Abstract
The objective of this study is to evaluate, for the first time, the chemical composition and the antioxidant, enzyme inhibitory, photoprotective and antibacterial properties of the Tamarix boveana essential oil (EO) as well as its organic extracts. The analysis of the EO obtained from the aerial parts of T. boveana was carried out employing the technique of gas chromatography with flame ionization detection (GC-FID) and mass spectrometry (GC-MS). Forty-four constituents were identified, constituting 91.18% of the oil, with the major compounds being γ-cadinene (9.41%), β-caryophyllene (6.71%), limonene (6.5%), p-cymene (6.16%), copaene (4.37%), terpinen-4-ol (4.23%), δ-cadinene (4.21%) and γ-terpinene (4.11%). The antioxidant activity of T. boveana essential oil and organic extracts (hydroalcoholic, CHCl3, AcOEt, n-BuOH) was evaluated by different tests, including DPPH, ABTS, phenanthroline, SNP and ferric reducing power. The findings indicated that T. boveana essential oil possesses moderate antioxidant capacity, with IC50 values of 223.59 ± 1.01 μg/mL according to the DPPH test. The extracts and essential oil also demonstrated notable inhibitory impacts against α-amylase and butyrylcholinesterase. Antimicrobial activity was determined regarding four bacterial strains, determining the minimum inhibitory concentrations (MICs) and bactericidal concentrations (MBCs). The geometry and electronic properties of the main EO compounds were determined using density functional theory (DFT) calculations. Furthermore, docking studies were conducted to investigate the interaction and binding affinity of these molecules with the active sites of BuChE and α-amylase enzymes. The results highlight the value of Tamarix boveana as a medicinal plant and indicate its effectiveness as an important source of bioactive compounds for many uses.
1. Introduction
Since ancient times, humans have used nature, mainly plants, to develop remedies for a variety of diseases. Thus, natural compounds have been the foundation of drug development over the decade [1]. Phytochemicals possessing biological properties, such as antioxidants, antimicrobials, antimutagens and anti-inflammatories, are extensively utilized to treat various human disorders, including Alzheimer’s disease (AD) and diabetes mellitus [2].
Essential oils are natural volatile secondary metabolites obtained from aromatic plants, recognized for their unique smells and diverse biological effects [3]. Owing to their prospective health advantages and natural sources, these oils are utilized across multiple sectors, including medicine [4], food [5] and cosmetics [6]. Essential oils are increasingly employed in food preservation due to their capacity to suppress microbiological proliferation, hence prolonging shelf life and maintaining food safety [7]. Pharmaceutically, they exhibit promise as natural therapies for infections, with studies demonstrating their efficacy against multidrug-resistant bacteria, thereby establishing them as significant contenders in combating antibiotic resistance [8]. To this fact, the production of essential oils from plants and the assessment of their efficacy remain in high demand due to their significant worth.
Polyphenols present in plants have been recognized for their diverse pharmacological effects, including antioxidant, antibacterial, anticancer, antihypertensive and anti-inflammatory actions [9,10,11]. These compounds are essential in the prevention of oxidative stress caused by the excessive generation of free radicals that can harm biological components. This damage causes aging and the development of several diseases associated with age, including cancer, cardiovascular disease and degenerative disorders.
In addition, polyphenols can provide skin protection against UV rays by absorbing and reducing the generated free radicals [12].
Butyrylcholinesterase and α-amylase are essential targets for drugs and extracts because their inhibition is involved in the management of Alzheimer’s disease (AD) and diabetes mellitus (DM) [13,14]. Cholinesterase inhibitors, such as galantamine, are used for AD, while acarbose regulates glucose levels in diabetes mellitus. However, these treatments have side effects, such as hepatotoxicity and gastrointestinal disorders [15,16,17]. Therefore, there is a necessity for new natural inhibitors without unwanted side effects to treat these two diseases. These alternatives could offer safer and more effective solutions.
The genus Tamarix, of the family Tamaricaceae, includes approximately 104 species taxonomically characterized and accepted [18]. These species are known to thrive in saline–alkaline soils as they tolerate harsh abiotic conditions, including high temperatures, salinity and drought [19,20]. Tamarix species are distributed in Asia, North Africa, North America and Europe. In traditional medicine, Tamarix species are used to treat various diseases [21,22]. For instance, T. gallica has demonstrated a range of biological activities, including anti-Alzheimer’s, anticancer, antidiabetic and antibacterial properties [18]. As well, T. articulata has been used as a therapeutic option against several diseases for decades; this plant has shown antibacterial, antiviral, anticancer, antioxidant and anti-inflammatory properties [22,23,24]. Other species, like T. aphylla, provide antimicrobial, antioxidant and anti-inflammatory properties [25,26]. In particular, T. boveana is well recognized for its antibacterial and insecticidal activities [18,27,28].
Numerous phytochemical investigations conducted on different Tamarix species have shown the presence of several active compounds, notably polyphenols [21]. On the other hand, no studies have been conducted on essential oils and organic extracts from Algerian T. boveana. As far as we know, there are no reports on the phytochemical composition or studies on the biological activity of this species, especially regarding enzyme inhibition and photoprotective properties. This paucity of scientific information reveals the novelty and interest of the present study. To this end, efforts will be made to include a unified assessment of such aspects for the first time. As part of ongoing research on Algerian medicinal plants [29,30,31], GC/MS and GC-FID analyses were used to determine the chemical composition of the essential oil of T. boveana aerial parts. This study also intended to evaluate the antioxidant, enzyme inhibitory, photoprotective and antimicrobial activities of the essential oil as well as the hydroalcoholic, CHCl3, AcOEt and n-BuOH extracts of the aerial parts of T. boveana. This polarity gradient approach was intended to maximize the chemical diversity of the extracts and enable correlation between individual extract types and their respective observed biological activities.
2. Results and Discussion
2.1. T. boveana Essential Oil Chemical Composition
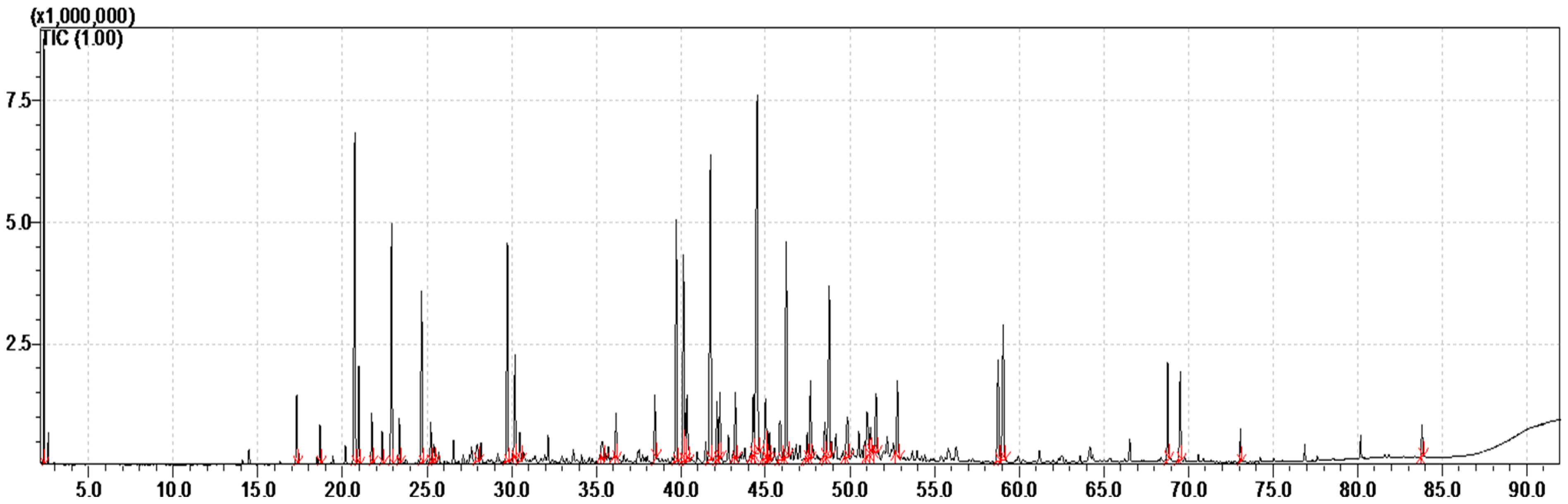
The essential oil of the whole aerial parts of the species T. boveana is obtained by steam distillation, yielding 0.33 ± 0.01% (w/w) relative to the dry plant matter. It is a yellowish oil with an aromatic smell. The results of GC-FID and GC/MS analyses of essential oil are presented in Table 1. Furthermore, the chromatogram presented in Figure 1 illustrates the abundance of secondary metabolites in the essential oil.

Table 1.
Compounds identified from T. boveana essential oil.
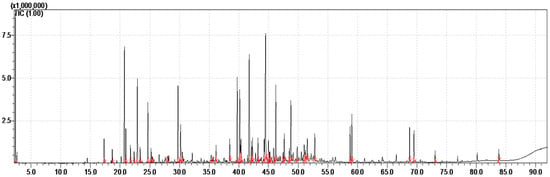
Figure 1.
GC-MS Chromatogram of the EO obtained from Algerian T. boveana (the integrated and identified peaks are in red).
A total of 44 compounds were identified in the whole aerial part of T. boveana, representing 91.18% of the volatile compounds. The major constituents of the essential oil are γ-Cadinene (9.41%), β-caryophyllene (6.71%), Limonene (6.5%), p-Cymene (6.16%), Copaene (4.37%), Terpinen-4-ol (4.23%), δ-Cadinene (4.21%) and γ-Terpinene (4.11%) (Table 1).
The predominant chemical components were observed to be sesquiterpene hydrocarbons (37.9%), followed by a significant amount of monoterpene hydrocarbons (26.01%). Additionally, a small amount of oxygenated sesquiterpenes (9.47%) and oxygenated monoterpenes (9.15%) were identified (Table 1).
The chemical profile of T. boveana essential oil, harvested in Algeria, is reported here for the first time. This allows for a comparative study with the same species harvested in other regions or with other Tamarix species whose essential oil chemical composition has been reported. For instance, Saïdana et al. [27] published GC-FID and GC/MS analysis of the essential oil from the whole aerial parts of T. boveana harvested in Tunisia. Initially, it is apparent that the essential oil of Tunisian T. boveana is richer in secondary metabolites containing 62 compounds (93.32% of the total oil), dominated by hexadecanoic acid (18.14%), docosane (13.34%), germacrene D (7.68%) and fenchyl acetate (7.34%). The Tunisian essential oil is abundant in fatty acids, fatty acid esters and hydrocarbons, while the Algerian T. boveana essential oil is characterized by a higher terpene content. Unique compounds like Edusma-4 (15),7-diene-1-β-ol and cis-Muurola-4 (14),5-diene are found in the Algerian T. boveana essential oil. In contrast, 2.4-Nonadienal is specific to Tunisian oil.
On the other hand, Alhourani et al. [32] analyzed the GC and GC-MS profiles of the essential oil from the aerial parts of T. aphylla (L.). At first sight, T. boveana essential oil has more identified compounds than T. aphylla (33 identified compounds representing 89.75% of the total oil). T. aphylla oil is dominated by non-terpenoid non-aromatic hydrocarbons (52.39%), with 6,10,14-trimethyl-2-pentadecanone (32.39%) as the predominant component, followed by β-ionone (13.74%) and dodecanoic acid (6.00%). T. boveana oil shows a higher fraction of sesquiterpenes (47.7%) compared to monoterpenes (35.78%), while T. aphylla oil is rich in oxygenated sesquiterpenes (26.53%).
Unique compounds like Edusma-4 (15),7-diene-1-β-ol and cis-Muurola-4 (14),5-diene are found in T. boveana oil, with γ-Cadinene as the major component (9.41%), which is not reported in T. aphylla oil. Conversely, T. aphylla contains a small amount of α-thujone (0.57%), which is not mentioned in our study. This dissimilarity can be explained by variances in extraction methodology, along with variations in geographical conditions and the fact that it is a distinct species [33,34,35].
Several of the major compounds identified in Tamarix boveana essential oil, such as β-caryophyllene [36], Terpinen-4-ol [37] and γ-cadinene, are known for their strong antioxidant, anti-inflammatory and antimicrobial activities [38]. β-Caryophyllene has demonstrated significant antioxidant [36] and anti-inflammatory properties [39], while Terpinen-4-ol contributes to microbial inhibition and wound healing [40]. Although less studied, γ-cadinene has shown notable antioxidant activity in the essential oils of other plant species [41]. These bioactivities likely explain, at least in part, the in vitro and in silico effects observed in this study. The synergistic action of these volatile compounds enhances this oil’s pharmacological potential.
2.2. In Vitro Antioxidant Ability
Plant antioxidants can inhibit or prevent reactive oxygen species (ROSs), acting as a regulator of antioxidant defense, thus protecting the human body from oxidative stress. In this study, the antioxidant capacity of the essential oil and the different extracts of T. boveana was evaluated and compared to many references (Table 2). The antioxidant capacity of the essential oil and extracts was evaluated by five methods: DPPH radical scavenging, ABTS, Phenanthroline, Silver Nanoparticles SNP and the reducing power assay.

Table 2.
Antioxidant activity of T. boveana extracts and EO.
Table 2 indicates that the n-BuOH extract has the most significant antioxidant activity (p < 0.05) in DPPH radical scavenging (IC50 = 47.45 ± 4.82 μg/mL), although this value is lower than that of the reference substances (BHT, BHA, α-tocopherol, tannic acid and ascorbic acid, Table 2). The hydroalcoholic extract exhibited an IC50 of 64.16 ± 1.77 μg/mL, followed by the AcOEt extract (IC50 = 91.34 ± 0.65 μg/mL), the CHCl3 extract (IC50 = 131.68 ± 0.01 μg/mL) and, finally, the essential oil (IC50 = 223.59 ± 1.01 μg/mL), which showed the lowest antioxidant activity. The essential oil’s low antioxidant activity may be due to its limited content of polyphenolic and flavonoid compounds, which are generally responsible for radical-scavenging mechanisms and are more prevalent in polar extracts. The other tests, ABTS, reducing power, Phenanthroline and SNP assays, also confirmed the antioxidant potential of the n-BuOH extract, which showed higher activity than the hydroalcoholic extract, succeeded by the AcOEt extract, the CHCl3 extract and, finally, the essential oil, which showed the lowest activity in all tests (Table 2, p < 0.05). Furthermore, in some tests, the n-BuOH extract demonstrated superior antioxidant capacity compared to reference antioxidants. In the reducing power test, the n-BuOH extract (IC50 = 23.42 ± 0.5 μg/mL) showed better activity than BHT (IC50 = 152.24 ± 2.43 μg/mL, p < 0.05), tannic acid (IC50 = 41.07 ± 2.36 μg/mL, p < 0.05) and α-tocopherol (IC50 = 34.93 ± 2.38 μg/mL, p < 0.01). Similarly, for the SNP assay, the n-BuOH extract (IC50 = 32.21 ± 0.73 μg/mL) showed higher activity than α-tocopherol (IC50 = 34.93 ± 2.38 μg/mL, p < 0.001), used as the standard.
A correlation study was conducted among DPPH, ABTS, Phenanthroline, SNP and the reducing power tests of n-BuOH extract (Table 3). Table 3 reveals highly positive linear correlations (R > 0.88) among all tests, particularly between the reducing power, DPPH and ABTS assays. These correlations indicate that these tests have comparable predictive capabilities to assess the antioxidant activities of n-BuOH extract.

Table 3.
R2 linear correlation coefficient that shows the correlations between the tests of T. boveanan-BuOH extracts.
The total phenol and flavonoid contents (Table 4) showed that the species T. boveana is a significant source of phenolic compounds, particularly the n-BuOH extract, which exhibits the highest concentrations of total phenols and flavonoids (563.70 ± 3.40 μg AGE/mg and 124.79 ± 0.26 μg QE/mg, respectively, p < 0.05) compared to the hydroalcoholic, AcOEt and CHCl3 extracts.

Table 4.
Quantification of total phenolic and flavonoid constituents in T. boveana extracts.
Based on previous research, the n-butanol extract was found to be more effective for retrieving phenolic compounds [42]. Indeed, the n-butanolic extract demonstrated the best antioxidant activity, primarily due to its polarity. This characteristic improves the efficient extraction of phenolic compounds and flavonoids, which are known for their antioxidant properties [43].
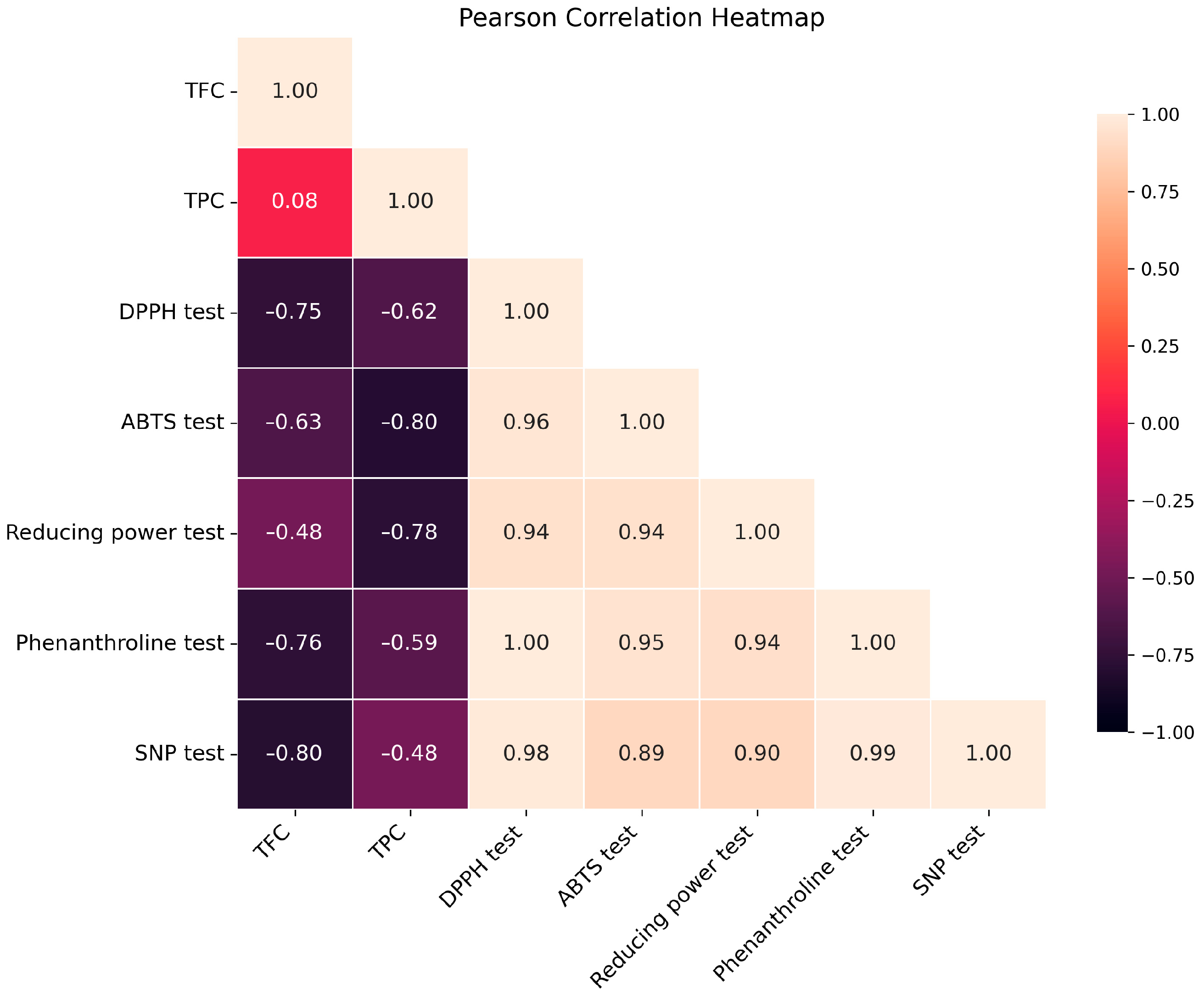
A Pearson correlation analysis was conducted to examine the correlations between antioxidant assay results and phenolic/flavonoid concentrations (Figure 2). The heatmap demonstrated significant negative associations between TPC/TFC and IC50 or A0.5 values, indicating that increased phenolic/flavonoid content correlates with enhanced antioxidant activity. The n-BuOH extract, demonstrating the highest total phenolic content (TPC) and total flavonoid content (TFC), displayed the most significant antioxidant effects in all experiments. This corroborates the concept that antioxidant activity is predominantly influenced by the concentration of phenolic and flavonoid compounds [44,45]. These results are presented as preliminary indicators of polyphenolic and flavonoid content, intended to support a statistically grounded correlation with the observed biological activities, rather than as definitive evidence of chemical composition.
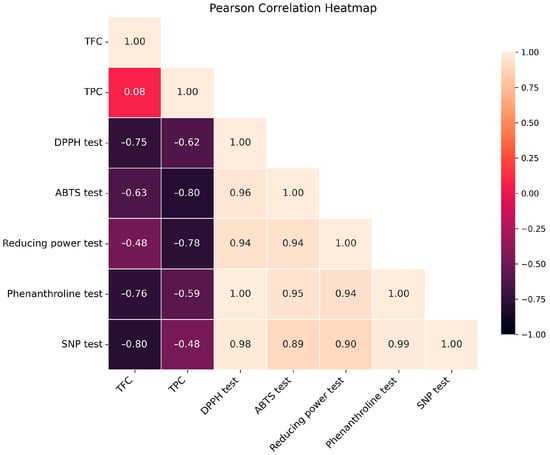
Figure 2.
Pearson’s correlation heatmap between antioxidant parameters and TPC/TFC of n-BuOH extract.
Through our study, the antioxidant activity of extracts and essential oil of the Algerian T. boveana was evaluated for the first time. However, some antioxidant tests (DPPH, ABTS) on T. boveana extracts harvested in Tunisia have already been confirmed by Saidana Naija et al. [46], who reported that ethyl acetate and methanolic fractions exhibited DPPH IC50 values close to 80 µg/mL. In contrast, our n-BuOH and hydroalcoholic extracts demonstrated superior antioxidant potential, with significantly lower IC50 values. Additionally, while the Tunisian study focused on a limited number of radical-scavenging assays, our antioxidant assessment was based on five complementary tests (DPPH, ABTS, Phenanthroline, SNP and reducing power), allowing for a more comprehensive evaluation of antioxidant mechanisms.
2.3. Enzyme Inhibition Effects
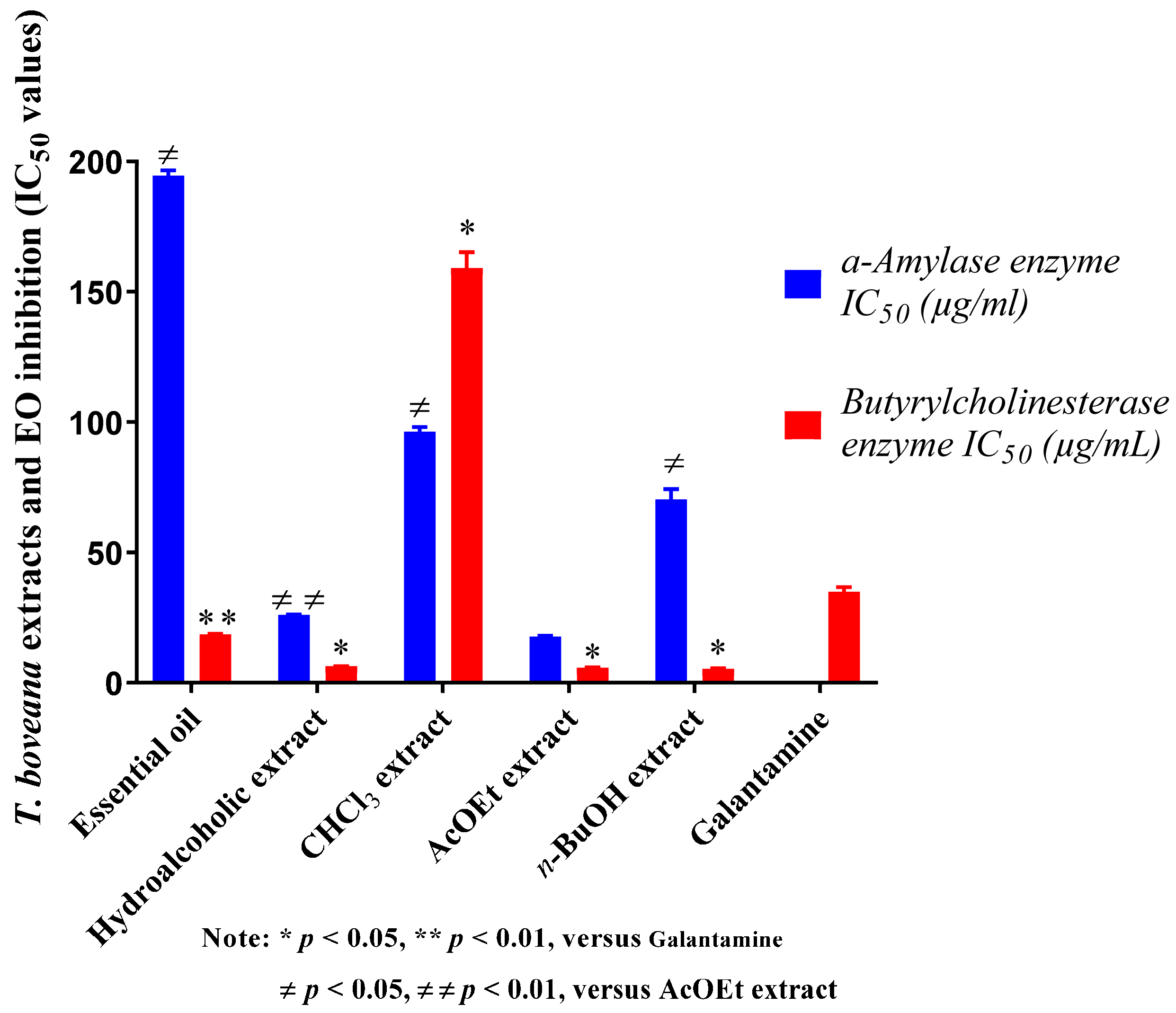
Inhibition of butyrylcholinesterase is considered an effective means to treat Alzheimer’s disease (AD) [47]. Therefore, the essential oil and all extracts of T. boveana were evaluated for their ability to inhibit BChE. The results are provided in Figure 3 with IC50 in μg/mL. The samples demonstrated dose-dependent inhibitory effects against BChE. n-BuOH, AcOEt and hydroalcoholic extracts exhibited extreme BChE inhibitory effects with an IC50 of 5.44 ± 0.40 µg/mL, 5.92 ± 0.40 μg/mL and 6.44 ± 0.06 μg/mL, respectively, in contrast to the standard Galantamine (IC50 = 34.75 ± 1.99 μg/mL, p < 0.05) (Figure 3). The essential oil also demonstrated significant activity against BChE (IC50 = 18.35 ± 0.96 μg/mL), unlike the standard Galantamine (p < 0.01). The CHCl3 extract (IC50 = 159.17 ± 1.74 μg/mL, p < 0.05) showed the lowest activity. The anticholinesterase activity of T. boveana essential oil was evidently associated with its high concentrations of sesquiterpenes and monoterpenes, especially γ-Cadinene (9.41%), β-caryophyllene (6.71%), α-pinene (2.59%) and β-Phellandrene (1.12%), which have been suggested for their cholinesterase inhibitory activities [48,49].
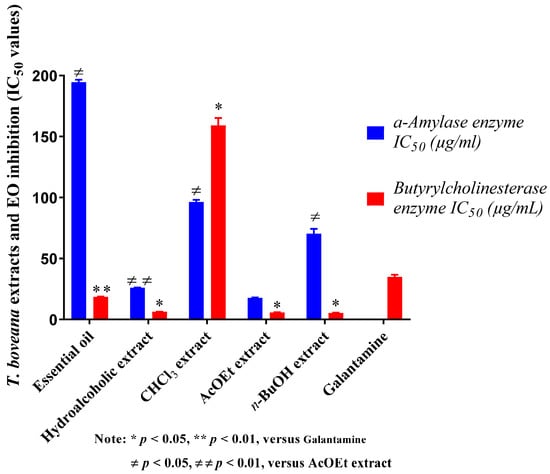
Figure 3.
The results of T. boveana extracts and EO inhibition (IC50 values) against α-Amylase and BuChE enzymes.
The α-amylase assay indicates that our extracts and essential oil exert dose-dependent antidiabetic activity. They were proven to have measurable inhibitory activity against the involved enzyme in diabetic disease. The results indicate that the AcOEt extract exhibited the most significant inhibitory activity against α-amylase (IC50 = 17.56 ± 1.38 μg/mL) followed by the hydroalcoholic extract (IC50 = 25.92 ± 0.18 μg/mL, p < 0.01), the n-BuOH extract (IC50 = 70.43 ± 4.38 μg/mL, p < 0.05) and, lastly, the CHCl3 extract (IC50 = 96.71 ± 1.66 μg/mL, p < 0.05). The essential oil exhibited the lowest activity with an IC50 of 194.67 ± 7.92 μg/mL, but this value remains higher than that of acarbose, which was used as a reference (IC50 = 3650.93 ± 10.70 μg/mL).
The activity of T. boveana essential oil against α-amylase is perhaps due to the presence of monoterpene and sesquiterpene molecules, including β-caryophyllene (6.71%), α-pinene (2.59%) and p-Cymene (6.16%), which are known for their α-amylase inhibitory activity [50,51].
Through this work, we present the first study concerning the enzymatic inhibitory activity of extracts and essential oil of Tamarix boveana, harvested in Algeria, against α-amylase and butyrylcholinesterase. To our knowledge, no assessment of α-amylase and BuChE inhibition exists yet for T. boveana grown in other regions. However, a literature search revealed that several species of the genus Tamarix have been assessed for their α-amylase and cholinesterase inhibitory activities, and the obtained results are positive [52].
2.4. Photoprotective Activity
The sun protection factor (SPF) of a sunscreen shows the protection degree offered by this product against UV-B radiation and measures the skin’s protection against sunburn. This factor is determined in the laboratory through standardized tests [53].
An effective sunscreen should cover a wide absorption range, from 290 to 400 nm. This study aims to evaluate for the first time in vitro the SPF of essential oil and extracts of T. boveana. The protection factors against UV-B radiation are summarized in Table 5. According to this table, the n-BuOH, hydroalcoholic and CHCl3 extracts presented the highest SPF values (46.40 ± 0.00, 46.10 ± 0.03 and 41.28 ± 0.3, respectively) compared to standards, including Vichy sunscreen (SPF = 44.22 ± 0.1) and Nivea sunscreen (SPF = 50.11 ± 0.22). In contrast, the essential oil showed the lowest SPF value (SPF = 9.08 ± 1.18, p < 0.05).

Table 5.
Sun protection factor determination in T. boveana extracts and EO.
The SPF values of the extracts appear to be directly related to their polyphenol content. Indeed, T. boveana extracts showed high SPF values, probably due to their richness in polyphenols, which can absorb UV radiation in the 280–320 nm wavelength range [54]. This UV absorption capacity makes these extracts promising candidates for developing potent photoprotective agents.
2.5. Antibacterial Activity
The antibacterial potential of T. boveana essential oil and extracts was confirmed by the appearance of a bacterial growth inhibition zone. The activity was determined by measuring minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs). The microbial resistance of Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Micrococcus luteus strains is presented in Table 6. The essential oil, as well as all extracts, showed inhibition of microbial growth, depending on the strains’ sensitivity and the sample concentration, as shown in Table 6.

Table 6.
Minimum bacterial concentration (MBC) and minimum inhibitory concentration (MIC) values of essential oil and aerial part extracts of T. boveana.
The results revealed that the essential oil (EO) effectively inhibited the growth of all tested strains, with MIC values between 1.5 and 3 μg/mL and MBC values between 4 and 8 μg/mL, which are similar to those of gentamicin (MIC: 2 μg/mL, MBC: 6 μg/mL, p < 0.01). The EO mainly showed high efficacy against Micrococcus luteus, with respective MIC and MBC values equal to 1.5 μg/mL and 4 μg/mL, for p < 0.01. However, the n-BuOH and hydroalcoholic extracts showed similar antibacterial efficacy against Micrococcus luteus, with MIC and MBC of 3 and 10 μg/mL, respectively, which are higher than CHCl3 and AcOEt extracts, for p < 0.01. Regarding Staphylococcus aureus, the CHCl3 extract showed more marked antibacterial activity, with an MIC of 3 μg/mL and MBC of 5 μg/mL, compared to the hydroalcoholic, n-BuOH and AcOEt extracts.
This study reports, for the first time, the antimicrobial properties of the essential oil and extracts of T. boveana harvested in Algeria. However, other research has documented similar antibacterial activities of the essential oil from T. boveana harvested in Tunisia [27].
The antimicrobial efficacy of T. boveana essential oil appears to be consistent with the presence of β-caryophyllene, a major component (6.71%) in this essential oil. This compound has shown in vitro activity against S. aureus, E. coli and P. aeruginosa. Furthermore, volatile compounds, such as β-caryophyllene, might be responsible for the noted antimicrobial properties, potentially related to their role in forming resins or complex menthols, which are also said to possess antibacterial characteristics [27].
2.6. Density Functional Theory Calculation
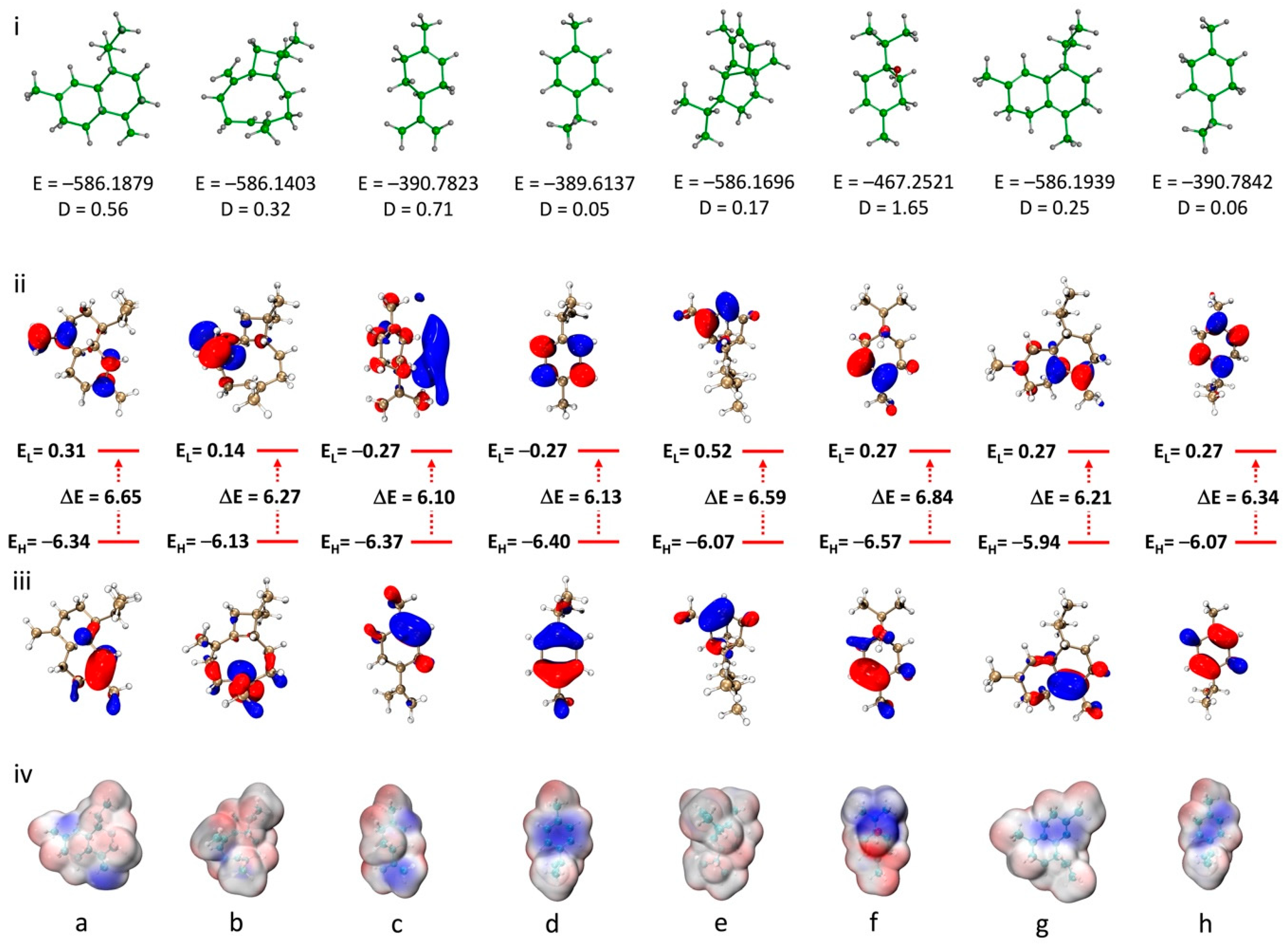
Quantum chemical calculations were performed for the main EO compounds to better understand their geometry, electronic properties and chemical reactivity. All calculations were performed using the DFT method at the B3LYP/6-311G (d,p) theoretical level. Analysis of the molecular geometry (Figure 4i) reveals variations in total energy and dipole moments, indicating differences in stability and polarity that may influence the chemical properties and reactivity of the molecules. Terpinen-4-ol, with the highest dipole moment (D = 1.65 Debye), shows a pronounced polar character, suggesting a greater tendency to interact with polar environments or molecular systems. In contrast, compounds such as copaene and γ-terpinene, with lower dipole moments (D = 0.17 and 0.06 Debye, respectively), reflect a more non-polar, hydrophobic nature.
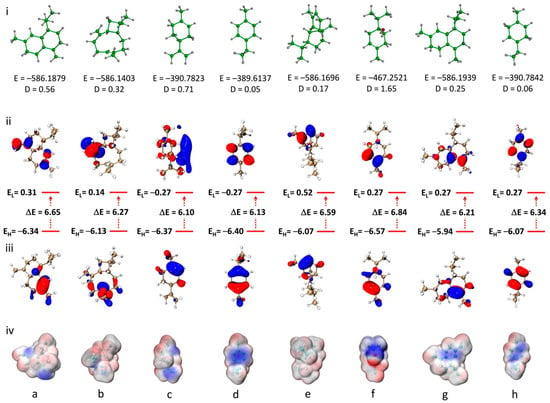
Figure 4.
(i) Computed molecular geometry; (ii) LUMO; (iii) HOMO; and (iv) ESP of: (a) γ-cadinene; (b) β-caryophyllene; (c) limonene; (d) p-cymene; (e) copaene; (f) terpinen-4-ol; (g) δ-cadinene; and (h) γ-terpinene at B3LYP/6-311G (d,p) level.
The frontier orbitals, HOMO (highest-occupied molecular orbital) and LUMO (lowest-occupied molecular orbital), play a crucial role in the chemical reactivity of organic compounds. For the studied compounds, as shown in Figure 4ii and iii, the energy gaps (ΔE) between HOMO and LUMO range from 6.10 to 6.84 eV, reflecting the electronic stability and potential reactivity of the molecules. For example, limonene and p-cymene, with moderate ΔE values (6.10 and 6.13 eV, respectively), suggest a good balance between chemical stability and reactivity compared to the other compounds. On the other hand, terpinen-4-ol, with a higher ΔE (6.84 eV), is associated with lower chemical reactivity due to its enhanced stability. The HOMO energy levels of the studied compounds, which reflect their ability to donate electrons and thus their antioxidant potential via the electron transfer mechanism, range from −5.94 to −6.57 eV. δ-cadinene shows the highest HOMO level (−5.94 eV), while terpinen-4-ol displays the lowest value (−6.57 eV), indicating that δ-cadinene may be the most potent antioxidant among the studied molecules. However, all HOMO values are lower than those of standard antioxidants such as Trolox (−5.39 eV) and BHT (−5.74 eV), which may explain the low antioxidant activity of the essential oil observed in the experimental studies [55].
Electrostatic potential (ESP) maps provide valuable insights into the distribution of electronic charges on the surface of molecules, highlighting regions likely to interact through electrostatic, nucleophilic or electrophilic interactions. The ESP maps of the studied compounds show varied charge distributions (Figure 4iv). For example, strongly negative regions (in blue) are observed around the terpinen-4-ol oxygen atom and double bonds, indicating potential sites for nucleophilic interactions. Conversely, slightly positive regions (in red), mainly observed on hydrogen atoms, correspond to sites likely to interact with nucleophilic species. Copaene and δ-cadinene, for example, show ESP distributions favoring hydrophobic interactions, while terpinen-4-ol, with a more heterogeneous distribution, suggests a higher polarity, implying the possibility of forming polar interactions. The Cartesian coordinates of the eight principal components of the calculated Tamarix boveana EO are reported in Table S1.
2.7. Docking Studies
EO has demonstrated significant inhibitory activity in vitro, corroborating its potential as a natural source of BuChE inhibitors. To elucidate the molecular basis of this activity, docking studies were carried out on the eight main components of EO towards the BuChE active site (PDB ID: 4bds). Docking analysis showed that most of the compounds exhibited favorable binding affinities, as reflected by their docking scores (Table 7).

Table 7.
List of the major constituents of T. boveana EO with their docking scores, interaction types between interacting residues and bond distances towards the BuChE enzyme (PDE 4bds).
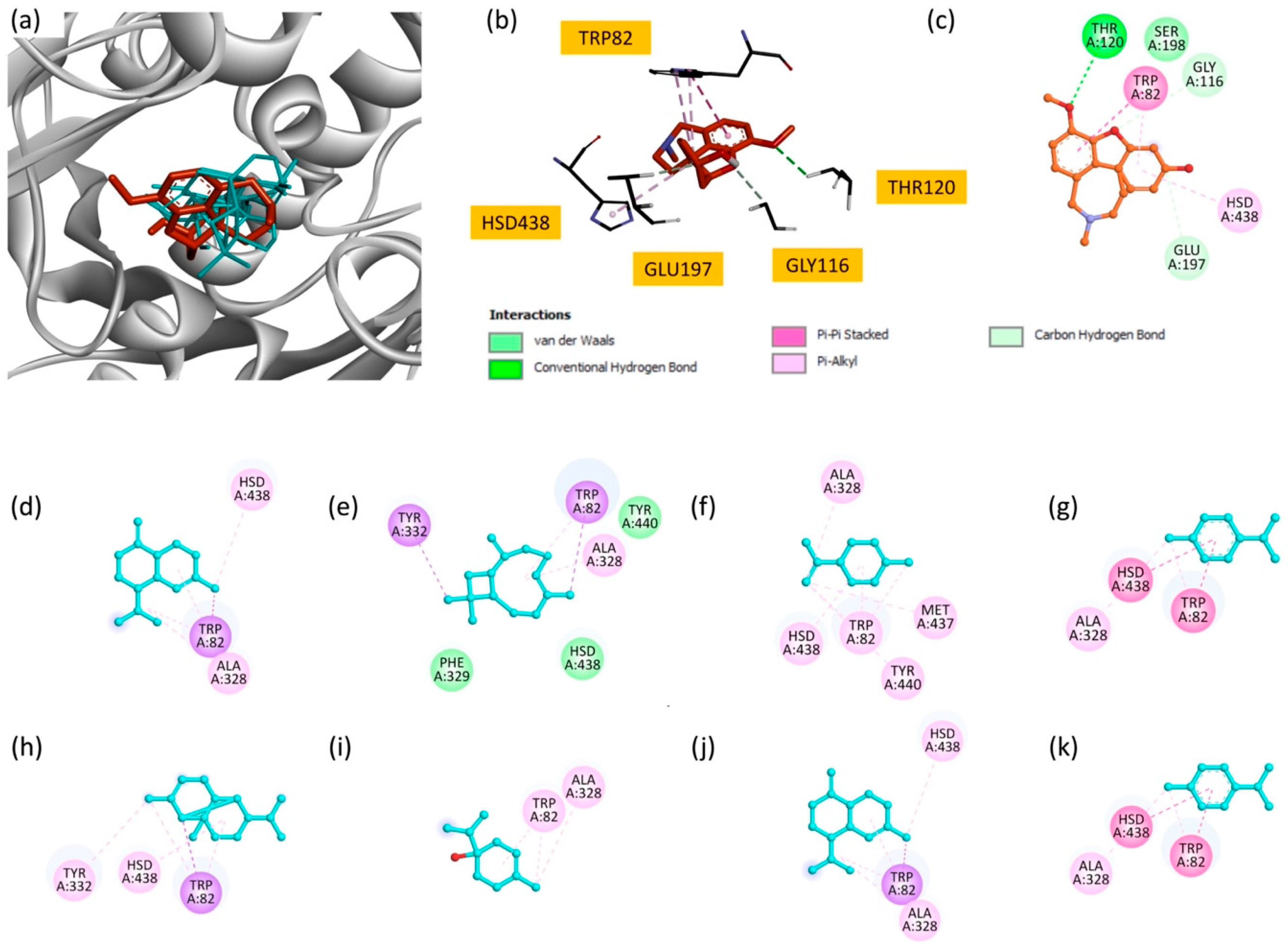
Among the studied molecules, γ-cadinene and δ-cadinene displayed the highest binding scores (−7.6 kcal/mol), suggesting their predominant role in the observed inhibitory activity. These compounds interacted primarily with TRP82 and HSD438 via Pi–alkyl and Pi–sigma interactions, highlighting the importance of hydrophobic interactions in stabilizing these molecules within the BuChE active site. Other significant contributors include β-caryophyllene and copaene, with docking scores of −7.5 kcal/mol and −7.3 kcal/mol, respectively. β-caryophyllene formed multiple interactions, including Pi–sigma and Pi–alkyl interactions, involving key residues such as TRP82, TYR332 and ALA328. Similarly, copaene exhibits interactions with TRP82, TYR332 and HSD438.
Compounds with moderate binding affinity, such as limonene, p-cymene, terpinen-4-ol and γ-terpinene, also demonstrated notable interactions with the BuChE active site. These interactions mainly involved Pi–alkyl and Pi–sigma contacts with residues such as TRP82, HSD438, ALA328 and TYR440.
Superimposition of all compounds and the reference inhibitor, galantamine (Figure 5a), revealed overlapping binding modes, suggesting that EO components may act through mechanisms similar to known inhibitors. Two-dimensional interaction maps (Figure 5c–k) further illustrate the involvement of critical active site residues, in particular TRP82 and HSD438, which are essential for BuChE activity [56].
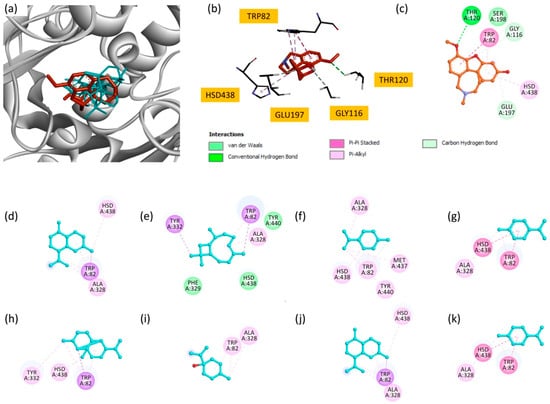
Figure 5.
(a) Superimposition of all compounds and the reference molecule at the active site of BuChE; (b) bonding modes of galantamine; and (c) 2D representations of the interactions of galantamine; (d) γ-cadinene; (e) β-caryophyllene; (f) limonene; (g) p-cymene; (h) copaene; (i) terpinen-4-ol; (j) δ-cadinene; and (k) γ-terpinene.
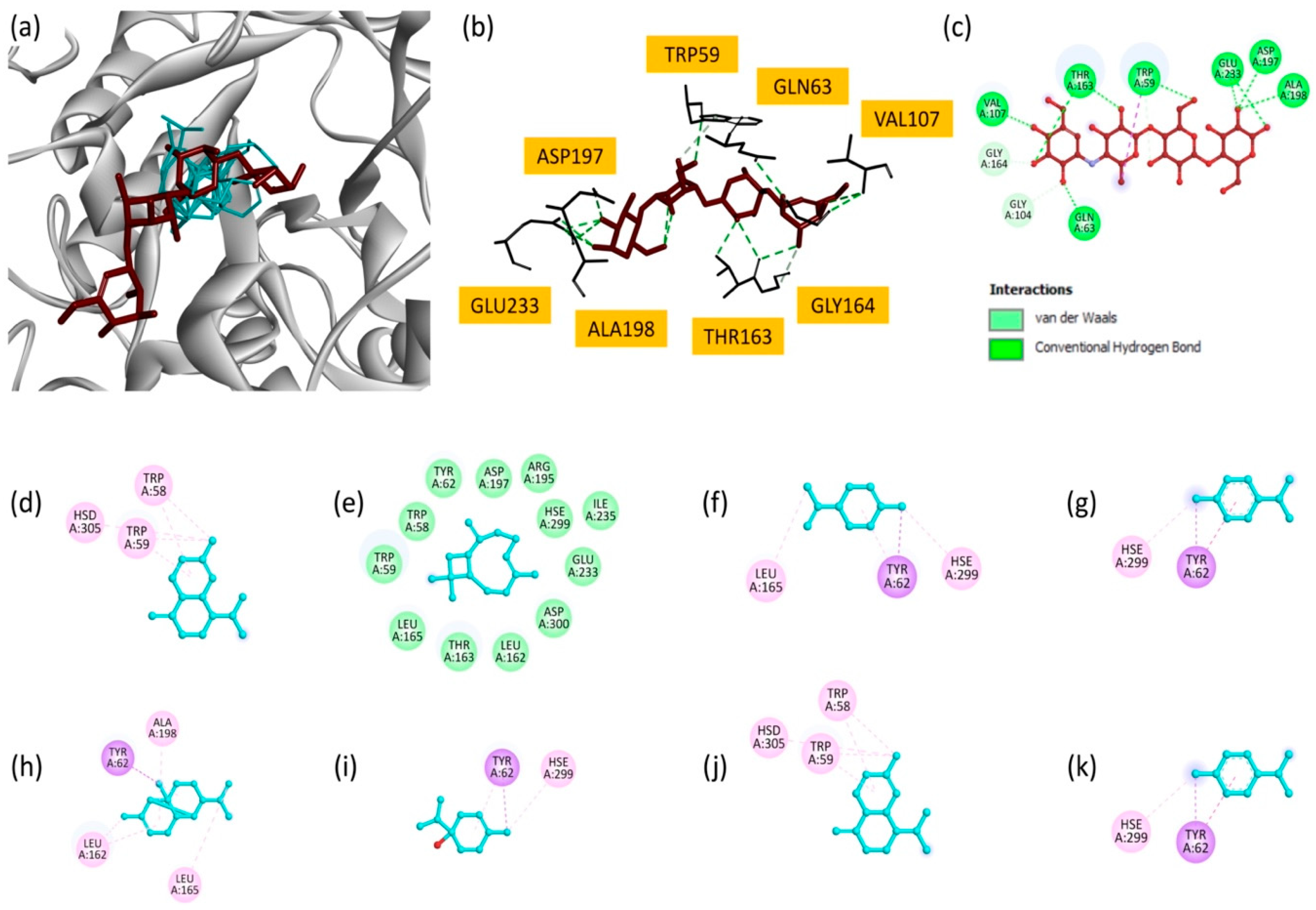
EO also showed promising results against the α-amylase enzyme in vitro, although it was less effective than the extracts. Consequently, we also examined the interaction of the oil’s main components with α-amylase (PDB ID: 4gqr) via molecular docking. Among the analyzed compounds, γ-cadinene and copaene showed the highest docking scores (−7.3 kcal/mol), suggesting their strong binding to the α-amylase active site (Table 8). γ-cadinene mainly formed Pi–alkyl interactions with critical residues, such as TRP58, TRP59 and HSD305. Similarly, copaene exhibited Pi–sigma and Pi–alkyl interactions involving TYR62, ALA198, LEU165 and LEU162. These results indicate the importance of hydrophobic interactions in stabilizing these molecules in the enzyme’s active site. δ-Cadinene (−7.0 kcal/mol) and β-caryophyllene (−6.9 kcal/mol) also made significant contributions. δ-Cadinene formed multiple interactions, including Pi–alkyl and Pi–sigma contacts, with residues TRP59, TRP58, TYR62 and HSE299, highlighting its ability to interact with key catalytic residues. β-caryophyllene, despite its slightly lower binding score, formed stabilizing interactions consistent with its moderate inhibitory potential. Moderate binding affinities were observed for p-cymene (−5.9 kcal/mol), γ-terpinene (−5.9 kcal/mol), limonene (−5.8 kcal/mol) and terpinen-4-ol (−5.1 kcal/mol). These compounds interact primarily with residues such as TYR62, HSE299, LEU165 and LEU162 via Pi–alkyl and Pi–sigma interactions.

Table 8.
List of the major constituents of T. boveana EO with their docking scores, interaction types between interacting residues and bond distances toward α-amylase enzyme (PDE 4gqr).
Superimposition of all docked compounds with the reference inhibitor, acarbose (Figure 6a), revealed overlapping binding modes, suggesting that EO components may share similar inhibitory mechanisms. Two-dimensional interaction maps (Figure 6d–k) highlighted the critical involvement of active site residues, in particular TRP58, TRP59, TYR62 and HSE299. These residues are essential for the enzyme’s catalytic function, and their interactions with EO components highlight the potential of these molecules to disrupt α-amylase activity [57].
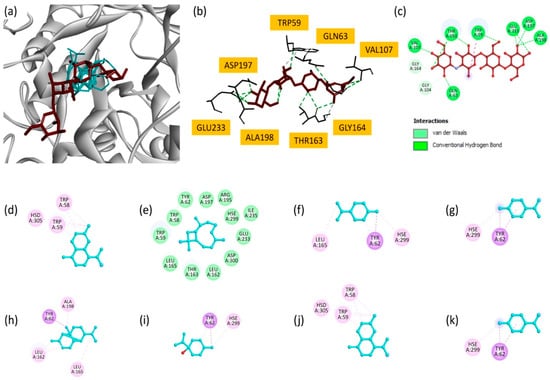
Figure 6.
(a) Superimposition of all compounds and the reference molecule at the active site of α-amylase; (b) bonding modes of acarbose; and (c) 2D representations of the interactions of acarbose; (d) γ-cadinene; (e) β-caryophyllene; (f) limonene; (g) p-cymene; (h) copaene; (i) terpinen-4-ol; (j) δ-cadinene; and (k) γ-terpinene.
In summary, docking studies revealed that the main components of the EO exhibit notable inhibitory potential against both BuChE and α-amylase, with favorable binding affinities and diverse interaction modes with the active sites of these enzymes. γ-cadinene and δ-cadinene were found to be the most potent inhibitors of BuChE (−7.6 kcal/mol), interacting mainly with TRP82 and HSD438 via hydrophobic interactions, while γ-cadinene and copaene showed the greatest affinity for α-amylase (−7.3 kcal/mol), forming Pi–alkyl and Pi–sigma interactions with residues such as TRP58, TYR62 and HSE299. Other constituents, such as β-caryophyllene and p-cymene, also demonstrated moderate activity towards both enzymes. The overlap in binding modes with the reference inhibitors suggests that the EO components may act via similar mechanisms, supporting their potential as natural inhibitors of both enzymes.
3. Conclusions
This study presents the first investigation on the chemical composition and biological properties of essential oil and extracts of Tamarix boveana, harvested in Algeria. The essential oil, which is rich in γ-cadinene, β-caryophyllene, limonene and p-cymene, exhibited noteworthy antimicrobial and enzyme inhibitory activities. The n-butanol extract, containing the highest concentrations of phenolic and flavonoid compounds, demonstrated strong antioxidant potential. Furthermore, docking studies revealed that the main components of the EO exhibit potential inhibitory activity against the BuChE and α-amylase enzymes, with notable binding affinities and various interaction modes.
This work offers significant new insights compared to previous studies, such as the Tunisian study conducted by Saïdana et al. [27]. It highlights a distinct chemical profile and provides a broader biological evaluation, including antioxidant activity, enzymatic inhibition (α-amylase and butyrylcholinesterase) and photoprotective potential. Additionally, this study is innovative in its integration of in silico methods, including DFT calculations and molecular docking, which simulate the interactions of the major compounds with the enzymatic active sites. These contributions considerably enrich existing knowledge, suggesting new potential therapeutic applications for this plant.
Although the results are promising, it is important to acknowledge certain limitations, such as the lack of in vivo validation and the absence of detailed toxicological data.
Further studies are warranted to confirm the biological activities through animal models and to investigate the action mechanisms of the active compounds. Additionally, broader safety and pharmacokinetic assessments will be required before potential therapeutic applications can be considered.
4. Materials and Methods
4.1. Plant Material
T. boveana aerial parts were collected in March 2022 at the flowering time from El-Bayadh region (33°40′49″ north, 1°01′13″ east) located in western Algeria in the steppe zone. The plant was identified by Pr. Mohamed Kaabache from Ferhat Abbas University in Setif. A sample specimen was placed in the herbarium of the research unit “VARENBIOMOL” in Constantine-1 University under the number TB/03/22. The plant material was dried for ten days in the shade at room temperature in the open air.
4.2. Essential Oil Extraction and Organic Extracts Preparation
Dried aerial parts of T. boveana (229 ± 4.96 g) were distilled for 3 h by steam distillation using a Kaiser Lang apparatus. The obtained essential oil was collected, extracted with hexane and then dried over anhydrous sodium sulfate (Na2SO4). The hexane was then allowed to evaporate at room temperature in open air. The resulting oil was stored at 4 °C for further analysis. The essential oil yield was calculated relative to the plant material weight, based on three replicates.
In addition, air-dried parts (697.1 ± 8.96 g) were cut into small pieces and macerated at room temperature in a MeOH–H2O (70:30 v/v) mixture at a rate of 1:40 (w/v) for 48 h, and this was repeated three times with solvent renewal. The filtrate was then concentrated and dissolved in water following filtration in order to obtain a hydroalcoholic extract (23.88 g) (yield was 3.43% ± 0.96). The resultant solution was sequentially extracted using organic solvents: CHCl3, AcOEt and n-butanol. The organic phases were dried using Na2SO4, filtered and then concentrated under vacuum, thus giving the different extracts as follows: chloroform (3.62 ± 0.96 g with a yield of 0.52 ± 0.02%), AcOEt (2.44 ± 0.46 g with a yield of 0.35 ± 0.03%) and n-butanol (15.6 ± 2.27 g with a yield of 2.24 ± 0.31%).
4.3. GC-FID Analysis
Quantitative analysis of the essential oil was determined using a Shimadzu gas chromatography (GC-FID) Model 2010, linked with a fused silica capillary column HP-5MS (30 m length × 0.25 mm ID. 0.25 μm film thickness, 5%-diphenyl-95%-dimethylpolysiloxane), set to go from 50 °C (5 min) to 250 °C at 3 °/min and held for 10 min. The column was coupled to an injector (split mode 1/60) and a flame ionization detector (FID). The temperatures of the injector and the flame ionization detector were 280 and 300 °C, respectively. Acetone at a concentration of 3.5% v/v was used to dilute the essential oil. The used carrier gas was helium (1.0 mL/min).
Retention indices (RI) were determined using the Van den Dool and Kratz equation by analyzing standard alkanes (C8–C20) solutions under identical conditions.
4.4. GC/MS Analysis
GC-MS analysis of the EO was conducted using a Shimadzu gas chromatograph–mass spectrometer (model 7890/5975), combined with a capillary column HP-5MS (25 m length × 0.25 mm ID. 0.25 μm film thickness). The identical conditions program, referenced above in the GC-FID analysis section, was employed. MS quadrupole and ion source temperatures were 230 °C and 180 °C, respectively. The mass spectrometer was set to positive electron impact mode with an ionization voltage of 70 eV, and the electron multiplier was adjusted to 2200 V. Identification of the essential oil components was performed through comparing their retention indices (RI) and mass spectra with those of the reference compounds from the NIST 20 and Wiley 12 MS libraries. The relative proportions of each component were determined from the GC peak areas without the application of response factor correction; these percentages refer to the relative abundance of each compound based on the area under their respective peaks on the chromatogram (GC-MS), and not their absolute concentration in the essential oil. In other words, these values reflect the proportion of each compound in relation to the total peak area detected, rather than the actual mass or volume percentage in the oil [58].
4.5. Antioxidant Activity
4.5.1. Total Flavonoid Content
Total flavonoid content was measured using the aluminum trichloride technique, with Quercetin as a reference component [59]. This approach involves generating a complex between flavonoids and aluminum, with a maximum absorption at 415 nm. The results are reported as µg equivalent of Quercetin per mg of extract (μg EQ/mg of extract).
4.5.2. Total Polyphenol Content
Total phenolic content is usually evaluated colorimetrically with the Folin–Ciocalteu (FCR) assay using a 96-well microplate. The produced coloration is related to the amount of polyphenols present in the plant extracts determined spectrophotometrically [60]. Results are presented as µg gallic acid equivalent per milligram of extract (µg EAG/mg of extract).
4.5.3. Scavenging Activity on DPPH Radical
The activity of extracts and EO to neutralize free radicals (DPPH) was evaluated using the method defined by Blois [61].
4.5.4. ABTS Test
The ABTS scavenging activity of extracts and EO was evaluated at 734 nm, following the methodology described by Re et al. [62].
4.5.5. Ferric Reducing Antioxidant Power
The reducing power of extracts and EO was evaluated using the Oyaizu [63] method with a slight modification.
4.5.6. Phenanthroline Test
The reduction activity of the Phenanthroline complex [Fe (phen)2]2+ was determined by the method of Mansur et al. [64]. The results were set at A0.50 (μg/mL).
4.5.7. Silver Nanoparticles SNP Activity
This activity is determined according to the method of Özyürek et al. [65]. The absorbance was recorded at 423 nm.
4.6. Photoprotective Activity (SPF)
Ultraviolet spectrophotometry serves as a supportive and preliminary in vitro approach for estimating the sun protection factor (SPF) of plant extracts and essential oils. The photoprotective effect was determined by the methodology of Mansur et al. [66,67]. The samples were put in ethanol at a concentration of 1 mg/mL (1000 ppm) and then ultrasonicated for 2 min to dissolve them and make a uniform solution. We used a multimode microplate reader (PerkinElmer Enspire, Singapore) to record absorbance across a range of 280–320 nm. We took three measurements for each and used the Mansur equation to figure out the SPF [53].
4.7. Enzyme Inhibitory Effect
4.7.1. α-Amylase Inhibition
α-Amylase inhibitory activity was determined employing the method of Zengin et al. [68]. Acarbose is used as a standard.
4.7.2. Cholinesterase Inhibition
The inhibitory activity of Butyrylcholinesterase (BChE) was determined by adopting Ellman’s technique [69], using galanthamine as a reference.
4.8. Antimicrobial Activity
The in vitro antimicrobial activity of extracts and essential oil was evaluated using the disk diffusion method [70]. Antimicrobial properties were tested against 4 bacterial strains, including Gram-positive bacteria (Staphylococcus aureus (ATCC 25923) and Micrococcus luteus strain (MM DSM 113600)) and Gram-negative bacteria (Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853)).
MBC and MIC Determination
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MCB) were determined by applying the micro-dilution technique [71,72]. All analyses were repeated three times.
4.9. DFT Calculations
Quantum chemical calculations were conducted using Gaussian 09 software, employing the density functional theory (DFT) method at the B3LYP/6-311G (d,p) level [73]. Frequency calculations were performed to confirm that all compounds are in their ground states. Multiwfn 3.8 and VMD 1.9.3 software were used for the analysis and visualization of computational results [74,75,76]. Cartesian coordinates of the optimized structures for all compounds are provided in Table S1 of the Supporting Information.
4.10. Molecular Docking
The coordinates of the studied compounds were obtained from DFT calculations, while those of the proteins (human butyrylcholinesterase (BuChE) and human pancreatic α-amylase) were retrieved from the Protein Data Bank with identifiers 4bds and 4gqr, respectively. The proteins were prepared by removing ligands, water molecules, heteroatoms and co-crystallized solvents, followed by the addition of partial charges and hydrogens. The docking search space was defined as a 25 Å cube with grid points 1 Å apart, centered on the active site of the proteins. Docking studies were performed using AutoDock vina 1.1.2 software [77]. Figures were generated using BIOVIA Discovery Studio. The docking protocol was validated by comparing crystallographic and theoretical data for the native ligands, yielding RMSD values of 0.52 Å and 2.20 Å for 4bds and 4gqr, respectively.
4.11. Statistical Analysis
All calculated parameters were tested using the one-way analysis of variance (ANOVA). This analysis was repeated three times [78,79]. In case of statistical significance of the ANOVA test (p < 0.05), the differences in means between each treatment were examined using Tukey’s multiple comparison test.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14162497/s1 Table S1: The cartesian coordinates of the eight main components of the EO of Tamarix boveana computed at B3LYP/6-311G(d,p) level in the gas phase.
Author Contributions
Conceptualization, E.L. and I.M.; methodology, E.L., I.M., H.H. and C.B.; software, H.B. and C.B.; validation, E.L., I.M., S.A. and G.N.; formal analysis, N.S. and C.B.; investigation, E.L., I.M., S.A. and G.N.; resources, E.L. and N.S.; data curation, H.B., C.B. and H.H.; writing—original draft preparation, E.L., I.M. and H.B.; writing—review and editing, E.L., I.M., H.B., S.A. and G.N.; visualization, E.L., S.A. and G.N.; supervision, E.L., I.M., S.A. and G.N.; project administration, E.L. and I.M.; funding acquisition, G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained in the article.
Acknowledgments
The supercomputing resources used in this work were supported by the HPC of UCI-UFMC (Unité de Calcul Intesif of the University Fréres Mentouri Constantine 1).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EO | Essential Oil |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GC-FID | Gas Chromatography–Flame Ionization Detector |
| CHCl3 | Chloroform |
| AcOEt | Ethyl Acetate |
| n-BuOH | n-Butanol |
| MIC | Minimum Inhibitory Concentration |
| MIB | Minimum Bactericidal Concentration |
| DFT | Density Functional Theory |
| BuChE | Butyryl Cholinesterase Enzyme |
| AD | Alzheimer’s Disease |
| DM | Diabetes Mellitus |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| IC50 | Half Maximal Inhibitory Concentration |
| RT | Retention Time |
| RI | Retention Index |
| Relat. | Conc. Relative Concentration Expressed as a Percentage |
| TPC | Total Phenolic Content |
| AGE | Gallic Acid Equivalents |
| QE | Quercetin Equivalents |
| TFC | Total Flavonoids Content |
| ROS | Reactive Oxygen Species |
| SNP | Silver Nanoparticles |
| ABTS | 2,2′-Azino-Bis(3-ethylbenzoThiazoline-6-Sulfonic Acid) |
| BHA | Butylated HydroxyAnisole |
| BHT | Butylated HydroxyToluene |
| SPF | Sun Protection Factor |
| HOMO | Highest-Occupied Molecular Orbital |
| LUMO | Lowest-Occupied Molecular Orbital |
| ESP | Electrostatic Potential Maps |
References
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Mici, D.; Ðurovi, S.; Riabov, P.; Tomi, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinovi, B.; Božovi, R.; Jovanovi, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Salanță, L.C.; Cropotova, J. An Update on Effectiveness and Practicability of Plant Essential Oils in the Food Industry. Plants 2022, 11, 2488. [Google Scholar] [CrossRef]
- Achagar, R.; Ait-Touchente, Z.; El Ati, R.; Boujdi, K.; Thoume, A.; Abdou, A.; Touzani, R. A Comprehensive Review of Essential Oil–Nanotechnology Synergy for Advanced Dermocosmetic Delivery. Cosmetics 2024, 11, 48. [Google Scholar] [CrossRef]
- Rodilla, J.M.; Rosado, T.; Gallardo, E. Essential Oils: Chemistry and Food Applications. Foods 2024, 13, 1074. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Coatings Based on Essential Oils for Combating Antibiotic Resistance. Antibiotics 2024, 13, 625. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial Effects of Polyphenols on Cardiovascular Disease. Pharmacol. Res. 2013, 68, 125–131. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and Health: What Compounds Are Involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Ajila, C.M.; Jaganmohan Rao, L.; Prasada Rao, U.J.S. Characterization of Bioactive Compounds from Raw and Ripe Mangifera Indica L. Peel Extracts. Food Chem. Toxicol. 2010, 48, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Houghton, P.J. Plants Used in Chinese and Indian Traditional Medicine for Improvement of Memory and Cognitive Function. Pharmacol. Biochem. Behav. 2003, 75, 513–527. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert. Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A Review of Alpha-Glucosidase Inhibitors from Plants as Potential Candidates for the Treatment of Type-2 Diabetes; Springer: Dordrecht, The Netherlands, 2022; Volume 21, ISBN 0123456789. [Google Scholar]
- Thompson, S.; Lanctôt, K.L.; Hermann, N. The Benefits and Risks Associated with Cholinesterase Inhibitor Therapy in Alzheimer’s Disease. Expert Opin. Drug Saf. 2004, 3, 425–440. [Google Scholar] [CrossRef]
- Li, F.; Xie, W.; Ding, X.; Xu, K.; Fu, X. Phytochemical and Pharmacological Properties of the Genus Tamarix: A Comprehensive Review. Arch. Pharm. Res. 2024, 47, 410–441. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Xu, X.; Shi, X.; Ji, X.; Wang, Y. Revealing the Salt Tolerance Mechanism of Tamarix Hispida by Large-Scale Identification of Genes Conferring Salt Tolerance. Tree Physiol. 2021, 41, 2153–2170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Effects of Exogenous (K+) Potassium Application on Plant Hormones in the Roots of Tamarix Ramosissima under NaCl Stress. Genes 2022, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Sajadimajd, S.; Iranpanah, A.; Khazaei, M.; Pourjabar, Z.; Hajimahmoodi, M.; Rahimi, R. In Vitro and in Vivo Antidiabetic Activity of Tamarix Stricta Boiss.: Role of Autophagy. J. Ethnopharmacol. 2021, 269, 113692. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Almutary, A.G.; Alsahli, M.A.; Alnasser, S.; Rah, B. Tamarix Articulata Induced Prevention of Hepatotoxicity Effects of In Vivo Carbon Tetrachloride by Modulating Pro-Inflammatory Serum and Antioxidant Enzymes to Reverse the Liver Fibrosis. Antioxidants 2022, 11, 1824. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Rah, B. Tamarix Articulata (T. Articulata)—An Important Halophytic Medicinal Plant with Potential. Pharmacological Properties. Curr. Pharm. Biotechnol. 2019, 20, 285–292. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Ali Zainy, F.M.; Almutary, A.G.; Sadier, N.S.; Rah, B. Tamarix Articulata Extract Offers Protection against Toxicity Induced by Beauty Products in Hs27 Human Skin Fibroblasts. PLoS ONE 2023, 18, e0287071. [Google Scholar] [CrossRef]
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix Aphylla: A Comprehensive Review. Plants 2022, 11, 118. [Google Scholar] [CrossRef]
- Fayez, N.; Khalil, W.; Abdel-Sattar, E.; Abdel-Fattah, A.F.M. Involvement of TNFα, IL-1β, COX-2 and NO in the Anti-Inflammatory Activity of Tamarix Aphylla in Wistar Albino Rats: An in-Vivo and in-Vitro Study. BMC Complement. Med. Ther. 2024, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Saïdana, D.; Mahjoub, M.A.; Boussaada, O.; Chriaa, J.; Chéraif, I.; Daami, M.; Mighri, Z.; Helal, A.N. Chemical Composition and Antimicrobial Activity of Volatile Compounds of Tamarix Boveana (Tamaricaceae). Microbiol. Res. 2008, 163, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Saïdana, D.; Ben Halima-Kamel, M.; Mahjoub, M.A.; Haouas, D.; Mighri, Z.; Helal, A.N. Insecticidal Activities of Tunisian Halophytic Plant Extracts against Larvae and Adults of Tribolium Confusum. Tropicultura 2007, 25, 193–199. [Google Scholar]
- Mennai, I.; Lamera, E.; Slougui, N.; Benaicha, B.; Gasmi, S.; Samai, Z.; Rahmouni, N.; Bensouici, C.; Pinto, D.C.G.A. Chemical Composition and Antioxidant, Antiparasitic, Cytotoxicity and Antimicrobial Potential of the Algerian Limonium Oleifolium Mill. Essential Oil and Organic Extracts. Chem. Biodivers. 2021, 18, e2100278. [Google Scholar] [CrossRef]
- Mennai, I.; Hanfer, M.; Esseid, C.; Benayache, S.; Ameddah, S.; Menad, A.; Benayache, F. Chemical Composition, in Vitro Antiparasitic, Antimicrobial and Antioxidant Activities of Frankenia Thymifolia Desf. Nat. Prod. Res. 2020, 34, 3363–3368. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Santos, S.A.O.; Beghidja, N.; Silva, A.M.S. Lipophilic Composition of Scabiosa Stellata L.: An Underexploited Plant from Batna (Algeria). Chem. Pap. 2018, 72, 753–762. [Google Scholar] [CrossRef]
- Alhourani, N.; Kasabri, V.; Bustanji, Y.; Abbassi, R.; Hudaib, M. Potential Antiproliferative Activity and Evaluation of Essential Oil Composition of the Aerial Parts of Tamarix Aphylla (L.) H.Karst.: A Wild Grown Medicinal Plant in Jordan. Evid. Based Complement. Alternat. Med. 2018, 2018, 9363868. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in Essential Oil Composition and Antioxidant Activity in Perovskia Abrotanoides Kar. Collected from Different Regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef]
- Sözmen, F.; Uysal, B.; Köse, E.O.; Aktaş, Ö.; Cinbilgel, I.; Oksal, B.S. Extraction of the Essential Oil from Endemic Origanum Bilgeri P.H. Davis with Two Different Methods: Comparison of the Oil Composition and Antibacterial Activity. Chem. Biodivers. 2012, 9, 1356–1363. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-Ol, A Volatile Terpene Molecule, Extensively Electrifies the Biological Systems against the Oxidative Stress-Linked Pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Santos, E.L.; Freitas, P.R.; Araújo, A.C.J.; Almeida, R.S.; Tintino, S.R.; Paulo, C.L.R.; Silva, A.C.A.; Silva, L.E.; do Amaral, W.; Deschamps, C.; et al. Enhanced Antibacterial Effect of Antibiotics by the Essential Oil of Aloysia Gratissima (Gillies & Hook.) Tronc. and Its Major Constituent Beta-Caryophyllene. Phytomedicine Plus 2021, 1, 100100. [Google Scholar] [CrossRef]
- Prerna, P.; Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A Comprehensive Review on the Pharmacological Prospects of Terpinen-4-Ol: From Nature to Medicine and Beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Saha, S.; Walia, S.; Ahluwalia, V.; Kaur, C. Antioxidant Potential of Essential Oil and Cadinene Sesquiterpenes of Eupatorium Adenophorum. Toxicol. Environ. Chem. 2013, 95, 127–137. [Google Scholar] [CrossRef]
- Nakamura, M.; Ra, J.H.; Jee, Y.; Kim, J.S. Impact of Different Partitioned Solvents on Chemical Composition and Bioavailability of Sasa Quelpaertensis Nakai Leaf Extract. J. Food Drug Anal. 2017, 25, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Jayakody, J.T.M.; Kim, J.I.; Jeong, J.W.; Choi, K.M.; Kim, T.S.; Seo, C.; Azimi, I.; Hyun, J.M.; Ryu, B.M. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Naija, D.S.; Helal, A.N. The Antioxidant and Free-Radical Scavenging Activities of Tamarix Boveana and Suaeda Fruticosa Fractions and Related Active Compound. Eur. Sci. J. 2014, 10, 201–219. [Google Scholar]
- Spinelli, R.; Sanchis, I.; Aimaretti, F.M.; Attademo, A.M.; Portela, M.; Humpola, M.V.; Tonarelli, G.G.; Siano, A.S. Natural Multi-Target Inhibitors of Cholinesterases and Monoamine Oxidase Enzymes with Antioxidant Potential from Skin Extracts of Hypsiboas Cordobae and Pseudis Minuta (Anura: Hylidae). Chem. Biodivers. 2019, 16, e1800472. [Google Scholar] [CrossRef]
- Srief, M.; Bani, M.; Mokrani, E.H.; Mennai, I.; Hamdi, M.; Boumechhour, A.; Mustapha, M.A.; Derdour, M.; Kerkatou, M.; El-Shazly, M.; et al. Evaluation of In Vitro and In Silico Anti-Alzheimer Potential of Nonpolar Extracts and Essential Oil from Mentha Piperita. Foods 2023, 12, 190. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Ayaz, M.; Ghufran, M.; Ullah, F.; Sadiq, A.; Ullah, I.; Haq, I.U.; et al. Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria Hydropiper L. Leaves Essential Oils. Evid. Based Complement. Alternat. Med. 2022, 2022, 7924171. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef]
- Mahfoudhi, A.; Grosso, C.; Gonçalves, R.F.; Khelifi, E.; Hammami, S.; Achour, S.; Trabelsi-Ayadi, M.; Valentão, P.; Andrade, P.B.; Mighri, Z. Evaluation of Antioxidant, Anticholinesterase, and Antidiabetic Potential of Dry Leaves and Stems in Tamarix Aphylla Growing Wild in Tunisia. Chem. Biodivers. 2016, 13, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic Contents and in Vitro Investigation of the Antioxidant, Enzyme Inhibitory, Photoprotective, and Antimicrobial Effects of the Organic Extracts of Pelargonium Graveolens Growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Animal. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Boulebd, H. Comparative Study of the Radical Scavenging Behavior of Ascorbic Acid, BHT, BHA and Trolox: Experimental and Theoretical Study. J. Mol. Struct. 2020, 1201, 127210. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Evaristus, N.A.; Abdullah, W.N.W.; Gan, C.-Y. Extraction and Identification of α-Amylase Inhibitor Peptides from Nephelium Lappacheum and Nephelium Mutabile Seed Protein Using Gastro-Digestive Enzymes. Peptides 2018, 102, 61–67. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Topçu, G.; Ay, M.; Bilici, A.; Sarikürkcü, C.; Öztürk, M.; Ulubelen, A. A New Flavone from Antioxidant Extracts of Pistacia Terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- Mansur, J.d.S.; Breder, M.N.; Mansur, M.C.; Azulay, R.D. Determination of Sun Protection Factor by Spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Dutra, E.A.; Oliveira, D.A.G.D.C.E.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of Sun Protection Factor (SPF) of Sunscreens by Ultraviolet Spectrophotometry. Rev. Bras. Cienc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R. Sideritis Galatica Bornm.: A Source of Multifunctional Agents for the Management of Oxidative Damage, Alzheimer’s’s and Diabetes Mellitus. J. Funct. Foods 2014, 11, 538–547. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y. Chemical Composition and Antibacterial Activity of Essential Oils from Different Parts of Litsea Cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; López, S.; Luna, L.; Aguero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential Oils of Medicinal Plants from the Central Andes of Argentina: Chemical Composition, and Antifungal, Antibacterial, and Insect-Repellent Activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Syu, W.; Shen, C.; Lu, J.; Lee, G.; Sun, C. Antimicrobial and Cytotoxic Activities of Neolignans from Magnolia Officinalis. Chem. Biodivers. 2004, 1, 530–537. [Google Scholar] [CrossRef]
- Frisch, M.J. Gaussian 09, Revision D. 01/Gaussian 2009; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Nieto, G.; Banón, S.; Garrido, M. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).