Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment
Abstract
1. Introduction
2. The Plant Circadian Clock
2.1. Light and Clock: A Bidirectional Relationship
2.2. Clock Modulation of Light Signaling and Sensitivity
3. Clock and Metabolism: Ticking Organelles
3.1. Circadian Control of Chloroplast
3.2. Circadian Control of Mitochondria
3.3. The Organelle Entrainment of the Clock
3.3.1. Chloroplast Entrainment
3.3.2. Mitochondrial Entrainment
3.3.3. Synergic Entrainment: The Intracellular Dynamic Duo
4. Light, Clock, and Metabolism Integration: The Three-Body Problem
5. From Circadian Control to Agricultural Innovation
Author Contributions
Funding
Conflicts of Interest
References
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Cell Biology: Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef]
- Green, R.M.; Tingay, S.; Wang, Z.Y.; Tobin, E.M. Circadian Rhythms Confer a Higher Level of Fitness to Arabidopsis Plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef]
- Yerushalmi, S.; Green, R.M. Evidence for the Adaptive Significance of Circadian Rhythms. Ecol. Lett. 2009, 12, 970–981. [Google Scholar] [CrossRef]
- Haydon, M.J.; Hearn, T.J.; Bell, L.J.; Hannah, M.A.; Webb, A.A.R. Metabolic Regulation of Circadian Clocks. Semin. Cell Dev. Biol. 2013, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Steed, G.; Ramirez, D.C.; Hannah, M.A.; Webb, A.A.R. Chronoculture, Harnessing the Circadian Clock to Improve Crop Yield and Sustainability. Science 2021, 372, eabc9141. [Google Scholar] [CrossRef] [PubMed]
- Nohales, M.A.; Kay, S.A. Molecular Mechanisms at the Core of the Plant Circadian Oscillator. Nat. Struct. Mol. Biol. 2016, 23, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Laosuntisuk, K.; Elorriaga, E.; Doherty, C.J. The Game of Timing: Circadian Rhythms Intersect with Changing Environments. Annu. Rev. Plant Biol. 2023, 74, 511–538. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Harmer, S.L. Wheels within Wheels: The Plant Circadian System. Trends Plant Sci. 2014, 19, 240–249. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Kay, S.A. The Plant Circadian Clock: From a Simple Timekeeper to a Complex Developmental Manager. Cold Spring Harb. Perspect. Biol. 2016, 8, a027748. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular Mechanisms and Physiological Importance of Circadian Rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Wu, J.F.; Wang, Y.; Wu, S.H. Two New Clock Proteins, LWD1 and LWD2, Regulate Arabidopsis Photoperiodic Flowering. Plant Physiol. 2008, 148, 948–959. [Google Scholar] [CrossRef]
- Rawat, R.; Takahashi, N.; Hsu, P.Y.; Jones, M.A.; Schwartz, J.; Salemi, M.R.; Phinney, B.S.; Harmer, S.L. REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock. PLoS Genet. 2011, 7, e1001350. [Google Scholar] [CrossRef] [PubMed]
- Más, P.; Kim, W.Y.; Somers, D.E.; Kay, S.A. Targeted Degradation of TOC1 by ZTL Modulates Circadian Function in Arabidopsis thaliana. Nature 2003, 426, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, P.; Liu, X.; Yuan, L.; Wang, L.; Zhang, C.; Li, Y.; Xing, H.; Zhi, L.; Yue, Z.; et al. LNK1 and LNK2 Are Transcriptional Coactivators in the Arabidopsis Circadian Oscillator. Plant Cell 2014, 26, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Fujiwara, S.; Suh, S.S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Nam, H.G.; Somers, D.E. ZEITLUPE Is a Circadian Photoreceptor Stabilized by GIGANTEA in Blue Light. Nature 2007, 449, 356–360. [Google Scholar] [CrossRef]
- Pruneda-Paz, J.L.; Breton, G.; Para, A.; Kay, S.A. A Functional Genomics Approach Reveals CHE as a Component of the Arabidopsis Circadian Clock. Science 2009, 323, 1481–1485. [Google Scholar] [CrossRef]
- Kusakina, J.; Dodd, A.N. Phosphorylation in the Plant Circadian System. Trends Plant Sci. 2012, 17, 575–583. [Google Scholar] [CrossRef]
- Seo, P.J.; Mas, P. Multiple Layers of Posttranslational Regulation Refine Circadian Clock Activity in Arabidopsis. Plant Cell 2014, 26, 79–87. [Google Scholar] [CrossRef]
- Cervela-Cardona, L.; Alary, B.; Mas, P. The Arabidopsis Circadian Clock and Metabolic Energy: A Question of Time. Front. Plant Sci. 2021, 12, 2899. [Google Scholar] [CrossRef]
- Chen, W.W.; Takahashi, N.; Hirata, Y.; Ronald, J.; Porco, S.; Davis, S.J.; Nusinow, D.A.; Kay, S.A.; Mas, P. A Mobile ELF4 Delivers Circadian Temperature Information from Shoots to Roots. Nat. Plants 2020, 6, 416–426. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light Perception: A Matter of Time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef]
- Oakenfull, R.J.; Davis, S.J. Shining a Light on the Arabidopsis Circadian Clock. Plant Cell Environ. 2017, 40, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Sánchez-Lamas, M.; Yanovsky, M.J.; Casal, J.J.; Cerdán, P.D. Arabidopsis thaliana Life without Phytochromes. Proc. Natl. Acad. Sci. USA 2010, 107, 4776–4781. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Hu, W.; Litthauer, S.; Lagarias, J.C.; Harmer, S.L. A Constitutively Active Allele of Phytochrome B Maintains Circadian Robustness in the Absence of Light. Plant Physiol. 2015, 169, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Yanovsky, M.J.; Mazzella, M.A.; Casal, J.J. A Quadruple Photoreceptor Mutant Still Keeps Track of Time. Curr. Biol. 2000, 10, 1013–1015. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Koehl, K.; Lunn, J.E.; Stitt, M. Response of Arabidopsis Primary Metabolism and Circadian Clock to Low Night Temperature in a Natural Light Environment. J. Exp. Bot. 2018, 69, 4881–4895. [Google Scholar] [CrossRef]
- Lai, A.G.; Doherty, C.J.; Mueller-Roeber, B.; Kay, S.A.; Schippers, J.H.M.; Dijkwel, P.P. Circadian Clock-Associated 1 Regulates ROS Homeostasis and Oxidative Stress Responses. Proc. Natl. Acad. Sci. USA 2012, 109, 17129–17134. [Google Scholar] [CrossRef]
- Leivar, P.; Quail, P.H. PIFs: Pivotal Components in a Cellular Signaling Hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef]
- Shor, E.; Paik, I.; Kangisser, S.; Green, R.; Huq, E. PHYTOCHROME INTERACTING FACTORS Mediate Metabolic Control of the Circadian System in Arabidopsis. New Phytol. 2017, 215, 217–228. [Google Scholar] [CrossRef]
- Seluzicki, A.; Burko, Y.; Chory, J. Dancing in the Dark: Darkness as a Signal in Plants. Plant Cell Environ. 2017, 40, 2487–2501. [Google Scholar] [CrossRef]
- Barak, S.; Tobin, E.M.; Green, R.M.; Andronis, C.; Sugano, S. All in Good Time: The Arabidopsis Circadian Clock. Trends Plant Sci. 2000, 5, 517–522. [Google Scholar] [CrossRef]
- Hassidim, M.; Dakhiya, Y.; Turjeman, A.; Hussien, D.; Shor, E.; Anidjar, A.; Goldberg, K.; Green, R.M. CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and the Circadian Control of Stomatal Aperture. Plant Physiol. 2017, 175, 1864–1877. [Google Scholar] [CrossRef]
- Tóth, R.; Kevei, É.; Hall, A.; Millar, A.J.; Nagy, F.; Kozma-Bognár, L. Circadian Clock-Regulated Expression of Phytochrome and Cryptochrome Genes in Arabidopsis. Plant Physiol. 2001, 127, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Findlay, K.M.W.; Jenkins, G.I. Regulation of UVR8 Photoreceptor Dimer/Monomer Photo-Equilibrium in Arabidopsis Plants Grown under Photoperiodic Conditions. Plant Cell Environ. 2016, 39, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Kircher, S.; Terecskei, K.; Wolf, I.; Sipos, M.; Adam, E. Phytochrome A-Specific Signaling in Arabidopsis thaliana. Plant Signal. Behav. 2011, 6, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Kozma-Bognár, L.; Tóth, R.; Nagy, F.; Millar, A.J. Conditional Circadian Regulation of Phytochrome A Gene Expression. Plant Physiol. 2001, 127, 1808–1818. [Google Scholar] [CrossRef]
- Lin, R.; Ding, L.; Casola, C.; Ripoll, D.R.; Feschotte, C.; Wang, H. Transposase-Derived Transcription Factors Regulate Light Signaling in Arabidopsis. Science 2007, 318, 1302–1305. [Google Scholar] [CrossRef]
- Kircher, S.; Gil, P.; Kozma-Bognár, L.; Fejes, E.; Speth, V.; Husselstein-Muller, T.; Bauer, D.; Ádám, É.; Schäfer, E.; Nagy, F. Nucleocytoplasmic Partitioning of the Plant Photoreceptors Phytochrome A, B, C, D, and E Is Regulated Differentially by Light and Exhibits a Diurnal Rhythm. Plant Cell 2002, 14, 1541–1555. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, M.; Li, G.; Yuan, L.; Xie, Y.; Wei, H.; Ma, X.; Li, Q.; Devlin, P.F.; Xu, X.; et al. Transcription Factors FHY3 and FAR1 Regulate Light-Induced CIRCADIAN CLOCK ASSOCIATED1 Gene Expression in Arabidopsis. Plant Cell 2020, 32, 1464–1478. [Google Scholar] [CrossRef]
- Hiltbrunner, A.; Tscheuschler, A.; Viczián, A.; Kunkel, T.; Kircher, S.; Schäfer, E. FHY1 and FHL Act Together to Mediate Nuclear Accumulation of the Phytochrome A Photoreceptor. Plant Cell Physiol. 2006, 47, 1023–1034. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Yao, H.; Zheng, Y.; Cao, S.; Wang, H. Arabidopsis Circadian Clock Repress Phytochrome a Signaling. Front. Plant Sci. 2022, 13, 809563. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Khan, S.; Rhodes, B.M.; Devlin, P.F. FHY3 and FAR1 Act Downstream of Light Stable Phytochromes. Front. Plant Sci. 2016, 7, 180238. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive Oxygen Species, Oxidative Signaling and the Regulation of Photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Zavafer, A.; Cheah, M.H.; Hillier, W.; Chow, W.S.; Takahashi, S. Photodamage to the Oxygen Evolving Complex of Photosystem II by Visible Light. Sci. Rep. 2015, 5, 16363. [Google Scholar] [CrossRef]
- Yarkhunova, Y.; Guadagno, C.R.; Rubin, M.J.; Davis, S.J.; Ewers, B.E.; Weinig, C. Circadian Rhythms Are Associated with Variation in Photosystem II Function and Photoprotective Mechanisms. Plant Cell Environ. 2018, 41, 2518–2529. [Google Scholar] [CrossRef]
- Rochaix, J.D. Regulation and Dynamics of the Light-Harvesting System. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef]
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global Transcriptome Analysis Reveals Circadian Regulation of Key Pathways in Plant Growth and Development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef]
- Khan, S.; Rowe, S.C.; Harmon, F.G. Coordination of the Maize Transcriptome by a Conserved Circadian Clock. BMC Plant Biol. 2010, 10, 126. [Google Scholar] [CrossRef]
- Facella, P.; Lopez, L.; Carbone, F.; Galbraith, D.W.; Giuliano, G.; Perrotta, G. Diurnal and Circadian Rhythms in the Tomato Transcriptome and Their Modulation by Cryptochrome Photoreceptors. PLoS ONE 2008, 3, e2798. [Google Scholar] [CrossRef]
- Lu, D.; Wang, T.; Persson, S.; Mueller-Roeber, B.; Schippers, J.H.M. Transcriptional Control of ROS Homeostasis by KUODA1 Regulates Cell Expansion during Leaf Development. Nat. Commun. 2014, 5, 3767. [Google Scholar] [CrossRef]
- Dalchau, N.; Baek, S.J.; Briggs, H.M.; Robertson, F.C.; Dodd, A.N.; Gardner, M.J.; Stancombe, M.A.; Haydon, M.J.; Stan, G.B.; Gonçalves, J.M.; et al. The Circadian Oscillator Gene GIGANTEA Mediates a Long-Term Response of the Arabidopsis thaliana Circadian Clock to Sucrose. Proc. Natl. Acad. Sci. USA 2011, 108, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Murakami, M.; Nakamura, Y.; Ito, S.; Nakamichi, N.; Yamashino, T.; Mizuno, T. Mutants of Circadian-Associated PRR Genes Display a Novel and Visible Phenotype as to Light Responses during de-Etiolation of Arabidopsis thaliana Seedlings. Biosci. Biotechnol. Biochem. 2007, 71, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Hirata, Y.; Aihara, K.; Mas, P. A Hierarchical Multi-Oscillator Network Orchestrates the Arabidopsis Circadian System. Cell 2015, 163, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, K.; Mori, F.; Yamauchi, S.; Kubota, A.; Takahashi, N.; Egashira, H.; Kunimoto, Y.; Araki, T.; Takemiya, A.; Ito, H.; et al. Root PRR7 Improves the Accuracy of the Shoot Circadian Clock through Nutrient Transport. Plant Cell Physiol. 2023, 64, 352–362. [Google Scholar] [CrossRef]
- Haydon, M.J.; Mielczarek, O.; Robertson, F.C.; Hubbard, K.E.; Webb, A.A.R. Photosynthetic Entrainment of the Arabidopsis thaliana Circadian Clock. Nature 2013, 502, 689–692. [Google Scholar] [CrossRef]
- Frank, A.; Matiolli, C.C.; Viana, A.J.C.; Hearn, T.J.; Kusakina, J.; Belbin, F.E.; Wells Newman, D.; Yochikawa, A.; Cano-Ramirez, D.L.; Chembath, A.; et al. Circadian Entrainment in Arabidopsis by the Sugar-Responsive Transcription Factor BZIP63. Curr. Biol. 2018, 28, 2597–2606.e6. [Google Scholar] [CrossRef]
- Mair, A.; Pedrotti, L.; Wurzinger, B.; Anrather, D.; Simeunovic, A.; Weiste, C.; Valerio, C.; Dietrich, K.; Kirchler, T.; Nägele, T.; et al. SnRK1-Triggered Switch of BZIP63 Dimerization Mediates the Low-Energy Response in Plants. Elife 2015, 4, e05828. [Google Scholar] [CrossRef][Green Version]
- Shin, J.; Sánchez-Villarreal, A.; Davis, A.M.; Du, S.X.; Berendzen, K.W.; Koncz, C.; Ding, Z.; Li, C.; Davis, S.J. The Metabolic Sensor AKIN10 Modulates the Arabidopsis Circadian Clock in a Light-Dependent Manner. Plant Cell Environ. 2017, 40, 997–1008. [Google Scholar] [CrossRef]
- Haydon, M.J.; Mielczarek, O.; Frank, A.; Román, Á.; Webb, A.A.R. Sucrose and Ethylene Signaling Interact to Modulate the Circadian Clock. Plant Physiol. 2017, 175, 947–958. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Kay, S.A. Molecular Basis of Seasonal Time Measurement in Arabidopsis. Nature 2002, 419, 308–312. [Google Scholar] [CrossRef]
- Suárez-López, P.; Wheatley, K.; Robson, F.; Onouchi, H.; Valverde, F.; Coupland, G. CONSTANS Mediates between the Circadian Clock and the Control of Flowering in Arabidopsis. Nature 2001, 410, 1116–1120. [Google Scholar] [CrossRef]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS Gene of Arabidopsis Promotes Flowering and Encodes a Protein Showing Similarities to Zinc Finger Transcription Factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Gendron, J.M.; Staiger, D. New Horizons in Plant Photoperiodism. Annu. Rev. Plant Biol. 2023, 74, 481–509. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor Regulation of CONSTANS Protein in Photoperiodic Flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feke, A.; Leung, C.C.; Tarté, D.A.; Yuan, W.; Vanderwall, M.; Sager, G.; Wu, X.; Schear, A.; Clark, D.A.; et al. A Metabolic Daylength Measurement System Mediates Winter Photoperiodism in Plants. Dev. Cell 2021, 56, 2501–2515.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Leung, C.C.; Tarté, D.A.; Gendron, J.M. Plants Distinguish Different Photoperiods to Independently Control Seasonal Flowering and Growth. Science 2024, 383, 605. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.R.; Li, X.; Martí, M.C.; Haydon, M.J. A Bittersweet Symphony: Metabolic Signals in the Circadian System. Curr. Opin. Plant Biol. 2023, 73, 102333. [Google Scholar] [CrossRef]
- Lim, S.L.; Voon, C.P.; Guan, X.; Yang, Y.; Gardeström, P.; Lim, B.L. In Planta Study of Photosynthesis and Photorespiration Using NADPH and NADH/NAD+ Fluorescent Protein Sensors. Nat. Commun. 2020, 11, 3238. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Dahal, K.; Alber, N.A.; Chadee, A. Photosynthesis, Respiration and Growth: A Carbon and Energy Balancing Act for Alternative Oxidase. Mitochondrion 2020, 52, 197–211. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate Valves: Old Shuttles with New Perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Ng, S.; De Clercq, I.; Van Aken, O.; Law, S.R.; Ivanova, A.; Willems, P.; Giraud, E.; Van Breusegem, F.; Whelan, J. Anterograde and Retrograde Regulation of Nuclear Genes Encoding Mitochondrial Proteins during Growth, Development, and Stress. Mol. Plant 2014, 7, 1075–1093. [Google Scholar] [CrossRef]
- Hernández-Verdeja, T.; Strand, Å. Retrograde Signals Navigate the Path to Chloroplast Development. Plant Physiol. 2018, 176, 967–976. [Google Scholar] [CrossRef]
- Hwang, Y.; Han, S.; Yoo, C.Y.; Hong, L.; You, C.; Le, B.H.; Shi, H.; Zhong, S.; Hoecker, U.; Chen, X.; et al. Anterograde Signaling Controls Plastid Transcription via Sigma Factors Separately from Nuclear Photosynthesis Genes. Nat. Commun. 2022, 13, 7440. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Berkowitz, O.; Hu, S.; Zhao, Y.; Qian, K.; Shou, H.; Whelan, J.; Wang, Y. Co-Regulation of Mitochondrial and Chloroplast Function: Molecular Components and Mechanisms. Plant Commun. 2023, 4, 100496. [Google Scholar] [CrossRef] [PubMed]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated Transcription of Key Pathways in Arabidopsis by the Circadian Clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Kenigsbuch, D.; Sun, L.; Harel, E.; Ong, M.S.; Tobin, E.M. A Myb-Related Transcription Factor Is Involved in the Phytochrome Regulation of an Arabidopsis Lhcb Gene. Plant Cell 1997, 9, 491–507. [Google Scholar] [CrossRef]
- Andronis, C.; Barak, S.; Knowles, S.M.; Sugano, S.; Tobin, E.M. The Clock Protein CCA1 and the BZIP Transcription Factor HY5 Physically Interact to Regulate Gene Expression in Arabidopsis. Mol. Plant 2008, 1, 58–67. [Google Scholar] [CrossRef]
- Lu, S.X.; Knowles, S.M.; Andronis, C.; Ong, M.S.; Tobin, E.M. Circadian Clock Associated1 and Late Elongated Hypocotyl Function Synergistically in the Circadian Clock of Arabidopsis. Plant Physiol. 2009, 150, 834–843. [Google Scholar] [CrossRef]
- Noordally, Z.B.; Ishii, K.; Atkins, K.A.; Wetherill, S.J.; Kusakina, J.; Walton, E.J.; Kato, M.; Azuma, M.; Tanaka, K.; Hanaoka, M.; et al. Circadian Control of Chloroplast Transcription by a Nuclear-Encoded Timing Signal. Science 2013, 339, 1316–1319. [Google Scholar] [CrossRef]
- Gould, P.D.; Diaz, P.; Hogben, C.; Kusakina, J.; Salem, R.; Hartwell, J.; Hall, A. Delayed Fluorescence as a Universal Tool for the Measurement of Circadian Rhythms in Higher Plants. Plant J. 2009, 58, 893–901. [Google Scholar] [CrossRef]
- Dakhiya, Y.; Hussien, D.; Fridman, E.; Kiflawi, M.; Green, R. Correlations between Circadian Rhythms and Growth in Challenging Environments. Plant Physiol. 2017, 173, 1724–1734. [Google Scholar] [CrossRef]
- Dodd, A.N.; Parkinson, K.; Webb, A.A.R. Independent Circadian Regulation of Assimilation and Stomatal Conductance in the Ztl-1 Mutant of Arabidopsis. New Phytol. 2004, 162, 63–70. [Google Scholar] [CrossRef]
- de Dios, V.R.; Goulden, M.L.; Ogle, K.; Richardson, A.D.; Hollinger, D.Y.; Davidson, E.A.; Alday, J.G.; Barron-Gafford, G.A.; Carrara, A.; Kowalski, A.S.; et al. Endogenous Circadian Regulation of Carbon Dioxide Exchange in Terrestrial Ecosystems. Glob. Change Biol. 2012, 18, 1956–1970. [Google Scholar] [CrossRef]
- Hubbard, K.E.; Webb, A.A.R. Circadian Rhythms: FLOWERING LOCUS T Extends Opening Hours. Curr. Biol. 2011, 21, R636–R638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Britz, S.J.; Briggs, W.R. Circadian Rhythms of Chloroplast Orientation and Photosynthetic Capacity in Ulva. Plant Physiol. 1976, 58, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hassidim, M.; Yakir, E.; Fradkin, D.; Hilman, D.; Kron, I.; Keren, N.; Harir, Y.; Yerushalmi, S.; Green, R.M. Mutations in CHLOROPLAST RNA BINDING Provide Evidence for the Involvement of the Chloroplast in the Regulation of the Circadian Clock in Arabidopsis. Plant J. 2007, 51, 551–562. [Google Scholar] [CrossRef]
- Atkins, K.A.; Dodd, A.N. Circadian Regulation of Chloroplasts. Curr. Opin. Plant Biol. 2014, 21, 43–50. [Google Scholar] [CrossRef]
- Wu, G.Z.; Bock, R. GUN Control in Retrograde Signaling: How GENOMES UNCOUPLED Proteins Adjust Nuclear Gene Expression to Plastid Biogenesis. Plant Cell 2021, 33, 457–474. [Google Scholar] [CrossRef]
- Calderon, R.H.; Strand, Å. How Retrograde Signaling Is Intertwined with the Evolution of Photosynthetic Eukaryotes. Curr. Opin. Plant Biol. 2021, 63, 102093. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Tetrapyrrole Biosynthesis in Higher Plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef]
- Matsumoto, F.; Obayashi, T.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.I.; Masuda, T. Gene Expression Profiling of the Tetrapyrrole Metabolic Pathway in Arabidopsis with a Mini-Array System. Plant Physiol. 2004, 135, 2379–2391. [Google Scholar] [CrossRef]
- Legnaioli, T.; Cuevas, J.; Mas, P. TOC1 Functions as a Molecular Switch Connecting the Circadian Clock with Plant Responses to Drought. EMBO J. 2009, 28, 3745–3757. [Google Scholar] [CrossRef]
- Fu, X.X.; Zhang, J.; Zhang, G.Q.; Liu, Z.J.; Chen, Z.D. Insights into the Origin and Evolution of Plant Sigma Factors. J. Syst. Evol. 2021, 59, 326–340. [Google Scholar] [CrossRef]
- Nagashima, A.; Hanaoka, M.; Shikanai, T.; Fujiwara, M.; Kanamaru, K.; Takahashi, H.; Tanaka, K. The Multiple-Stress Responsive Plastid Sigma Factor, SIG5, Directs Activation of the PsbD Blue Light-Responsive Promoter (BLRP) in Arabidopsis thaliana. Plant Cell Physiol. 2004, 45, 357–368. [Google Scholar] [CrossRef]
- Shimizu, M.; Kato, H.; Ogawa, T.; Kurachi, A.; Nakagawa, Y.; Kobayashi, H. Sigma Factor Phosphorylation in the Photosynthetic Control of Photosystem Stoichiometry. Proc. Natl. Acad. Sci. USA 2010, 107, 10760–10764. [Google Scholar] [CrossRef] [PubMed]
- Favory, J.J.; Kobayshi, M.; Tanaka, K.; Peltier, G.; Kreis, M.; Valay, J.G.; Lerbs-Mache, S. Specific Function of a Plastid Sigma Factor for NdhF Gene Transcription. Nucleic Acids Res. 2005, 33, 5991–5999. [Google Scholar] [CrossRef] [PubMed]
- Belbin, F.E.; Noordally, Z.B.; Wetherill, S.J.; Atkins, K.A.; Franklin, K.A.; Dodd, A.N. Integration of Light and Circadian Signals That Regulate Chloroplast Transcription by a Nuclear-Encoded Sigma Factor. New Phytol. 2017, 213, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Cano-Ramirez, D.L.; Panter, P.E.; Takemura, T.; de Fraine, T.S.; de Barros Dantas, L.L.; Dekeya, R.; Barros-Galvão, T.; Paajanen, P.; Bellandi, A.; Batstone, T.; et al. Low-Temperature and Circadian Signals Are Integrated by the Sigma Factor SIG5. Nat. Plants 2023, 9, 661–672. [Google Scholar] [CrossRef]
- Strand, Å.; Asami, T.; Alonso, J.; Ecker, J.R.; Chory, J. Chloroplast to Nucleus Communication Triggered by Accumulation of Mg-Protoporphyrinix. Nature 2003, 421, 79–83. [Google Scholar] [CrossRef]
- Yang, E.J.; Yoo, C.Y.; Liu, J.; Wang, H.; Cao, J.; Li, F.W.; Pryer, K.M.; Sun, T.-p.; Weigel, D.; Zhou, P.; et al. NCP Activates Chloroplast Transcription by Controlling Phytochrome-Dependent Dual Nuclear and Plastidial Switches. Nat. Commun. 2019, 10, 2630. [Google Scholar] [CrossRef]
- Waters, M.T.; Langdale, J.A. The Making of a Chloroplast. EMBO J. 2009, 28, 2861–2873. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK Transcription Factors Coordinate Expression of the Photosynthetic Apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Leivar, P.; Ludevid, D.; Tepperman, J.M.; Quail, P.H.; Monte, E. Phytochrome and Retrograde Signalling Pathways Converge to Antagonistically Regulate a Light-Induced Transcriptional Network. Nat. Commun. 2016, 7, 11431. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, Y.; Kuai, B.; Li, L. Circadian Clock-Associated 1 Inhibits Leaf Senescence in Arabidopsis. Front. Plant Sci. 2018, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, R.; Abe, S.; Marugami, M.; Yamagami, A.; Akema, R.; Ohashi, T.; Nishida, K.; Nosaki, S.; Miyakawa, T.; Tanokura, M.; et al. BPG4 Regulates Chloroplast Development and Homeostasis by Suppressing GLK Transcription Factors and Involving Light and Brassinosteroid Signaling. Nat. Commun. 2024, 15, 370. [Google Scholar] [CrossRef]
- Seaton, D.D.; Graf, A.; Baerenfaller, K.; Stitt, M.; Millar, A.J.; Gruissem, W. Photoperiodic Control of the Arabidopsis Proteome Reveals a Translational Coincidence Mechanism. Mol. Syst. Biol. 2018, 14, 7962. [Google Scholar] [CrossRef]
- Kay, H.; Grünewald, E.; Feord, H.K.; Gil, S.; Peak-Chew, S.Y.; Stangherlin, A.; O’Neill, J.S.; van Ooijen, G. Deep-Coverage Spatiotemporal Proteome of the Picoeukaryote Ostreococcus tauri Reveals Differential Effects of Environmental and Endogenous 24-Hour Rhythms. Commun. Biol. 2021, 4, 1147. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between Metabolism and Circadian Clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Bass, J. Circadian Topology of Metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef]
- Scrima, R.; Cela, O.; Merla, G.; Augello, B.; Rubino, R.; Quarato, G.; Fugetto, S.; Menga, M.; Fuhr, L.; Relógio, A.; et al. Clock-Genes and Mitochondrial Respiratory Activity: Evidence of a Reciprocal Interplay. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1344–1351. [Google Scholar] [CrossRef]
- Womac, A.D.; Burkeen, J.F.; Neuendorff, N.; Earnest, D.J.; Zoran, M.J. Circadian Rhythms of Extracellular ATP Accumulation in Suprachiasmatic Nucleus Cells and Cultured Astrocytes. Eur. J. Neurosci. 2009, 30, 869–876. [Google Scholar] [CrossRef]
- De Goede, P.; Wefers, J.; Brombacher, E.C.; Schrauwen, P.; Kalsbeek, A. Circadian Rhythms in Mitochondrial Respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M.; et al. Circadian Control of Oscillations in Mitochondrial Rate-Limiting Enzymes and Nutrient Utilization by PERIOD Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.S.; Valera-Alberni, M.; Cantó, C.; Pillon, N.J. Circadian Rhythms and Mitochondria: Connecting the Dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef]
- Pracharoenwattana, I.; Zhou, W.; Keech, O.; Francisco, P.B.; Udomchalothorn, T.; Tschoep, H.; Stitt, M.; Gibon, Y.; Smith, S.M. Arabidopsis Has a Cytosolic Fumarase Required for the Massive Allocation of Photosynthate into Fumaric Acid and for Rapid Plant Growth on High Nitrogen. Plant J. 2010, 62, 785–795. [Google Scholar] [CrossRef]
- Robison, M.M.; Ling, X.; Smid, M.P.L.; Zarei, A.; Wolyn, D.J. Antisense Expression of Mitochondrial ATP Synthase Subunits OSCP (ATP5) and γ (ATP3) Alters Leaf Morphology, Metabolism and Gene Expression in Arabidopsis. Plant Cell Physiol. 2009, 50, 1840–1850. [Google Scholar] [CrossRef]
- León, G.; Holuigue, L.; Jordana, X. Mitochondrial Complex II Is Essential for Gametophyte Development in Arabidopsis. Plant Physiol. 2007, 143, 1534–1546. [Google Scholar] [CrossRef]
- Huang, S.; Taylor, N.L.; Ströher, E.; Fenske, R.; Millar, A.H. Succinate Dehydrogenase Assembly Factor 2 Is Needed for Assembly and Activity of Mitochondrial Complex II and for Normal Root Elongation in Arabidopsis. Plant J. 2013, 73, 429–441. [Google Scholar] [CrossRef]
- Kühn, K.; Richter, U.; Meyer, E.H.; Delannoy, E.; De Longevialle, A.F.; Otoole, N.; Börner, T.; Millar, A.H.; Small, I.D.; Whelan, J. Phage-Type RNA Polymerase RPOTmp Performs Gene-Specific Transcription in Mitochondria of Arabidopsis thaliana. Plant Cell 2009, 21, 2762–2779. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Lindén, P.; Lee, C.P.; Carroll, A.J.; Ströher, E.; Smith, S.M.; Gardeström, P.; Millar, A.H. Mitochondrial Malate Dehydrogenase Lowers Leaf Respiration and Alters Photorespiration and Plant Growth in Arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Carrari, F.; Nunes-Nesi, A.; Gibon, Y.; Lytovchenko, A.; Loureiro, M.E.; Fernie, A.R. Reduced Expression of Aconitase Results in an Enhanced Rate of Photosynthesis and Marked Shifts in Carbon Partitioning in Illuminated Leaves of Wild Species Tomato. Plant Physiol. 2003, 133, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Brotman, Y.; Riewe, D.; Lisec, J.; Meyer, R.C.; Willmitzer, L.; Altmann, T. Identification of Enzymatic and Regulatory Genes of Plant Metabolism through QTL Analysis in Arabidopsis. J. Plant Physiol. 2011, 168, 1387–1394. [Google Scholar] [CrossRef]
- Fromm, S.; Braun, H.P.; Peterhansel, C. Mitochondrial Gamma Carbonic Anhydrases Are Required for Complex I Assembly and Plant Reproductive Development. New Phytol. 2016, 211, 194–207. [Google Scholar] [CrossRef]
- Cervela-Cardona, L.; Yoshida, T.; Zhang, Y.; Okada, M.; Fernie, A.; Mas, P. Circadian Control of Metabolism by the Clock Component TOC1. Front. Plant Sci. 2021, 12, 1126. [Google Scholar] [CrossRef]
- Missra, A.; Ernest, B.; Lohoff, T.; Jia, Q.; Satterlee, J.; Ke, K.; von Arnim, A.G. The Circadian Clock Modulates Global Daily Cycles of Mrna Ribosome Loading. Plant Cell 2015, 27, 2582–2599. [Google Scholar] [CrossRef]
- Lee, C.P.; Eubel, H.; Millar, A.H. Diurnal Changes in Mitochondrial Function Reveal Daily Optimization of Light and Dark Respiratory Metabolism in Arabidopsis. Mol. Cell. Proteom. 2010, 9, 2125–2139. [Google Scholar] [CrossRef]
- Uhrig, R.G.; Echevarría-Zomeño, S.; Schlapfer, P.; Grossmann, J.; Roschitzki, B.; Koerber, N.; Fiorani, F.; Gruissem, W. Diurnal Dynamics of the Arabidopsis Rosette Proteome and Phosphoproteome. Plant Cell Environ. 2021, 44, 821–841. [Google Scholar] [CrossRef]
- Rugen, N.; Schaarschmidt, F.; Eirich, J.; Finkemeier, I.; Braun, H.P.; Eubel, H. Protein Interaction Patterns in Arabidopsis thaliana Leaf Mitochondria Change in Dependence to Light. Biochim. Biophys. Acta-Bioenerg. 2021, 1862, 148443. [Google Scholar] [CrossRef]
- Giraud, E.; Ng, S.; Carrie, C.; Duncan, O.; Low, J.; Lee, C.P.; van Aken, O.; Harvey Millar, A.; Murcha, M.; Whelan, J. TCP Transcription Factors Link the Regulation of Genes Encoding Mitochondrial Proteins with the Circadian Clock in Arabidopsis thaliana. Plant Cell 2010, 22, 3921–3934. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Coman, D.; Uhrig, R.G.; Walsh, S.; Flis, A.; Stitt, M.; Gruissem, W. Parallel Analysis of Arabidopsis Circadian Clock Mutants Reveals Different Scales of Transcriptome and Proteome Regulation. Open Biol. 2017, 7, 160333. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Usadel, B.; Blaesing, O.E.; Kamlage, B.; Hoehne, M.; Trethewey, R.; Stitt, M. Integration of Metabolite with Transcript and Enzyme Activity Profiling during Diurnal Cycles in Arabidopsis Rosettes. Genome Biol. 2006, 7, R76. [Google Scholar] [CrossRef] [PubMed]
- Flis, A.; Mengin, V.; Ivakov, A.A.; Mugford, S.T.; Hubberten, H.M.; Encke, B.; Krohn, N.; Höhne, M.; Feil, R.; Hoefgen, R.; et al. Multiple Circadian Clock Outputs Regulate Diel Turnover of Carbon and Nitrogen Reserves. Plant Cell Environ. 2019, 42, 549–573. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Mengin, V.; Lauxmann, M.A.; Köhl, K.; Nikoloski, Z.; Stitt, M.; et al. Getting Back to Nature: A Reality Check for Experiments in Controlled Environments. J. Exp. Bot. 2017, 68, 4463–4477. [Google Scholar] [CrossRef]
- Sulpice, R.; Flis, A.; Ivakov, A.A.; Apelt, F.; Krohn, N.; Encke, B.; Abel, C.; Feil, R.; Lunn, J.E.; Stitt, M. Arabidopsis Coordinates the Diurnal Regulation of Carbon Allocation and Growth across a Wide Range of Photoperiods. Mol. Plant 2014, 7, 137–155. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Nakamichi, N.; Kobayashi, M.; Hayashi, N.; Sakakibara, H.; Mizuno, T.; Saito, K. Impact of Clock-Associated Arabidopsis Pseudo-response Regulators in Metabolic Coordination. Proc. Natl. Acad. Sci. USA 2009, 106, 7251–7256. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kusano, M.; Fukushima, A.; Kita, M.; Ito, S.; Yamashino, T.; Saito, K.; Sakakibara, H.; Mizuno, T. Transcript Profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR Arrhythmic Triple Mutant Reveals a Role for the Circadian Clock in Cold Ctress Response. Plant Cell Physiol. 2009, 50, 447–462. [Google Scholar] [CrossRef]
- Engel, N.; Van Den Daele, K.; Kolukisaoglu, Ü.; Morgenthal, K.; Weckwerth, W.; Pärnik, T.; Keerberg, O.; Bauwe, H. Deletion of Glycine Decarboxylase in Arabidopsis Is Lethal under Nonphotorespiratory Conditions. Plant Physiol. 2007, 144, 1328–1335. [Google Scholar] [CrossRef]
- Miyashita, Y.; Good, A.G. NAD(H)-Dependent Glutamate Dehydrogenase Is Essential for the Survival of Arabidopsis thaliana during Dark-Induced Carbon Starvation. J. Exp. Bot. 2008, 59, 667–680. [Google Scholar] [CrossRef]
- Ishizaki, K.; Larson, T.R.; Schauer, N.; Fernie, A.R.; Graham, I.A.; Leaver, C.J. The Critical Role of Arabidopsis Electron-Transfer Flavoprotein:Ubiquinone Oxidoreductase during Dark-Induced Starvation. Plant Cell 2005, 17, 2587–2600. [Google Scholar] [CrossRef]
- Pedrotti, L.; Weiste, C.; Nägele, T.; Wolf, E.; Lorenzin, F.; Dietrich, K.; Mair, A.; Weckwerth, W.; Teige, M.; Baena-González, E.; et al. Snf1-RELATED KINASE1-Controlled C/S1-BZIP Signaling Activates Alternative Mitochondrial Metabolic Pathways to Ensure Plant Survival in Extended Darkness. Plant Cell 2018, 30, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein Degradation—An Alternative Respiratory Substrate for Stressed Plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, J.H.F.; Quinhones, C.G.S.; Schertl, P.; Brito, D.S.; Eubel, H.; Hildebrandt, T.; Nunes-Nesi, A.; Braun, H.P.; Araújo, W.L. Differential Impact of Amino Acids on OXPHOS System Activity Following Carbohydrate Starvation in Arabidopsis Cell Suspensions. Physiol. Plant. 2017, 161, 451–467. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca-Pereira, P.; Pham, P.A.; Cavalcanti, J.H.F.; Omena-Garcia, R.P.; Barros, J.A.S.; Rosado-Souza, L.; Vallarino, J.G.; Mutwil, M.; Avin-Wittenberg, T.; Nunes-Nesi, A.; et al. The Arabidopsis Electron-Transfer Flavoprotein:Ubiquinone Oxidoreductase Is Required during Normal Seed Development and Germination. Plant J. 2022, 109, 196–214. [Google Scholar] [CrossRef]
- Peng, C.; Uygun, S.; Shiu, S.H.; Last, R.L. The Impact of the Branched-Chain Ketoacid Dehydrogenase Complex on Amino Acid Homeostasis in Arabidopsis. Plant Physiol. 2015, 169, 1807–1820. [Google Scholar] [CrossRef]
- Espinoza, C.; Degenkolbe, T.; Caldana, C.; Zuther, E.; Leisse, A.; Willmitzer, L.; Hincha, D.K.; Hannah, M.A. Interaction with Diurnal and Circadian Regulation Results in Dynamic Metabolic and Transcriptional Changes during Cold Acclimation in Arabidopsis. PLoS ONE 2010, 5, e14101. [Google Scholar] [CrossRef]
- Viana, A.J.C.; Matiolli, C.C.; Newman, D.W.; Vieira, J.G.P.; Duarte, G.T.; Martins, M.C.M.; Gilbault, E.; Hotta, C.T.; Caldana, C.; Vincentz, M. The Sugar-Responsive Circadian Clock Regulator BZIP63 Modulates Plant Growth. New Phytol. 2021, 231, 1875–1889. [Google Scholar] [CrossRef]
- Baena-González, E.; Lunn, J.E. SnRK1 and Trehalose 6-Phosphate—Two Ancient Pathways Converge to Regulate Plant Metabolism and Growth. Curr. Opin. Plant Biol. 2020, 55, 52–59. [Google Scholar] [CrossRef]
- Urrea-Castellanos, R.; Caldana, C.; Henriques, R. Growing at the Right Time: Interconnecting the TOR Pathway with Photoperiod and Circadian Regulation. J. Exp. Bot. 2022, 73, 7006–7015. [Google Scholar] [CrossRef]
- Zhang, N.; Meng, Y.; Li, X.; Zhou, Y.; Ma, L.; Fu, L.; Schwarzländer, M.; Liu, H.; Xiong, Y. Metabolite-Mediated TOR Signaling Regulates the Circadian Clock in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 25395–25397. [Google Scholar] [CrossRef]
- Boix, M.; Garcia-Rodriguez, A.; Castillo, L.; Miró, B.; Hamilton, F.; Tolak, S.; Pérez, A.; Monte-Bello, C.; Caldana, C.; Henriques, R. 40S Ribosomal Protein S6 Kinase Integrates Daylength Perception and Growth Regulation in Arabidopsis thaliana. Plant Physiol. 2024, 195, 3039–3052. [Google Scholar] [CrossRef]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and Primary Photosynthetic Metabolism—More than the Icing on the Cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef]
- Lu, Y.; Gehan, J.P.; Sharkey, T.D. Daylength and Circadian Effects on Starch Degradation and Maltose Metabolism. Plant Physiol. 2005, 138, 2280–2291. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Bell, L.J.; Webb, A.A.R. Interactions between Plant Circadian Clocks and Solute Transport. J. Exp. Bot. 2011, 62, 2333–2348. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Tian, X.Y.; Li, J.; Bai, S.; Zhang, Z.Y.; Li, Y.; Cao, H.R.; Chen, Z.C. Two Central Circadian Oscillators OsPRR59 and OsPRR95 Modulate Magnesium Homeostasis and Carbon Fixation in Rice. Mol. Plant 2022, 15, 1602–1614. [Google Scholar] [CrossRef] [PubMed]
- Salomé, P.A.; Oliva, M.; Weigel, D.; Krämer, U. Circadian Clock Adjustment to Plant Iron Status Depends on Chloroplast and Phytochrome Function. EMBO J. 2013, 32, 511–523. [Google Scholar] [CrossRef]
- de Melo, J.R.F.; Gutsch, A.; De Caluwé, T.; Leloup, J.C.; Gonze, D.; Hermans, C.; Webb, A.A.R.; Verbruggen, N. Magnesium Maintains the Length of the Circadian Period in Arabidopsis. Plant Physiol. 2021, 185, 519–532. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, Y.; Shin, L.J.; Wu, J.F.; Shanmugam, V.; Tsednee, M.; Lo, J.C.; Chen, C.C.; Wu, S.H.; Yeh, K.C. Iron Is Involved in the Maintenance of Circadian Period Length in Arabidopsis. Plant Physiol. 2013, 161, 1409–1420. [Google Scholar] [CrossRef]
- Li, J.; Yokosho, K.; Liu, S.; Cao, H.R.; Yamaji, N.; Zhu, X.G.; Liao, H.; Ma, J.F.; Chen, Z.C. Diel Magnesium Fluctuations in Chloroplasts Contribute to Photosynthesis in Rice. Nat. Plants 2020, 6, 848–859. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; Van Ooijen, G. Daily Magnesium Fluxes Regulate Cellular Timekeeping and Energy Balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Johnson, C.H.; Knight, M.R.; Kondo, T.; Masson, P.; Sedbrook, J.; Haley, A.; Trewavas, A. Circadian Oscillations of Cytosolic and Chloroplastic Free Calcium in Plants. Science 1995, 269, 1863–1865. [Google Scholar] [CrossRef]
- Dodd, A.N.; Gardner, M.J.; Hotta, C.T.; Hubbard, K.E.; Dalchau, N.; Love, J.; Assie, J.M.; Robertson, F.C.; Jakobsen, M.K.; Gonçalves, J.; et al. The Arabidopsis Circadian Clock Incorporates a CADPR-Based Feedback Loop. Science 2007, 318, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Love, J.; Dodd, A.N.; Webb, A.A.R. Circadian and Diurnal Calcium Oscillations Encode Photoperiodic Information in Arabidopsis. Plant Cell 2004, 16, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Martí Ruiz, M.C.; Hubbard, K.E.; Gardner, M.J.; Jung, H.J.; Aubry, S.; Hotta, C.T.; Mohd-Noh, N.I.; Robertson, F.C.; Hearn, T.J.; Tsai, Y.C.; et al. Circadian Oscillations of Cytosolic Free Calcium Regulate the Arabidopsis Circadian Clock. Nat. Plants 2018, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Martí Ruiz, M.C.; Jung, H.J.; Webb, A.A.R. Circadian Gating of Dark-Induced Increases in Chloroplast- and Cytosolic-Free Calcium in Arabidopsis. New Phytol. 2020, 225, 1993–2005. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Meier, S.; Petersen, L.N.; Ingle, R.A.; Roden, L.C. Defence Responses of Arabidopsis thaliana to Infection by Pseudomonas syringae Are Regulated by the Circadian Clock. PLoS ONE 2011, 6, e26968. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhou, M.; Yoo, H.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X. Spatial and Temporal Regulation of Biosynthesis of the Plant Immune Signal Salicylic Acid. Proc. Natl. Acad. Sci. USA 2015, 112, 9166–9173. [Google Scholar] [CrossRef]
- Goodspeed, D.; Chehab, E.W.; Min-Venditti, A.; Braam, J.; Covington, M.F. Arabidopsis Synchronizes Jasmonate-Mediated Defense with Insect Circadian Behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 4674–4677. [Google Scholar] [CrossRef]
- Ng, G.; Seabolt, S.; Zhang, C.; Salimian, S.; Watkins, T.A.; Lu, H. Genetic Dissection of Salicylic Acid-Mediated Defense Signaling Networks in Arabidopsis. Genetics 2011, 189, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, M.; Seitz, N.C.; Angel, W.; Hallworth, A.; Wiratan, L.; Darwish, O.; Alkharouf, N.; Dawit, T.; Lin, D.; et al. LUX ARRHYTHMO Mediates Crosstalk between the Circadian Clock and Defense in Arabidopsis. Nat. Commun. 2019, 10, 2543. [Google Scholar] [CrossRef] [PubMed]
- Fraser, O.J.P.; Cargill, S.J.; Spoel, S.H.; van Ooijen, G. Crosstalk between Salicylic Acid Signalling and the Circadian Clock Promotes an Effective Immune Response in Plants. npj Biol. Timing Sleep 2024, 1, 6. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, W.; Karapetyan, S.; Mwimba, M.; Marqués, J.; Buchler, N.E.; Dong, X. Redox Rhythm Reinforces the Circadian Clock to Gate Immune Response. Nature 2015, 523, 472–476. [Google Scholar] [CrossRef]
- Paeng, S.K.; Wi, S.D.; Chae, H.B.; Bae, S.B.; Phan, K.A.T.; Kim, M.G.; Yun, D.-J.; Kim, W.-Y.; McClung, C.R.; Lee, S.Y. NTRC Mediates the Coupling of Chloroplast Redox Rhythm with Nuclear Circadian Clock in Plant Cells. Mol. Plant 2025, 18, 468–484. [Google Scholar] [CrossRef]
- Michalska, J.; Zauber, H.; Buchanan, B.B.; Cejudo, F.J.; Geigenberger, P. NTRC Links Built-in Thioredoxin to Light and Sucrose in Regulating Starch Synthesis in Chloroplasts and Amyloplasts. Proc. Natl. Acad. Sci. USA 2009, 106, 9908–9913. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Schmitt, K.; Grimm, A.; Dallmann, R.; Oettinghaus, B.; Restelli, L.M.; Witzig, M.; Ishihara, N.; Mihara, K.; Ripperger, J.A.; Albrecht, U.; et al. Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics. Cell Metab. 2018, 27, 657–666.e5. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Li, B.; Zhang, Y.; Wang, L. Attenuated TOR Signaling Lengthens Circadian Period in Arabidopsis. Plant Signal. Behav. 2020, 15, 1710935. [Google Scholar] [CrossRef]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B.; et al. A Membrane-Bound NAC Transcription Factor, ANAC017, Mediates Mitochondrial Retrograde Signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Narsai, R.; He, C.; Wang, Y.; Berkowitz, O.; Whelan, J.; Liew, L.C. Coordinated Regulation of the Mitochondrial Retrograde Response by Circadian Clock Regulators and ANAC017. Plant Commun. 2023, 4, 100501. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Walker, B.J. Dynamic Response of Photorespiration in Fluctuating Light Environments. J. Exp. Bot. 2023, 74, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.K.A.; Yamori, W.; Takahashi, S.; Terashima, I.; Noguchi, K. Mitochondrial Alternative Pathway-Associated Photoprotection of Photosystem II Is Related to the Photorespiratory Pathway. Plant Cell Physiol. 2016, 57, 1426–1431. [Google Scholar] [CrossRef]
- Pilgrim, M.L.; McClung, C.R. Differential Involvement of the Circadian Clock in the Expression of Genes Required for Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Synthesis, Assembly, and Activation in Arabidopsis thaliana. Plant Physiol. 1993, 103, 553–564. [Google Scholar] [CrossRef]
- Zhong, H.H.; Young, J.C.; Pease, E.A.; Hangarter, R.P.; McClung, C.R. Interactions between Light and the Circadian Clock in the Regulation of CAT2 Expression in Arabidopsis. Plant Physiol. 1994, 104, 889–898. [Google Scholar] [CrossRef]
- Zhong, H.H.; McClung, C.R. The Circadian Clock Gates Expression of Two Arabidopsis Catalase Genes to Distinct and Opposite Circadian Phases. Mol. Gen. Genet. MGG 1996, 251, 196–203. [Google Scholar] [CrossRef]
- McClung, C.R.; Hsu, M.; Painter, J.E.; Gagne, J.M.; Karlsberg, S.D.; Salomé, P.A. Integrated Temporal Regulation of the Photorespiratory Pathway. Circadian Regulation of Two Arabidopsis Genes Encoding Serine Hydroxymethyltransferase. Plant Physiol. 2000, 123, 381–391. [Google Scholar] [CrossRef]
- Estavillo, G.M.; Crisp, P.A.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP Chloroplast Retrograde Pathway That Functions in Drought and High Light Signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef]
- Chan, K.X.; Mabbitt, P.D.; Phua, S.Y.; Mueller, J.W.; Nisar, N.; Gigolashvili, T.; Stroeher, E.; Grassl, J.; Arlt, W.; Estavillo, G.M.; et al. Sensing and Signaling of Oxidative Stress in Chloroplasts by Inactivation of the SAL1 Phosphoadenosine Phosphatase. Proc. Natl. Acad. Sci. USA 2016, 113, E4567–E4576. [Google Scholar] [CrossRef]
- Crisp, P.A.; Smith, A.B.; Ganguly, D.R.; Murray, K.D.; Eichten, S.R.; Millar, A.A.; Pogson, B.J. RNA Polymerase II Read-through Promotes Expression of Neighboring Genes in SAL1-PAP-XRN Retrograde Signaling. Plant Physiol. 2018, 178, 1614–1630. [Google Scholar] [CrossRef]
- Litthauer, S.; Jones, M.A. SAL1-PAP Retrograde Signalling Extends Circadian Period by Reproducing the Loss of Exoribonuclease (XRN) Activity. Plant Signal. Behav. 2018, 13, e1500066. Available online: https://www.tandfonline.com/doi/10.1080/15592324.2018.1500066?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed#d1e166 (accessed on 5 August 2025).
- Prasetyaningrum, P.; Litthauer, S.; Vegliani, F.; Battle, M.W.; Wood, M.W.; Liu, X.; Dickson, C.; Jones, M.A. Inhibition of RNA Degradation Integrates the Metabolic Signals Induced by Osmotic Stress into the Arabidopsis Circadian System. J. Exp. Bot. 2023, 74, 5805–5819. [Google Scholar] [CrossRef]
- Careno, D.A.; Perez Santangelo, S.; MacKnight, R.C.; Yanovsky, M.J. The 5′-3′ MRNA Decay Pathway Modulates the Plant Circadian Network in Arabidopsis. Plant Cell Physiol. 2022, 63, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Litthauer, S.; Chan, K.X.; Jones, M.A. 3′-Phosphoadenosine 5′-Phosphate Accumulation Delays the Circadian System. Plant Physiol. 2018, 176, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, H.; Zhou, J.M.; Smith, S.M.; Li, J. Malate Circulation: Linking Chloroplast Metabolism to Mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Jiménez, A.; Sevilla, F.; Martí, M.C. Reactive Oxygen Species Homeostasis and Circadian Rhythms in Plants. J. Exp. Bot. 2021, 72, 5825–5840. [Google Scholar] [CrossRef]
- Doherty, C.J.; Kay, S.A. Circadian Control of Global Gene Expression Patterns. Annu. Rev. Genet. 2010, 44, 419–444. [Google Scholar] [CrossRef]
- Martí, M.C.; Jiménez, A.; Sevilla, F. Thioredoxin Network in Plant Mitochondria: Cysteine S-Posttranslational Modifications and Stress Conditions. Front. Plant Sci. 2020, 11, 571288. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins Are Conserved Markers of Circadian Rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Finkemeier, I.; Goodman, M.; Lamkemeyer, P.; Kandlbinder, A.; Sweetlove, L.J.; Dietz, K.J. The Mitochondrial Type II Peroxiredoxin F Is Essential for Redox Homeostasis and Root Growth of Arabidopsis thaliana under Stress. J. Biol. Chem. 2005, 280, 12168–12180. [Google Scholar] [CrossRef]
- Román, Á.; Li, X.; Deng, D.; Davey, J.W.; James, S.; Graham, I.A.; Haydon, M.J. Superoxide Is Promoted by Sucrose and Affects Amplitude of Circadian Rhythms in the Evening. Proc. Natl. Acad. Sci. USA 2021, 118, e2020646118. [Google Scholar] [CrossRef]
- Giegé, P.; Sweetlove, L.J.; Cognat, V.; Leaver, C.J. Coordination of Nuclear and Mitochondrial Genome Expression during Mitochondrial Biogenesis in Arabidopsis. Plant Cell 2005, 17, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.M.; Lee, C.P.; Atkin, O.K.; Cheng, R.; Brown, T.B.; Millar, A.H. Variation in Leaf Respiration Rates at Night Correlates with Carbohydrate and Amino Acid Supply. Plant Physiol. 2017, 174, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Selinski, J.; Mao, C.; Zhu, Y.; Berkowitz, O.; Whelan, J. Linking Mitochondrial and Chloroplast Retrograde Signalling in Plants. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190410. [Google Scholar] [CrossRef] [PubMed]
- Blanco, N.E.; Guinea-Díaz, M.; Whelan, J.; Strand, Å. Interaction between Plastid and Mitochondrial Retrograde Signalling Pathways during Changes to Plastid Redox Status. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130231. [Google Scholar] [CrossRef]
- Ng, S.; Giraud, E.; Duncan, O.; Law, S.R.; Wang, Y.; Xu, L.; Narsai, R.; Carrie, C.; Walker, H.; Day, D.A.; et al. Cyclin-Dependent Kinase E1 (CDKE1) Provides a Cellular Switch in Plants between Growth and Stress Responses. J. Biol. Chem. 2013, 288, 3449–3459. [Google Scholar] [CrossRef]
- Knight, H.; Thomson, A.J.W.; McWatters, H.G. Sensitive to Freezing6 Integrates Cellular and Environmental Inputs to the Plant Circadian Clock. Plant Physiol. 2008, 148, 293–303. [Google Scholar] [CrossRef]
- Wathugala, D.L.; Hemsley, P.A.; Moffat, C.S.; Cremelie, P.; Knight, M.R.; Knight, H. The Mediator Subunit SFR6/MED16 Controls Defence Gene Expression Mediated by Salicylic Acid and Jasmonate Responsive Pathways. New Phytol. 2012, 195, 217–230. [Google Scholar] [CrossRef]
- Crawford, T.; Karamat, F.; Lehotai, N.; Rentoft, M.; Blomberg, J.; Strand, Å.; Björklund, S. Specific Functions for Mediator Complex Subunits from Different Modules in the Transcriptional Response of Arabidopsis thaliana to Abiotic Stress. Sci. Rep. 2020, 10, 5073. [Google Scholar] [CrossRef]
- Musielak, Z.E.; Quarles, B. The Three-Body Problem. Rep. Prog. Phys. 2014, 77, 065901. [Google Scholar] [CrossRef]
- Harmer, S.L. The Circadian System in Higher Plants. Annu. Rev. Plant Biol. 2009, 60, 357–377. [Google Scholar] [CrossRef]
- McClung, C.R. Beyond Arabidopsis: The Circadian Clock in Non-Model Plant Species. Semin. Cell Dev. Biol. 2013, 24, 430–436. [Google Scholar] [CrossRef]
- Müller, N.A.; Zhang, L.; Koornneef, M.; Jiménez-Gómez, J.M. Mutations in EID1 and LNK2 Caused Light-Conditional Clock Deceleration during Tomato Domestication. Proc. Natl. Acad. Sci. USA 2018, 115, 7135–7140. [Google Scholar] [CrossRef]
- Greenham, K.; Lou, P.; Puzey, J.R.; Kumar, G.; Arnevik, C.; Farid, H.; Willis, J.H.; McClung, C.R. Geographic Variation of Plant Circadian Clock Function in Natural and Agricultural Settings. J. Biol. Rhythms 2017, 32, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yarkhunova, Y.; Edwards, C.E.; Ewers, B.E.; Baker, R.L.; Aston, T.L.; Mcclung, C.R.; Lou, P.; Weinig, C. Selection during Crop Diversification Involves Correlated Evolution of the Circadian Clock and Ecophysiological Traits in Brassica rapa. New Phytol. 2016, 210, 133–144. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Tranquilli, G.; Lewis, S.; Kippes, N.; Dubcovsky, J. Genetic and Physical Mapping of the Earliness per Se Locus Eps-A m 1 in Triticum monococcum Identifies EARLY FLOWERING 3 (ELF3) as a Candidate Gene. Funct. Integr. Genom. 2016, 16, 365–382. [Google Scholar] [CrossRef]
- Saito, H.; Ogiso-Tanaka, E.; Okumoto, Y.; Yoshitake, Y.; Izumi, H.; Yokoo, T.; Matsubara, K.; Hori, K.; Yano, M.; Inoue, H.; et al. Ef7 Encodes an ELF3-like Protein and Promotes Rice Flowering by Negatively Regulating the Floral Repressor Gene Ghd7 under Both Short-and Long-Day Conditions. Plant Cell Physiol. 2012, 53, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Ariyadasa, R.; Himmelbach, A.; Poursarebani, N.; Kilian, B.; Stein, N.; Steuernagel, B.; Hensel, G.; Kumlehn, J.; Sehgal, S.K.; et al. A Distorted Circadian Clock Causes Early Flowering and Temperature-Dependent Variation in Spike Development in the Eps-3Am Mutant of Einkorn Wheat. Genetics 2014, 196, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Beales, J.; Faure, S.; Dunford, R.P.; Laurie, D.A. Botany: The Pseudo-Response Regulator Ppd-H1 Provides Adaptation to Photoperiod in Barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef]
- Mishra, P.; Panigrahi, K.C. GIGANTEA—An Emerging Story. Front. Plant Sci. 2015, 6, 8. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Van Poppel, P.M.J.A.; Heuvelink, E.; Millenaar, F.F. A Single Locus Confers Tolerance to Continuous Light and Allows Substantial Yield Increase in Tomato. Nat. Commun. 2014, 5, 4549. [Google Scholar] [CrossRef]
- Dodd, A.N.; Belbin, F.E.; Frank, A.; Webb, A.A.R. Interactions between Circadian Clocks and Photosynthesis for the Temporal and Spatial Coordination of Metabolism. Front. Plant Sci. 2015, 6, 245. [Google Scholar] [CrossRef]
- Nagel, D.H.; Kay, S.A. Complexity in the Wiring and Regulation of Plant Circadian Networks. Curr. Biol. 2012, 22, R648–R657. [Google Scholar] [CrossRef]
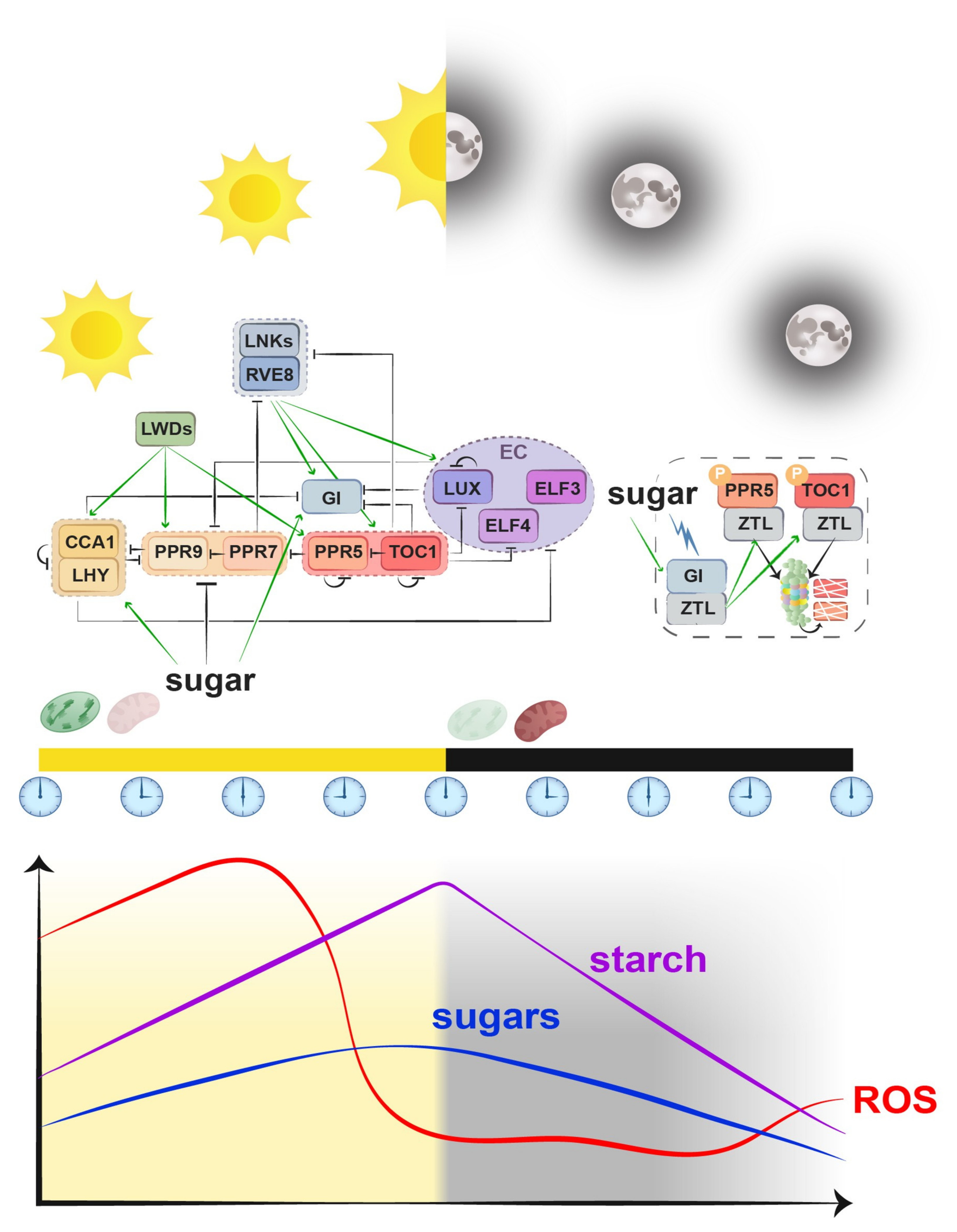

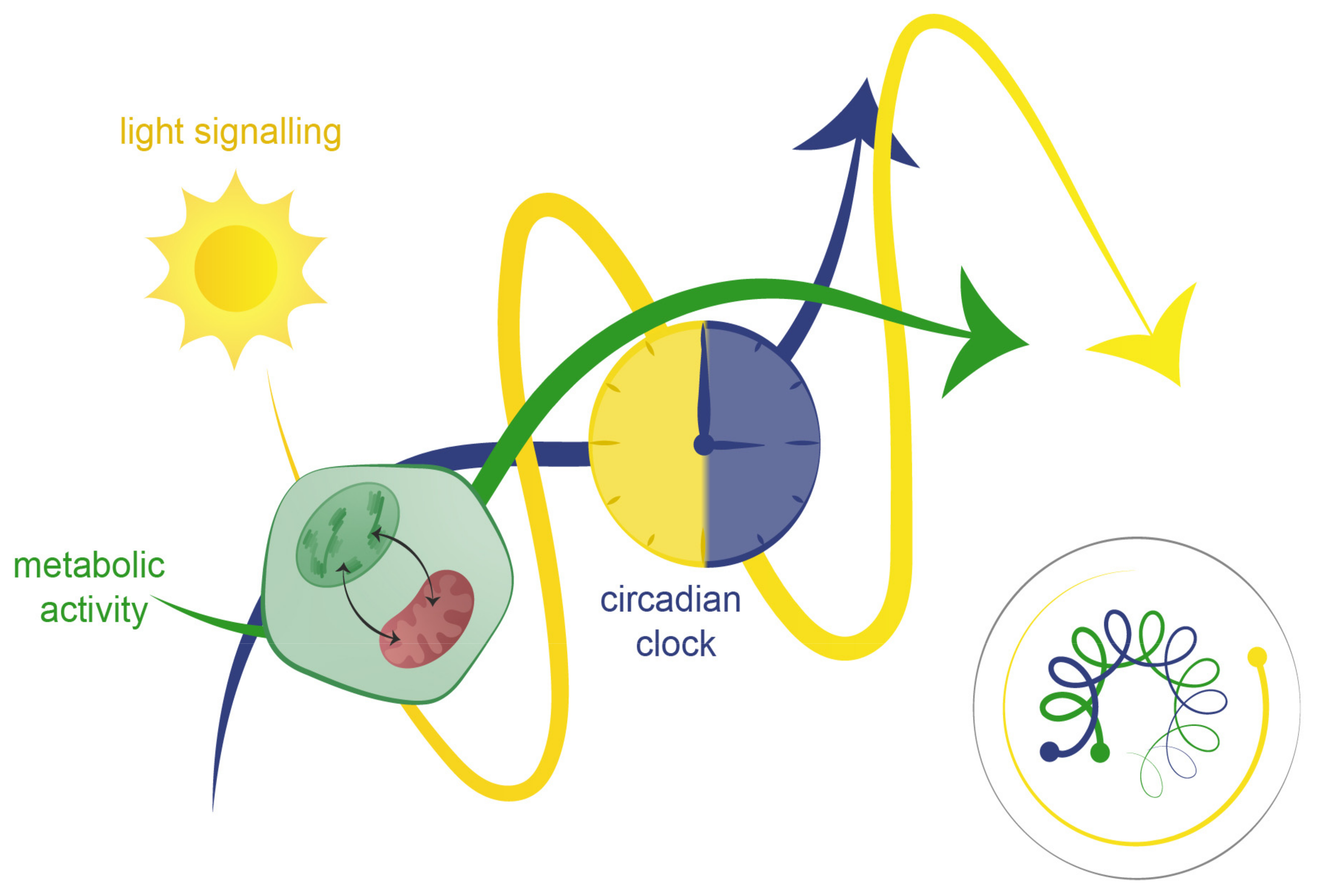
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervela-Cardona, L.; Francisco, M.; Strand, Å. Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment. Plants 2025, 14, 2464. https://doi.org/10.3390/plants14162464
Cervela-Cardona L, Francisco M, Strand Å. Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment. Plants. 2025; 14(16):2464. https://doi.org/10.3390/plants14162464
Chicago/Turabian StyleCervela-Cardona, Luis, Marta Francisco, and Åsa Strand. 2025. "Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment" Plants 14, no. 16: 2464. https://doi.org/10.3390/plants14162464
APA StyleCervela-Cardona, L., Francisco, M., & Strand, Å. (2025). Metabolism in Sync: The Circadian Clock, a Central Hub for Light-Driven Chloroplastic and Mitochondrial Entrainment. Plants, 14(16), 2464. https://doi.org/10.3390/plants14162464





