrossRef]
- Zhou, M.M.; Quek, S.Y.; Shang, X.L.; Fang, S.Z. Geographical variations of triterpenoid contents in Cyclocarya paliurus leaves and their inhibitory effects on Hela cells. Ind. Crops Prod. 2021, 162, 113314. [Google Scholar] [CrossRef]
- Zhou, M.M.; Chen, P.; Shang, X.L.; Fang, S.Z. Genotype-Environment interactions for tree growth and leaf phytochemical content of Cyclocarya paliurus (Batal.) Iljinskaja. Forests 2021, 12, 735. [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.E.; Yan, S.C.; Tong, L.L.; Gao, L.L.; Wang, Y.J. Content differences of condensed tannin in needles of Larix gmelinii by cutting needles and insect feeding. Acta Ecol. Sin. 2009, 29, 1415–1420. [Google Scholar]
- Meshram, S.G.; Alvandi, E.; Meshram, C.; Kahya, E.; Fadhil Al-Quraishi, A.M. Application of SAW and TOPSIS in prioritizing watersheds. Water Resour. Manag. 2020, 34, 715–732. [Google Scholar] [CrossRef]
- Fang, S.; Liu, Y.; Yue, J.; Tian, Y.; Xu, X. Assessments of growth performance, crown structure, stem form and wood property of introduced poplar clones: Results from a long-term field experiment at a lowland site. For. Ecol. Manag. 2021, 479, 118586. [Google Scholar] [CrossRef]
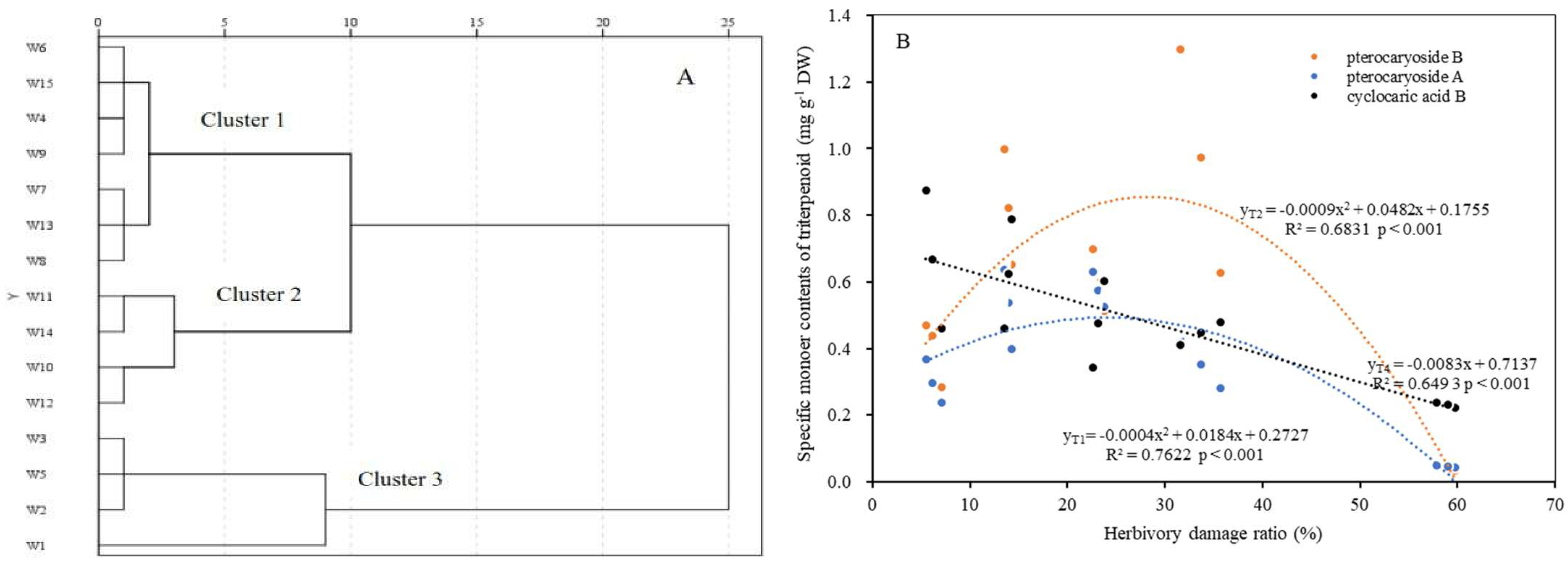
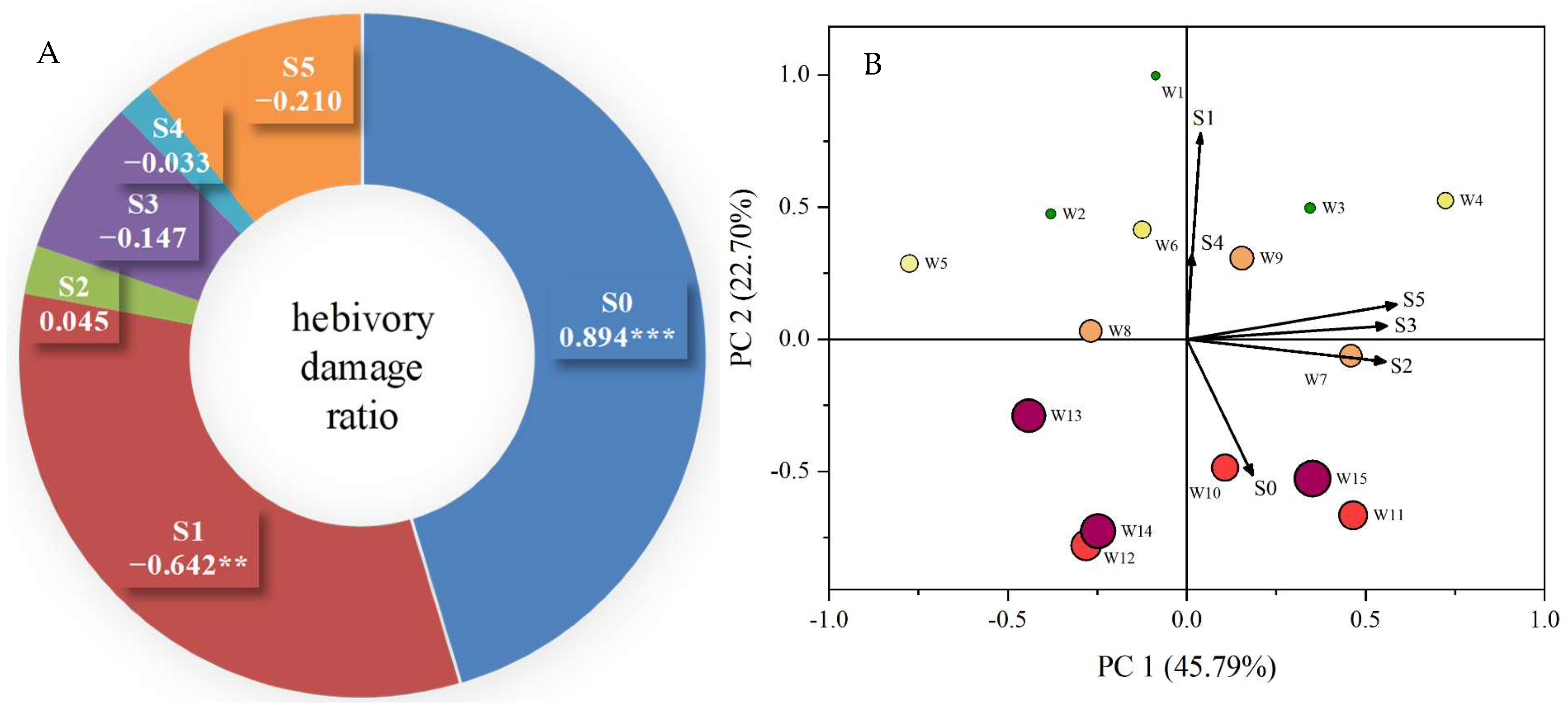
| Individual Code | Herbivory Damage Ratio (%) | di+ | di− | Ci | Ranking Order |
|---|---|---|---|---|---|
| W1 | 7.1 | 2.089 | 37.407 | 0.947 | 1 |
| W2 | 6.1 | 12.967 | 31.529 | 0.709 | 2 |
| W3 | 5.5 | 13.801 | 28.855 | 0.676 | 3 |
| W4 | 14.3 | 21.080 | 17.076 | 0.448 | 7 |
| W5 | 14.0 | 13.239 | 25.869 | 0.661 | 4 |
| W6 | 13.5 | 19.372 | 18.992 | 0.495 | 5 |
| W7 | 23.8 | 26.985 | 13.708 | 0.337 | 11 |
| W8 | 23.1 | 26.495 | 14.792 | 0.358 | 9 |
| W9 | 22.7 | 23.384 | 17.054 | 0.422 | 8 |
| W10 | 35.7 | 37.478 | 1.081 | 0.028 | 15 |
| W11 | 33.7 | 29.080 | 9.718 | 0.250 | 12 |
| W12 | 31.5 | 33.856 | 4.530 | 0.118 | 14 |
| W13 | 59.8 | 27.373 | 14.304 | 0.343 | 10 |
| W14 | 59.0 | 30.810 | 7.722 | 0.200 | 13 |
| W15 | 57.9 | 19.530 | 19.064 | 0.494 | 6 |
| Index | Secondary Metabolites | ||||
|---|---|---|---|---|---|
| Leaf Types | Total Flavonoids | Total Triterpenoids | Total Polyphenols | Condensed Tannin | |
| Herbivory damage ratio | Intact leaf | 0.498 * | −0.571 * | −0.265 | 0.722 ** |
| Damaged leaf | −0.822 *** | −0.536 * | −0.721 ** | 0.540 * | |
| Weighted average | −0.370 | −0.584 * | −0.581 * | 0.681 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Yang, W.; Shang, X.; Fu, X.; Sun, C.; Fang, S. Foliar Morphoanatomical and Phytochemical Variations Shape Resistance to Key Insect Herbivores and Leaf Quality in Cyclocarya paliurus. Plants 2025, 14, 2495. https://doi.org/10.3390/plants14162495
Xu Z, Yang W, Shang X, Fu X, Sun C, Fang S. Foliar Morphoanatomical and Phytochemical Variations Shape Resistance to Key Insect Herbivores and Leaf Quality in Cyclocarya paliurus. Plants. 2025; 14(16):2495. https://doi.org/10.3390/plants14162495
Chicago/Turabian StyleXu, Zhanhong, Wanxia Yang, Xulan Shang, Xiangxiang Fu, Caowen Sun, and Shengzuo Fang. 2025. "Foliar Morphoanatomical and Phytochemical Variations Shape Resistance to Key Insect Herbivores and Leaf Quality in Cyclocarya paliurus" Plants 14, no. 16: 2495. https://doi.org/10.3390/plants14162495
APA StyleXu, Z., Yang, W., Shang, X., Fu, X., Sun, C., & Fang, S. (2025). Foliar Morphoanatomical and Phytochemical Variations Shape Resistance to Key Insect Herbivores and Leaf Quality in Cyclocarya paliurus. Plants, 14(16), 2495. https://doi.org/10.3390/plants14162495







