The Endosperm-Specific Gene OsEnS-42 Regulates Seed Vigor and Grain Quality
Abstract
1. Introduction
2. Results
2.1. Expression Analysis and Subcellular Localization of OsEnS-42
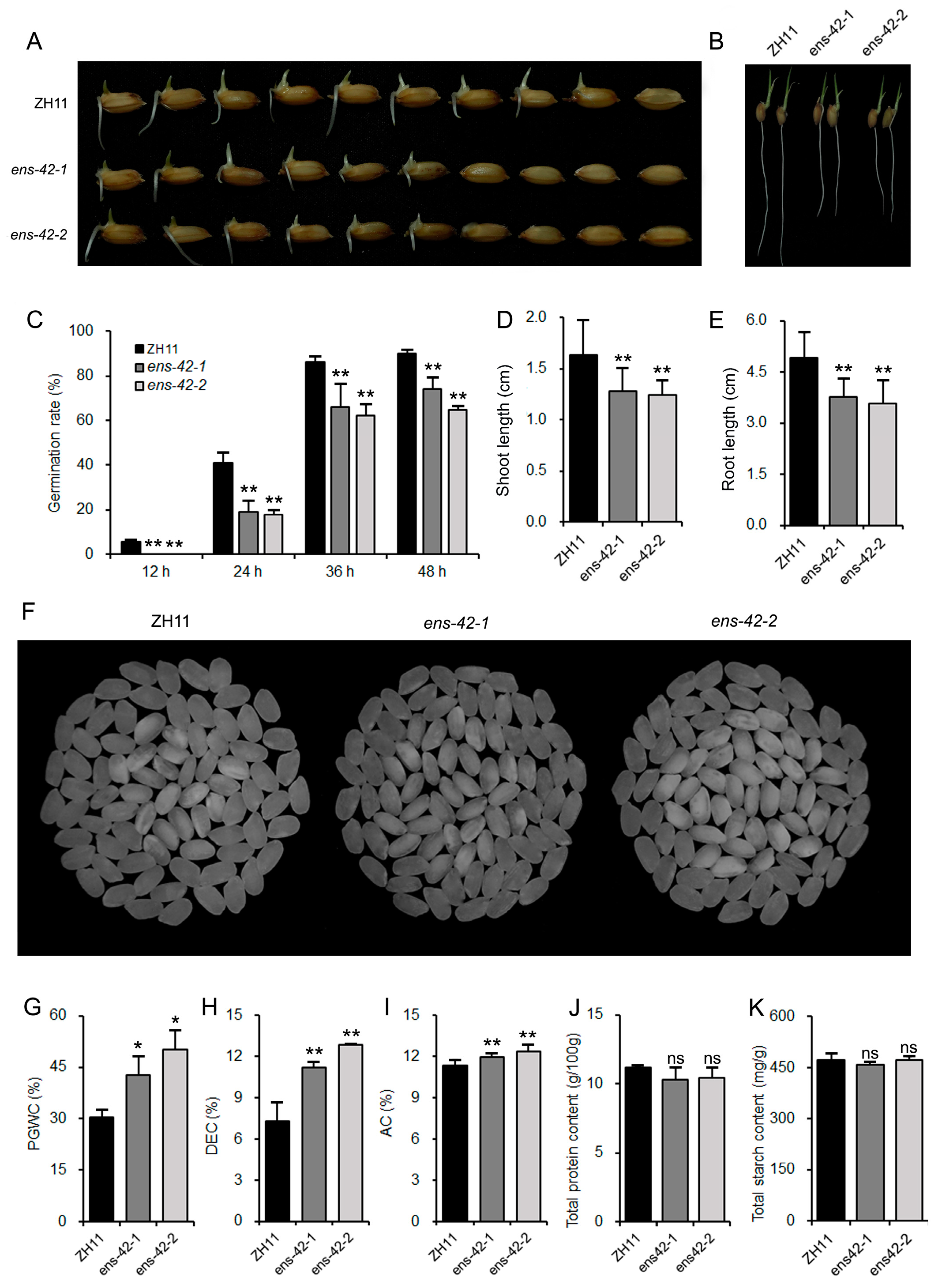
2.2. OsEnS-42 Regulates Seed Vigor and Grain Quality
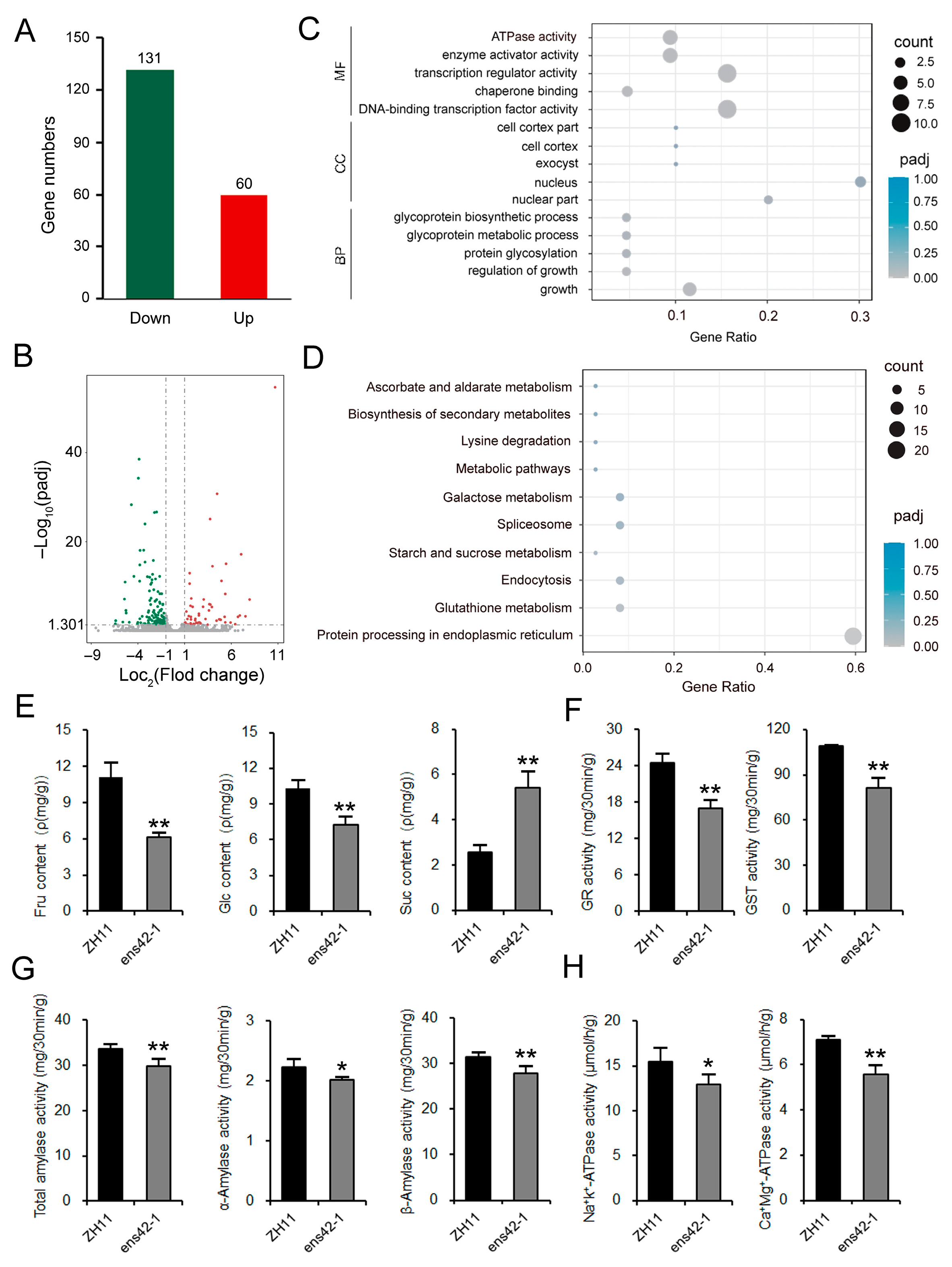
2.3. OsEnS-42 Regulates Soluble Sugar Metabolism and Redox Homeostasis During Seed Germination
2.4. OsEnS-42 Influences Soluble Sugar Content and Amylase Activity During Grain Filling
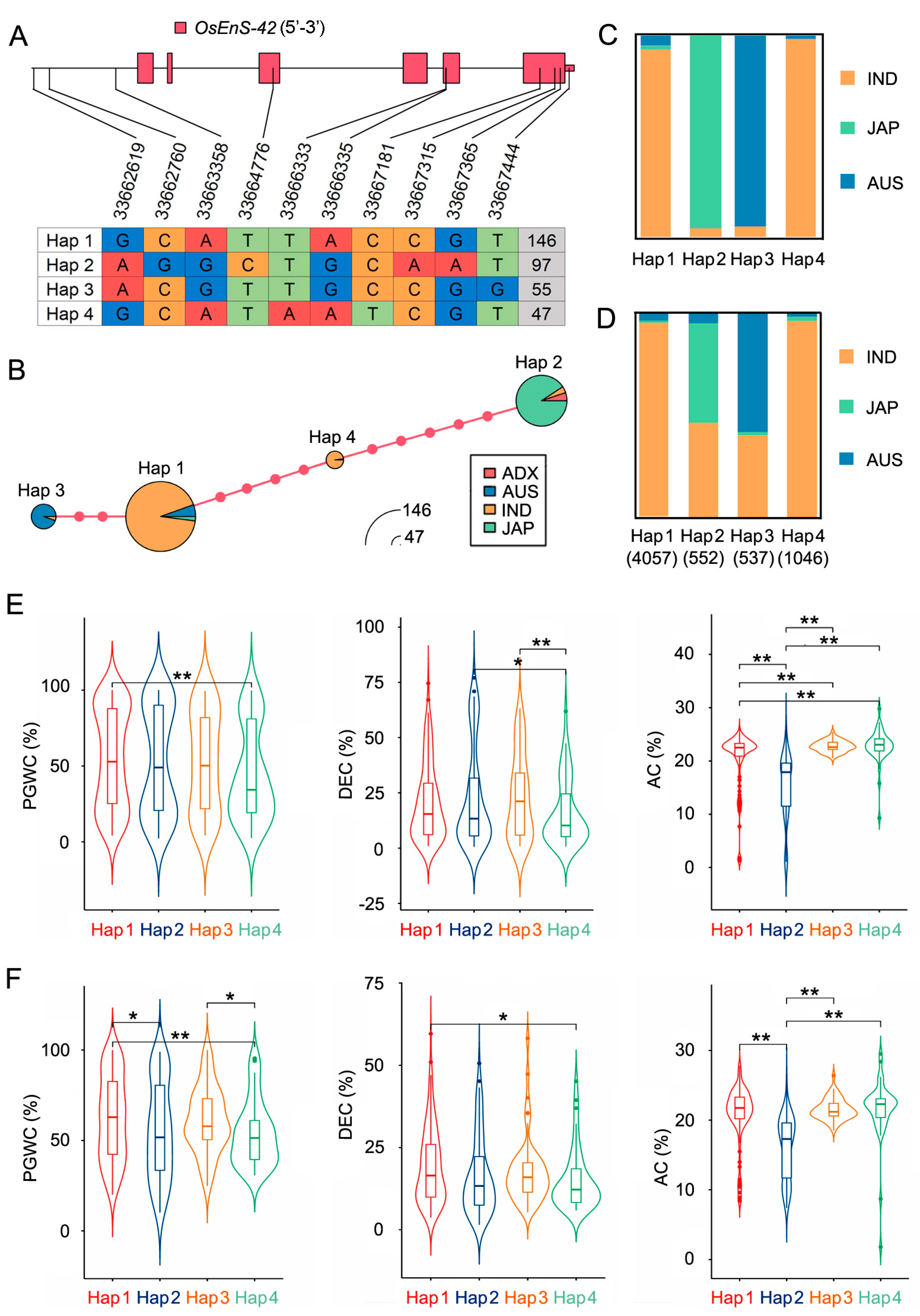
2.5. Haplotype Analysis of OsEnS-42
3. Discussion
4. Materials and Methods
4.1. RNA Extraction and qRT-PCR
4.2. Subcellular Localization of OsEnS-42
4.3. Construction of OsEnS-42 Knockout Lines
4.4. Seed Vigor Measurement
4.5. Grain Quality Analysis
4.6. Transcriptome Sequencing
4.7. Measurement of Physiological Indicators
4.8. Haplotype Analysis
4.9. Data Processing
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, R.; Bradford, K.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Lou, G.; Abbas, W.; Osti, R.; Ahmad, A.; Bista, S.; Ahiakpa, J.; He, Y. Improving rice grain quality through ecotype breeding for enhancing food and nutritional security in Asia–Pacific region. Rice 2024, 17, 47. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Yang, B.; Sun, S.; Peng, L.; Wang, Z. Indole-3-acetate beta glucosyl transferase OsIAGLU regulates seed vigour through mediating crosstalk between auxin and abscisic acid in rice. Plant Biotechnol. J. 2020, 18, 1933–1945. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Liu, K.; Chen, Y.; Cheng, Y.; Zeng, P.; Zhu, P.; Xie, T.; Chen, S.; Zhang, H.; et al. OsHIPL1, a hedgehog-interacting protein-like 1 protein, increases seed vigour in rice. Plant Biotechnol. J. 2022, 20, 1346–1362. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; He, D.; Damaris, R.; Yang, P. A stress-associated protein OsSAP8 modulates gibberellic acid biosynthesis by reducing the promotive effect of transcription factor OsbZIP58 on OsKO2. J. Exp. Bot. 2022, 73, 2420–2433. [Google Scholar] [CrossRef]
- He, Y.; Cheng, J.; He, Y.; Yang, B.; Cheng, Y.; Yang, C.; Zhang, H.; Wang, Z. Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotechnol. J. 2019, 17, 322–337. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M.; Zhan, C.; Liu, K.; Cheng, Y.; Xie, T.; Zhu, P.; He, Y.; Zeng, P.; Tang, H.; et al. Identification of OsPK5 involved in rice glycolytic metabolism and GA/ABA balance for improving seed germination via genome-wide association study. J. Exp. Bot. 2022, 73, 3446–3461. [Google Scholar] [CrossRef]
- Chen, C.; He, B.; Liu, X.; Ma, X.; Liu, Y.; Yao, H.; Zhang, P.; Yin, J.; Wei, X.; Koh, H.; et al. Pyrophosphate-fructose 6-phosphate 1-phosphotransferase (PFP1) regulates starch biosynthesis and seed development via heterotetramer formation in rice (Oryza sativa L.). Plant Biotechnol. J. 2020, 18, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Z.; Wang, X.; Ouyang, Y.; Chen, W.; Xie, K.; Wang, D.; Luo, M.; Luo, J.; Yao, J. Imprinted gene OsFIE1 modulates rice seed development by influencing nutrient metabolism and modifying genome H3K27me3. Plant J. 2016, 87, 305–317. [Google Scholar] [CrossRef]
- Stegner, M.; Wagner, J.; Roach, T. Antioxidant depletion during seed storage under ambient conditions. Seed Sci. Res. 2022, 32, 150–156. [Google Scholar] [CrossRef]
- Li, M.; He, Y. Research progress on the role of reactive oxygen species in seed germination. J. Zhejiang Univ. Sci. A 2023, 40, 254–264. [Google Scholar]
- Farooq, M.; Zhang, X.; Zafar, M.; Ma, W.; Zhao, J. Roles of reactive oxygen species and mitochondria in seed germination. Front. Plant Sci. 2021, 12, 781734. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Li, D.; Lv, B.; Chen, Y.; Chen, J.; Yan, X.; Liang, J. OsRACK1 is involved in abscisic acid- and H2O2-mediated signaling to regulate seed germination in rice (Oryza sativa L.). PLoS ONE 2014, 9, e97120. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Shao, G.; Jiao, G.; Sheng, Z.; Xie, L.; Hu, S.; Tang, S.; Wei, X.; Hu, P. Targeted mutagenesis of POLYAMINE OXIDASE 5 that negatively regulates mesocotyl elongation enables the generation of direct-seeding rice with improved grain yield. Mol. Plant 2021, 14, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sun, S.; Yang, B.; Zhao, J.; Li, W.; Huang, Z.; Li, Z.; He, Y.; Wang, Z. Genome-wide association study reveals that the cupin domain protein OsCDP3.10 regulates seed vigour in rice. Plant Biotechnol. J. 2022, 20, 485–498. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Sui, Y.; Ding, X.; Song, X. A multiomic study uncovers a bZIP23-PER1A-mediated detoxification pathway to enhance seed vigor in rice. Proc. Natl. Acad. Sci. USA 2022, 119, e2026355119. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.; Cuevas, R.; Ynion, J.; Laborte, A.; Velasco, M.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Tabassum, R.; Dosaka, T.; Ichida, H.; Morita, R.; Ding, Y.; Abe, T.; Katsube-Tanaka, T. FLOURY ENDOSPERM11-2 encodes plastid HSP70-2 involved with the temperature-dependent chalkiness of rice (Oryza sativa L.) grains. Plant J. 2020, 103, 604–616. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Xue, H. Rice aleurone layer specific OsNF-YB1 regulates grain filling and endosperm development by interacting with an ERF transcription factor. J. Exp. Bot. 2016, 67, 6399–6411. [Google Scholar] [CrossRef]
- Sun, X.; Ling, S.; Lu, Z.; Ouyang, Y.; Liu, S.; Yao, J. OsNF-YB1, a rice endosperm-specific gene, is essential for cell proliferation in endosperm development. Gene 2014, 551, 214–221. [Google Scholar] [CrossRef]
- Deng, H. Functional Analysis of the Seed Specific Gene OsbZIP76 of Rice (Oryza sativa). Master’s Thesis, Yangzhou University, Yangzhou, China, 2022. (In Chinese). [Google Scholar]
- Xiong, Y.; Ren, Y.; Wu, F.; Yang, W.; Huang, X.; Yao, J. NF-YC12 is a key multi-functional regulator of accumulation of seed storage substances in rice. J. Exp. Bot. 2019, 70, 3765–3780. [Google Scholar] [CrossRef]
- Xu, E.; Chen, M.; He, H.; Zhan, C.; Cheng, Y.; Zhang, H.; Wang, Z. Proteomic analysis reveals proteins in volved in seed imbibition under salt stress in rice. Front. Plant Sci. 2017, 7, 2006. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. Effects of Endosperm Specific Expression Gene OsEnS100 on Rice Grain. Ph.D. Thesis, Nanchang University, Nanchang, China, 2022. (In Chinese). [Google Scholar]
- Nie, D.; Ouyang, Y.; Wang, X.; Zhou, W.; Hu, C.; Yao, J. Genome-wide analysis of endosperm-specific genes in rice. Gene 2013, 530, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Chen, Y.; Zhang, P.; Tong, Q.; Zhu, Z.; Ma, T.; Wang, P.; Fu, K.; Chen, C.; Huang, Y.; et al. Prolongation of seed viability and grain quality in rice by editing OsLOX1 using CRISPR/Cas9. Mol. Plant Breed. 2024, 44, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, W.; Zhang, S.; Hu, H.; Yuan, Y.; Dong, J.; Chen, L.; Ma, Y.; Yang, T.; Zhou, L.; et al. A pangenome analysis pipeline provides insights into functional gene identification in rice. Genome Biol. 2023, 24, 19. [Google Scholar] [CrossRef]
- Huo, X.; Wang, J.; Chen, L.; Fu, H.; Yang, T.; Dong, J.; Ma, Y.; Zhou, L.; Chen, J.; Liu, D.; et al. Genome-wide association mapping and gene expression analysis reveal candidate genes for grain chalkiness in rice. Front. Plant Sci. 2023, 14, 1184276. [Google Scholar] [CrossRef]
- Nie, S.; Chen, L.; Zheng, M.; Dong, J.; Ma, Y.; Zhou, L.; Wang, J.; Chen, J.; Hu, H.; Yang, T.; et al. GWAS and transcriptomic analysis identify OsRING315 as a new candidate gene controlling amylose content and gel consistency in rice. Rice 2024, 17, 38. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Shi, H.; Yun, P.; Zhu, Y.; Wang, L.; Wang, Y.; Li, P.; Zhou, H.; Cheng, S.; Liu, R.; Gao, G.; et al. Natural variation of WBR7 confers rice high yield and quality by modulating sucrose supply in sink organs. Plant Biotechnol. J. 2024, 22, 2985–2999. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, X.; Xie, Y.; Chen, L.; Zhao, J.; Liu, B.; Zhang, S.; Liu, D. Transcriptome and metabolome analyses reveal new insights into the regulatory mechanism of head milled rice rate. Plants 2022, 11, 2838. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Zheng, M.; Hu, R.; Dong, J.; Zhou, L.; Liu, W.; Liu, D.; Yang, W. Genes controlling grain chalkiness in rice. Crop J. 2024, 12, 979–991. [Google Scholar] [CrossRef]
- Dong, J.F.; Chen, K.; Chen, L.; Zheng, M.H.; Nie, S.; Ye, C.J.; Li, X.Z.; Xie, G.W.; Chen, H.; Zhao, J.L.; et al. OsNRAMP7 positively regulates heat tolerance at seedling and reproductive stages in rice. Plant Stress 2025, 16, 100870. [Google Scholar] [CrossRef]
- Albaseer, S.S.; Rao, R.N.; Swamy, Y.V.; Mukkanti, K. An overview of sample preparation and extraction of synthetic pyrethroids from water, sediment and soil. J. Chromatogr. A 2010, 1217, 5537–5554. [Google Scholar] [CrossRef]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef]
- Glauser, G.; Grund, B.; Gassner, A.L.; Menin, L.; Henry, H.; Bromirski, M.; Schütz, F.; McMullen, J.; Rochat, B. Validation of the mass-extraction-window for quantitative methods using liquid chromatography high resolution mass spectrometry. Anal. Chem. 2016, 88, 3264–3271. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, W.J.; Yu, L.; Ding, J.; Feng, Y.Q. Comprehensive profiling of phytohormones in honey by sequential liquid-liquid extraction coupled with liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2017, 65, 575–585. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Guo, Q.; Wang, J.; Zhang, P.; Chen, W. Effects of nitric oxide on postharvest quality and soluble sugar content in papaya fruit during ripening. J. Food Process. Preserv. 2014, 38, 591–599. [Google Scholar] [CrossRef]
- Gangola, M.P.; Jaiswal, S.; Khedikar, Y.P.; Chibbar, R.N. A reliable and rapid method for soluble sugars and RFO analysis in chickpea using HPAEC–PAD and its comparison with HPLC–RI. Food Chem. 2014, 154, 127–133. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, S.; He, S.; Ma, Q.; Lu, X.; Hao, X.; Wang, H.; Yang, J.; Zhang, P. Production of very-high-amylose cassava by post-transcriptional silencing of branching enzyme genes. J. Integr. Plant Biol. 2020, 62, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jia, G.; Diao, X. geneHapR: An R package for gene haplotypic statistics and visualization. BMC Bioinform. 2023, 24, 199. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Hu, X.; Chen, L.; Xing, J.; Nie, S.; Ma, L.; Sun, W.; Liu, D.; Li, X.; Matthayatthaworn, W.; et al. The Endosperm-Specific Gene OsEnS-42 Regulates Seed Vigor and Grain Quality. Plants 2025, 14, 2492. https://doi.org/10.3390/plants14162492
Zheng M, Hu X, Chen L, Xing J, Nie S, Ma L, Sun W, Liu D, Li X, Matthayatthaworn W, et al. The Endosperm-Specific Gene OsEnS-42 Regulates Seed Vigor and Grain Quality. Plants. 2025; 14(16):2492. https://doi.org/10.3390/plants14162492
Chicago/Turabian StyleZheng, Minhua, Xiaodan Hu, Luo Chen, Jiale Xing, Shuai Nie, Lukai Ma, Wei Sun, Dilin Liu, Xiumei Li, Weerachai Matthayatthaworn, and et al. 2025. "The Endosperm-Specific Gene OsEnS-42 Regulates Seed Vigor and Grain Quality" Plants 14, no. 16: 2492. https://doi.org/10.3390/plants14162492
APA StyleZheng, M., Hu, X., Chen, L., Xing, J., Nie, S., Ma, L., Sun, W., Liu, D., Li, X., Matthayatthaworn, W., Yang, W., & Liu, W. (2025). The Endosperm-Specific Gene OsEnS-42 Regulates Seed Vigor and Grain Quality. Plants, 14(16), 2492. https://doi.org/10.3390/plants14162492





