Bioactivity of Essential Oils from Patagonian Wild Plants: Acaricidal and Insecticidal Effects on Varroa destructor and Apis mellifera
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis: Constituents of Essential Oils
2.2. Mite and Bee Bioassays
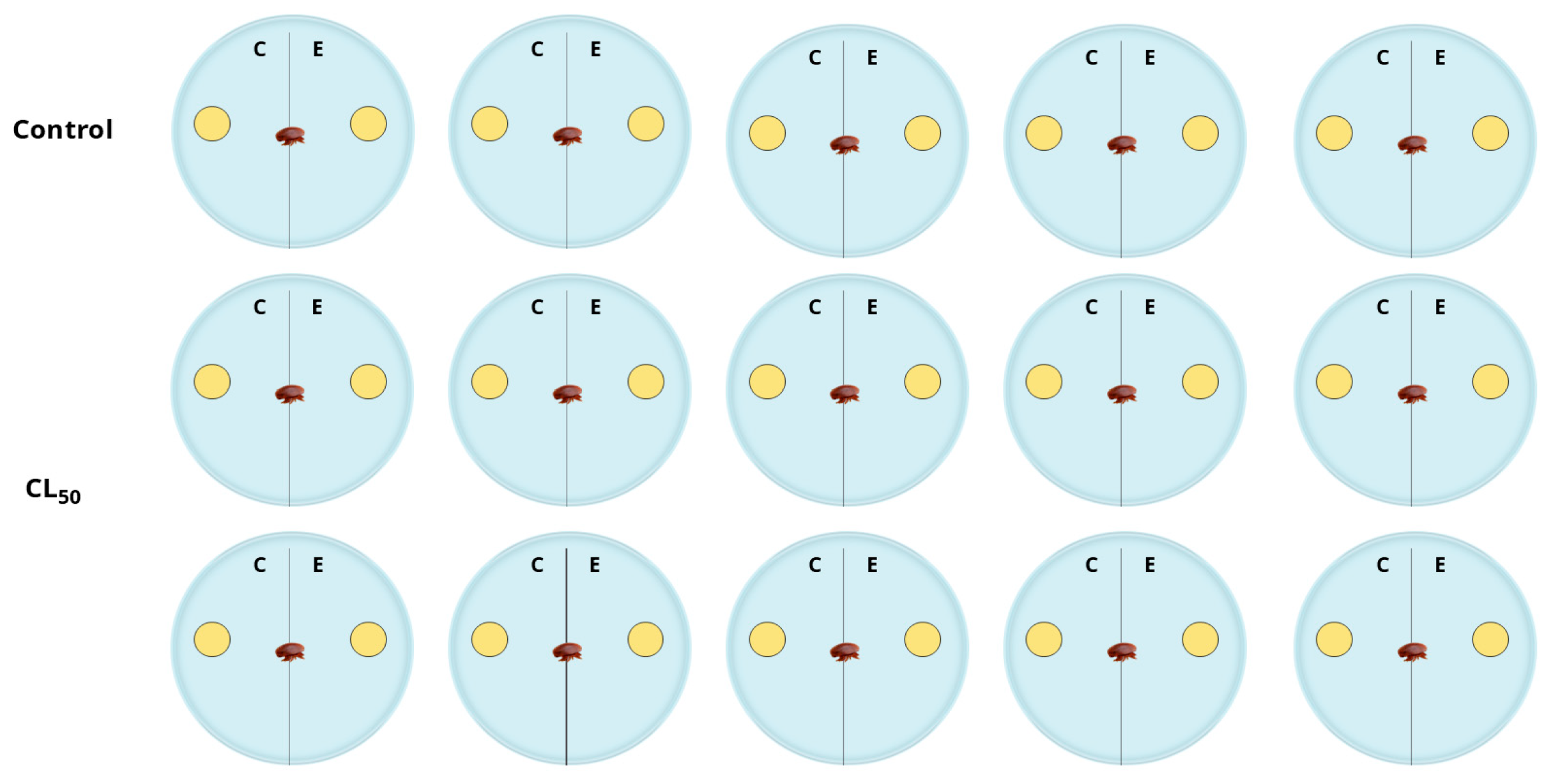
2.3. Attraction and Repellency Assay
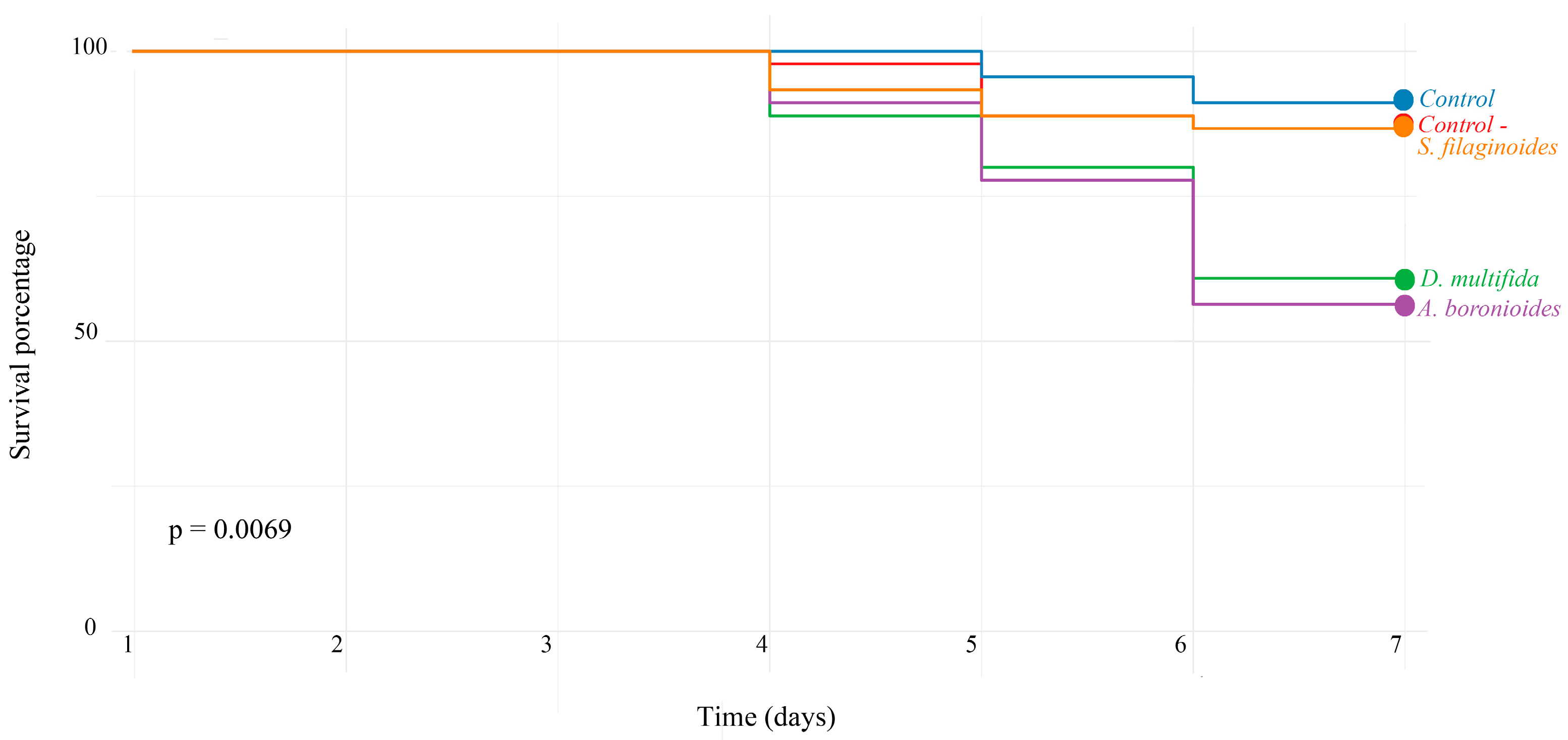
2.4. Toxicity Bioassay on Apis mellifera Larvae
3. Discussion
4. Materials and Methods
4.1. Collection of Plants and Extraction of Essential Oils (EOs)
4.2. Analysis of Essential Oils
4.3. Source of Mites and Bees
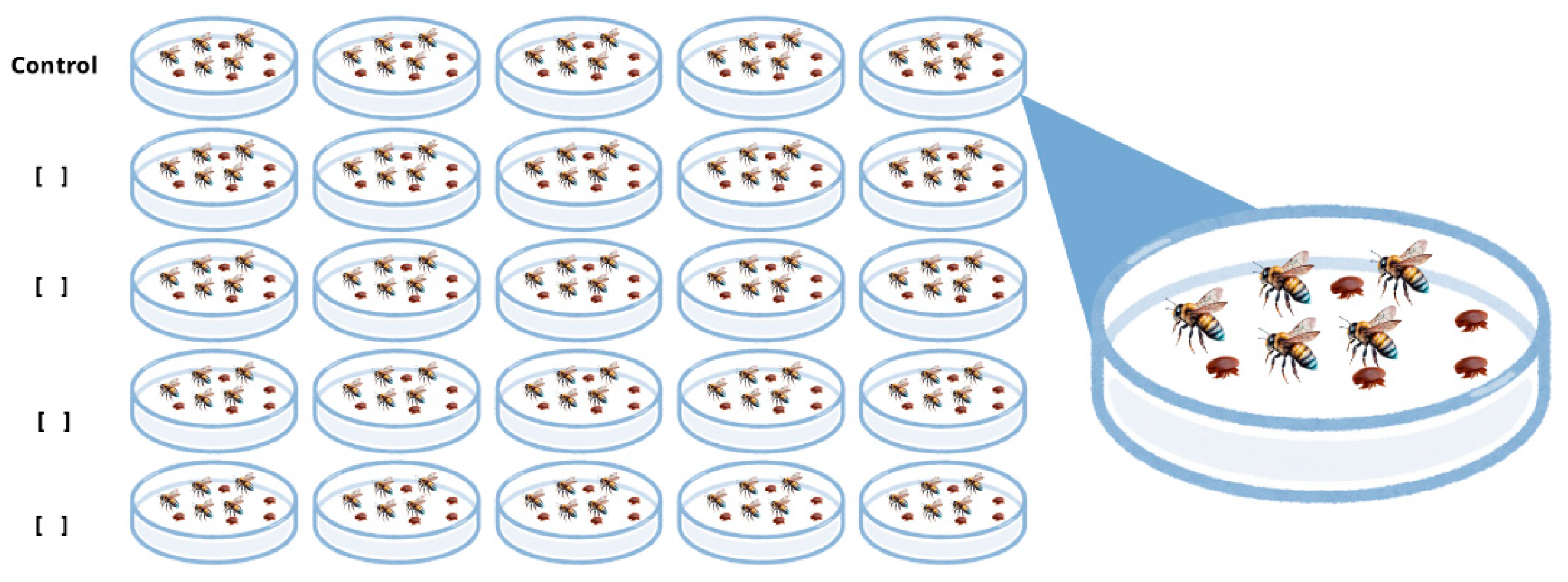
4.4. Experimental Design for Mite and Bee Bioassays
4.5. Attractive and Repellent Assay
4.6. Honeybee Larval Toxicity Bioassay
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef]
- Lorenzo- Felipe, I.; Blanco, C.; Corona, M. Impact of apoidea (Hymenoptera) on the world’s food production and diets. Ann. Entomol. Soc. Am. 2020, 113, 407–424. [Google Scholar] [CrossRef]
- Klein, A.M.; Müller, C.; Hoehn, P.; Kremen, C. Understanding the role of species richness for crop pollination services. In Biodiversity, Ecosystem Function and Human Wellbeing; Oxford University Press: Oxford, UK, 2009; pp. 195–208. [Google Scholar]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef]
- Singh, G.; Rana, A. Honeybees and colony collapse disorder: Understanding key drivers and economic implications. Proc. Indian Natl. Sci. Acad. 2025. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apicult. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Daughenbaugh, K.F.; Martin, M.; Brutscher, L.M.; Cavigli, I.; Garcia, E.; Lavin, M.; Flenniken, M.L. Honey bee infecting Lake Sinai viruses. Viruses 2015, 7, 3285–3309. [Google Scholar] [CrossRef] [PubMed]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Effect of Varroa destructor, wounding and varroa homogenate on gene expression in brood and adult honeybees. PLoS ONE 2017, 12, e0169669. [Google Scholar] [CrossRef] [PubMed]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef]
- Dubois, E.; Dardouri, M.; Schurr, F.; Cougoule, N.; Sircoulomb, F.; Thiéry, R. Outcomes of honeybee pupae inoculated with deformed wing virus genotypes A and B. Apidologie 2020, 51, 18–34. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Corona, M.; Alburaki, M.; Reynaldi, F.J.; Invernizzi, C.; Fernández de Landa, G.; Maggi, M. Honeybee populations surviving Varroa destructor parasitism in Latin America and their mechanisms of resistance. Front. Ecol. Evol. 2024, 12, 1434490. [Google Scholar] [CrossRef]
- Fries, I.; Rosenkranz, P. Number of reproductive cycles of Varroa jacobsoni in honeybee (Apis mellifera) colonies. Exp. Appl. Acarol. 1996, 20, 103–112. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honeybee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef]
- Thompson, H.M.; Brown, M.A.; Ball, R.F.; Bew, M.H. First report of Varroa destructor resistance to pyrethroids in U.K. Apidologie 2002, 33, 357–366. [Google Scholar] [CrossRef]
- Maggi, M.; Ruffinengo, S.; Damiani, N.; Sardella, N.; Eguaras, M. First detection of Varroa destructor resistance to coumaphos in Argentina. Exp. Appl. Acarol. 2009, 47, 317–320. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Negri, P.; Eguaras, M.J. Resistance phenomena to amitraz from populations of the ectoparasitic mite Varroa destructor of Argentina. Parasitol. Res. 2010, 107, 1189–1192. [Google Scholar] [CrossRef]
- Mitton, G.A.; Quintana, S.; Giménez Martínez, P.; Mendoza, Y.; Ramallo, G.; Brasesco, C.; Villalba, A.; Eguaras, M.J.; Maggi, M.; Ruffinengo, S.R. First record of resistance to flumethrin in a varroa population from Uruguay. J. Apic. Res. 2016, 55, 422–427. [Google Scholar] [CrossRef]
- Iglesias, A.; Mitton, G.; Szawarski, N.; Cooley, H.; Ramos, F.; Arcerito, F.M.; Brasesco, C.; Ramirez, C.; Gende, L.; Eguaras, M.; et al. Essential oils from Humulus lupulus as novel control agents against Varroa destructor. Ind. Crops. Prod. 2020, 158, 113043. [Google Scholar] [CrossRef]
- Medici, S.K.; Maggi, M.D.; Galetto, L.; del Rosario Iglesias, M.; Sarlo, E.G.; Recavarren, M.I.; Salar, P.E.; Eguaras, M.J. Influence of the agricultural landscape surrounding Apis mellifera colonies on the presence of pesticides in honey. Apidologie 2022, 53, 21. [Google Scholar] [CrossRef]
- Ferrero, A.A.; Werdin González, J.O.; Sánchez Chopa, C. Biological activity of Schinus molle on Triatoma infestans. Fitoterapia 2006, 77, 381–383. [Google Scholar] [CrossRef]
- Damiani, N.; Gende, L.; Maggi, M.; Palacios, S.; Marcangeli, J.; Eguaras, M. Repellent and acaricidal effects of botanical extracts on Varroa destructor. Parasitol. Res. 2011, 108, 79–86. [Google Scholar] [CrossRef]
- Iglesias, A.; Gimenez Martinez, P.; Ramirez, C.; Mitton, G.; Meroi Arcerito, F.R.; Fangio, M.F.; Churio, M.S.; Fuselli, S.; Fanovich, A.; Eguaras, M.; et al. Valorization of hop leaves for development of eco-friendly bee pesticides. Apidologie 2021, 52, 186–198. [Google Scholar] [CrossRef]
- Brasesco, C.; Gende, L.; Negri, P.; Szawarski, N.; Iglesias, A.; Eguaras, M.; Ruffinengo, S.; Maggi, M. Assessing In Vitro acaricidal effect and joint action of a binary mixture between essential oil compounds (Thymol, Phellandrene, Eucalyptol, Cinnamaldehyde, Myrcene, Carvacrol) over ectoparasitic mite Varroa destructor (Acari: Varroidae). J. Apic Sci. 2017, 61, 203–215. [Google Scholar] [CrossRef]
- González, S.B. Adesmia boronioides Hook. F.: Una especie aromática y medicinal nativa de la Patagonia. Naturalia 2005, 2, 85–91. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Guy, J.H.; Arkle, S.; Harrington, D.; De Luna, C.; Okello, E.J.; Shiel, R.S.; Port, G.; Sparagano, O.A. Use of plant derived products to control arthropods of veterinary importance: A review. Ann. N. Y. Acad. Sci. 2008, 1149, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Giordani, C.; Spinozzi, E.; Baldassarri, C.; Ferrati, M.; Cappellacci, L.; Santibañez Nieto, D.; Pavela, R.; Ricciardi, R.; Benelli, G.; Petrelli, R.; et al. Insecticidal activity of four essential oils extracted from Chilean Patagonian plants as potential organic pesticides. Plants 2022, 11, 2012. [Google Scholar] [CrossRef]
- Molares, S.; Ladio, A. Mapuche perceptions and conservation of Andean. Nothofagus forests and their medicinal plants: A case study from a rural community in Patagonia, Argentina. Biodivers. Conserv. 2012, 21, 1079–1093. [Google Scholar] [CrossRef]
- Burkart, A.E. Sinopsis del género sudamericano de Leguminosas Adesmia DC. Darwiniana 1967, 14, 463–468. [Google Scholar]
- González, S.B.; Houghton, P.J.; Hoult, J.R.S. The activity against leukocyte eicosanoid generation of essential oil and polar fractions of Adesmia boronioides Hook. F. Phytother. Res. 2003, 3, 290–293. [Google Scholar] [CrossRef]
- Hewis, L.G.; Daeli, G.B.C.; Tanoto, K.; Carlos, C.; Sahamastuti, A.A.T. A review of botany, phytochemical, and pharmacological effects of Dysphania ambrosioides. Indones. J. Life Sci. 2020, 2, 70–82. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Dysphania ambrosioides essential oils: From pharmacological agents to uses in modern crop protection—A review. Phytochem. Rev. 2022, 21, 141–159. [Google Scholar] [CrossRef]
- Arancibia, L.A.; Naspi, C.V.; Pucci, G.N.; Arce, M.E.; Colloca, C.B. Biological activity of 1-heneicosanol isolated from Senecio coluhuapiensis, an endemic species from Patagonia, Argentina. Pharm. Chem. J. 2016, 3, 73–77. [Google Scholar]
- Ruffinengo, S.; Eguaras, M.; Floris, I.; Faverin, C.; Bailac, P.; Ponzi, M. LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J. Econ. Entomol. 2005, 98, 651–655. [Google Scholar] [CrossRef]
- Maggi, M.D.; Mitton, G.A. Sustainable beekeeping: The impact of organic molecules on honey bee health and apiculture. Environ. Toxicol. Pharmacol. 2025, 117, 104739. [Google Scholar] [CrossRef]
- Imdorf, A.; Bogdanov, S.; Ibáñez Ochoa, R.; Calderone, N. Use of essential oils for the control of Varroa jacobsoni Oud. in honeybee colonies. Apidologie 1999, 30, 209–228. [Google Scholar] [CrossRef]
- Häußermann, C.K.; Ziegelmann, B.; Bergmann, P.; Rosenkranz, P. Male mites (Varroa destructor) perceive the female sex pheromone with the sensory pit organ on the front leg tarsi. Apidologie 2015, 46, 771–778. [Google Scholar] [CrossRef]
- Nganso, B.T.; Mani, K.; Altman, Y.; Rafaeli, A.; Soroker, V. How Crucial is the Functional Pit Organ for the Varroa Mite? Insects 2020, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Plettner, E.; Eliash, N.; Singh, N.K.; Pinnelli, G.R.; Soroker, V. The chemical ecology of host-parasite interaction as a target of Varroa destructor control agents. Apidologie 2017, 48, 78–92. [Google Scholar] [CrossRef]
- Gastaldi, B. Análisis de Los Compuestos Fenólicos y Volátiles de Plantas Medicinales y Aromáticas del Noroeste de la Patagonia Argentina: Estudio de Las Actividades Antioxidante y Citotóxica. Ph.D. Thesis, Universidad Nacional de la Patagonia San Juan Bosco, Esquel, Argentina, 2018. [Google Scholar]
- Kraus, B.; Koeniger, N.; Fuchs, S. Screening of substances for their effect on Varroa jacobsoni attractiveness, repellency, toxicity and masking effects of ethereal oils. J. Apic. Res. 1994, 33, 34–43. [Google Scholar] [CrossRef]
- Neira, M.; Heinsohn, P.; Carrillo, R.; Báez, A.; Fuentealba, J. Efecto de aceites esenciales de lavanda y laurel sobre el ácaro Varroa destructor Anderson y Trueman (Acari: Varroidae). Agric. Técnica 2004, 64, 238–244. [Google Scholar] [CrossRef]
- Auger, J.; Thibout, E. Susbtances soufrées des Allium et des Crucifères et leurs potentialités phytosanitaires. In Biopesticides D’origine Végétale; Regnault-Roger, C., Philogène, B.J.R., Vincent, C., Eds.; Lavoisier: Paris, France, 2002; pp. 7–95. [Google Scholar]
- Bendifallah, L.; Belguendouz, R.; Hamoudi, L.; Arab, K. Biological activity of the Salvia officinalis L. (Lamiaceae) essential oil on Varroa destructor infested honeybees. Plants 2018, 7, 44. [Google Scholar] [CrossRef]
- van Baren, C.M.; González, S.B.; Bandoni, A.L.; Di Leo Lira, P.; Bucio, M.A.; Hernández-Barragán, A.; Joseph-Nathan, P. GC-FID-MS and X-ray Diffraction for the Detailed Evaluation of the Volatiles From Senecio filaginoides. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Arantes, A.C.S.; Ribeiro, J.C.S.; Soares, D.S.; Reis, A.C.; Cardoso, M.D.G.; Remedio, R.N. Alpha-and beta-pinene isomers act differently to control Rhipicephalus microplus (Acari: Ixodidae). Parasitol. Res. 2024, 123, 164. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.S.; Arantes, A.C.S.; Soares, D.S.; das Graças Cardoso, M.; Campos, A.K.; Remedio, R.N. Alpha-and beta-pinene alter the morphology of oocytes in Rhipicephalus microplus ticks (Acari: Ixodidae). Vet. Parasitol. 2025, 337, 110507. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.P.; Parreira, L.A.; Venancio, A.N.; Santos, M.F.; Menini, L. Chemical characterization and evaluation of acaricidal potential of rosemary essential oil and its main compound α-pinene on the two-spotted spider mite, Tetranychus urticae. Nat. Prod. Res. 2023, 37, 2940–2944. [Google Scholar] [CrossRef]
- Giménez-Martínez, P.; Ramirez, C.; Mitton, G.; Arcerito, F.M.; Ramos, F.; Cooley, H.; Fuselli, S.; Maggi, M. Lethal concentrations of Cymbopogon nardus essential oils and their main component citronellal on Varroa destructor and Apis mellifera. Exp. Parasitol. 2022, 238, 108279. [Google Scholar] [CrossRef]
- Karasek, F.W.; Clement, R.E. Basic Gas Chromatography-Mass Spectrometry: Principles and Techniques; Elsevier Science: Amsterdam, The Netherlands, 2012; p. 220. [Google Scholar]
- Gruľová, D.; Baranová, B.; Sedlák, V.; De Martino, L.; Zheljazkov, V.D.; Konečná, M.; Poráčová, J.; Caputo, L.; De Feo, V. Juniperus horizontalis Moench: Chemical composition, herbicidal and insecticidal activities of its essential oil and of its main component, sabinene. Molecules 2022, 27, 8408. [Google Scholar] [CrossRef]
- Espinosa-García, F.J.; Langenheim, J.H. Effects of sabinene and γ-terpinene from coastal redwood leaves acting singly or in mixtures on the growth of some of their fungus endophytes. Biochem. Syst. Ecol. 1991, 19, 643–650. [Google Scholar] [CrossRef]
- Matias, E.F.; Alves, E.F.; Silva, M.K.; Carvalho, V.R.; Figueredo, F.G.; Ferreira, J.V.; Coutinho, H.D.; Silva, J.M.; Ribeiro-Filho, J.; Costa, J.G. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops. Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Macchioni, F.; Cioni, P.L.; Flamini, G.; Morelli, I.; Perrucci, S.; Franceschi, A.; Macchioni, G.; Ceccarini, L. Acaricidal activity of pine essential oils and their main components against Tyrophagus putrescentiae, a stored food mite. J. Agric. Food Chem. 2002, 50, 4586–4588. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Palma, E.; Musolino, V.; Carresi, C.; Cardamone, A.; Lupia, C.; Marrelli, M.; Conforti, F.; Roncada, P.; et al. Phytochemical Profile of Foeniculum vulgare Subsp. piperitum Essential Oils and Evaluation of Acaricidal Efficacy against Varroa destructor in Apis mellifera by In Vitro and Semi-Field Fumigation Tests. Vet. Sci. 2022, 9, 684. [Google Scholar] [CrossRef]
- Ramírez, L.; Negri, P.; Sturla, L.; Guida, L.; Vigliarolo, T.; Maggi, M.; Eguaras, M.; Zocchi, E.; Lamattina, L. Abscisic acid enhances cold tolerance in honeybee larvae. Proc. R. Soc. B 2017, 284, 20162140. [Google Scholar] [CrossRef] [PubMed]
- Aupinel, P.; Fortini, D.; Dufour, H.; Tasei, J.; Michaud, B.; Odoux, J.; Pham-Delegue, M. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 2005, 58, 107. [Google Scholar]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.P.; Claßen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Ellis, J.D. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci. Rep. 2018, 8, 5635. [Google Scholar] [CrossRef]
- Hewlett, P.; Packett, R. An Introduction to the Interpretation of Quantal Responses in Biology; Edwards Arnold: London, UK, 1979; p. 82. [Google Scholar]
- Stokes, M.E.; Davis, C.S.; Koch, G.G. Categorical Data Analysis Using the SAS System; SAS Institute Inc.: Cary, NC, USA, 1995; pp. 34–35. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Therneau, T.; Atkinson, E. The concordance statistic. A package for survival analysis in R, vignettes. R Package Version 2023, 3. [Google Scholar]

| Compound | A. boronioides | D. multifida | S. filaginoides | |||
|---|---|---|---|---|---|---|
| R.T. 1 | (%) | R.T. | (%) | R.T. | (%) | |
| Monoterpene | ||||||
| Tricyclene | 22.08 | 0.611 | ||||
| α-Pinene | 22.55 | 11.41 | 22.62 | 0.339 | 22.65 | 40.872 |
| Camphene | 23.52 | 0.333 | ||||
| Sabinene | 24.27 | 5.403 | ||||
| Myrcene | 24.58 | 1.255 | ||||
| β-Pinene | 24.60 | 1.95 | 24.69 | 0.246 | 24.78 | 32.962 |
| α-Terpinene | 25.55 | 0.192 | 26.08 | 0.490 | ||
| δ-3-Carene | 26.13 | 26.05 | 26.28 | 0.871 | ||
| Limonene | 26.63 | 3.18 | 26.83 | 4.126 | ||
| Z-β-Ocimene | 26.71 | 0.199 | ||||
| β-Phellandrene | 27.01 | 2.506 | ||||
| γ-Terpinene | 27.85 | 0.219 | 27.85 | 0.265 | ||
| Linalool | 28.30 | 0.35 | ||||
| Terpinolene | 29.16 | 0.988 | ||||
| 4-Terpineol | 33.47 | 0.847 | ||||
| Citronellol | 34.59 | 0.386 | ||||
| Ascaridole | 36.30 | 34.87 | ||||
| Piperitone epoxide | 38.40 | 8.26 | ||||
| Alkylbenzene | ||||||
| p-Cymene | 26.28 | 24.39 | ||||
| Sesquiterpene | ||||||
| β-Caryophyllene | 37.54 | 3.65 | ||||
| Guaiadiene | 44.23 | 10.23 | ||||
| 10-epi-γ-Eudesmol | 49.13 | 7.22 | ||||
| Esquelenone | 46.17 | 34.49 | ||||
| β-Furopelargane | 46.90 | 3.57 | ||||
| α-Furopelargane | 47.28 | 2.08 | ||||
| Isoesquelenone | 50.28 | 10.61 | ||||
| 4-α-Dihydroagarofuranol | 51.81 | 5.77 | ||||
| Essential Oil | Varroa destructor LC50 (µL/mL) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Adesmia boronioides | 1.1 (0.79–1.56) | 0.28 (0.16–0.50) | >0.5 |
| Dysphania multifida | 0.31 (0.02–1.91) | 0.95 (0.26–3.38) | >0.5 |
| Senecio filaginoides | 139.5 (69.2–280.1) | 78.6 (32.4–190.2) | 9.1 (5.4–14.8) |
| Essential Oil | Apis mellifera LC50 (µL/mL) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Adesmia boronioides | 28.02 (11.01–71.33) | 13.1 (6.64–25.9) | 14.4 (5.9–35.2) |
| Dysphania multifida | 0.98 (0.5–1.75) | 0.94 (0.25–3.46) | >20 |
| Senecio filaginoides | >10000 | >1000 | >1000 |
| Essential Oil | Effect | p-Value |
|---|---|---|
| Control | No effect | 0.581 |
| Adesmia boronioides | Repellency | 0.001 ** |
| Dysphania multifida | Repellency | 0.022 * |
| Senecio filaginoides | No effect | 1.00 |
| Treatments | HR (exp(coef)) | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Control- | 1.53 | 0.43–5.42 | 0.509 |
| Adesmia boronioides | 3.745 | 1.23–11.38 | 0.019 *1 |
| Dysphania multifida | 4.299 | 1.43–12.86 | 0.009 ** |
| Senecio filaginoides | 1.541 | 0.43–5.46 | 0.502 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, R.M.; Iglesias, A.E.; Guajardo, J.J.; Amaturi, C.A.; Freeman, B.D.; López de Armentia, J.; Rizzuto, S.; Maggi, M.D. Bioactivity of Essential Oils from Patagonian Wild Plants: Acaricidal and Insecticidal Effects on Varroa destructor and Apis mellifera. Plants 2025, 14, 2484. https://doi.org/10.3390/plants14162484
Manzo RM, Iglesias AE, Guajardo JJ, Amaturi CA, Freeman BD, López de Armentia J, Rizzuto S, Maggi MD. Bioactivity of Essential Oils from Patagonian Wild Plants: Acaricidal and Insecticidal Effects on Varroa destructor and Apis mellifera. Plants. 2025; 14(16):2484. https://doi.org/10.3390/plants14162484
Chicago/Turabian StyleManzo, Rosa M., Azucena E. Iglesias, Jimena J. Guajardo, Carolina A. Amaturi, Brenda D. Freeman, Juliana López de Armentia, Susana Rizzuto, and Matías D. Maggi. 2025. "Bioactivity of Essential Oils from Patagonian Wild Plants: Acaricidal and Insecticidal Effects on Varroa destructor and Apis mellifera" Plants 14, no. 16: 2484. https://doi.org/10.3390/plants14162484
APA StyleManzo, R. M., Iglesias, A. E., Guajardo, J. J., Amaturi, C. A., Freeman, B. D., López de Armentia, J., Rizzuto, S., & Maggi, M. D. (2025). Bioactivity of Essential Oils from Patagonian Wild Plants: Acaricidal and Insecticidal Effects on Varroa destructor and Apis mellifera. Plants, 14(16), 2484. https://doi.org/10.3390/plants14162484






