Screening and Application of Highly Efficient Rhizobia for Leguminous Green Manure Astragalus sinicus in Lyophilized Inoculants and Seed Coating
Abstract
1. Introduction
2. Results
2.1. Screening and Evaluation of High-Efficiency Nitrogen-Fixing Mesorhizobium Strains for A. sinicus
2.2. Optimization of Cryoprotectants for Enhanced Rhizobial Viability
2.3. Seed Coating Characteristics and Rhizobial Viability Assessment
3. Discussion
3.1. Symbiotic Efficiency and Environmental Adaptation of Mesorhizobium Strains in A. sinicus
3.2. Viability Preservation of Rhizobia: Effects of Cryoprotectant and Storage
3.3. Rhizobial Viability on Pelleted A. sinicus Seeds: Coating Effects and Survival Assessment
3.4. Current Limitations and Future Perspectives
4. Materials and Methods
4.1. Rhizobial Strains and Culture Conditions
4.2. Growth Response of A. sinicus to Inoculation with Diverse Mesorhizobium Strains
4.3. Measurement of A. sinicus Growth Traits and Nodulation Characteristics
4.4. Preparation of Cryoprotectant Solutions and the Freeze–Vacuum Drying Protocol
4.5. Rhizobial Cell Counting (CFU Enumeration)
4.6. Seed Coating and Rhizobial Viability Assessment
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCBAU | Culture Collection of Beijing Agricultural University, Beijing, China |
| CK | Control |
| PVP | Polyvinylpyrrolidone |
| CFU | Colony-forming Unit |
| Smp20 | 20% skimmed milk powder |
| Tre20 | 20% trehalose |
| M. | Mesorhizobium |
| M. huakuii | Mesorhizobium huakuii |
| A. sinicus | Astragalus sinicus |
| DMSO | Dimethyl sulfoxide |
| YM | Yeast extract–mannitol medium |
| TY | Tryptone–yeast extract medium |
| YMA | Yeast–extract mannitol agar medium |
| rpm | Rotation per minute |
| NA | Nalidixic acid |
| OD600 | Optical density at 600 nm |
References
- Zhang, J.; Shang, Y.; Liu, C.; Brunel, B.; Wang, E.; Li, S.; Peng, S.; Guo, C.; Chen, W. Mesorhizobium jarvisii is a dominant and widespread species symbiotically efficient on Astragalus sinicus L. in the Southwest of China. Syst. Appl. Microbiol. 2020, 43, 126102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.T.; Li, Y.; Wang, R.; Sui, X.H.; Zhang, X.X.; Zhang, J.J.; Wang, E.T.; Chen, W.X. Mesorhizobium qingshengii sp. nov., isolated from effective nodules of Astragalus sinicus. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 6, 2002–2007. [Google Scholar] [CrossRef]
- Fang, Y.; Yan, Z.L.; Chen, J.C.; Wang, F.; Wang, M.K.; Lin, X.J. Effect of chemical fertilization and green manure on the abundance and community structure of ammonia oxidizers in a paddy soil. Chil. J. Agric. Res. 2015, 75, 487–496. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Moudrý, J.; Konvalina, P.; Hörtenhuber, S.J.; Ghorbani, M.; Neugschwandtner, R.W.; Jiang, Z.; Krexner, T.; Kopecký, M. Environmental life cycle assessment in organic and conventional rice farming systems: Using a cradle to farm gate approach. Sustainability 2022, 14, 15870. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, K.D.; Jung, K.Y.; Ali, M.A.; Lee, D.; Gutierrez, J.; Kim, P.J. Effect of Chinese milk vetch (Astragalus sinicus L.) as a green manure on rice productivity and methane emission in paddy soil. Agric. Ecosyst. Environ. 2010, 138, 343–347. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, R.J.; Gao, J.S.; Wang, X.C.; Fan, F.L.; Ma, X.T.; Yin, H.Q.; Zhang, C.W.; Feng, K.; Deng, Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 2017, 104, 208–217. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Z.; Zhang, Y.; Chen, Z.; Du, X.; Mu, Y. Astragalus sinicus incorporated as green manure for weed control in corn. Front. Plant Sci. 2022, 13, 829421. [Google Scholar] [CrossRef]
- Gao, S.J.; Cao, W.D.; Gao, J.S.; Huang, J.; Bai, J.S.; Zeng, N.H.; Chang, D.N.; Shimizu, K. Effects of long-term application of different green manures on ferric iron reduction in a red paddy soil in Southern China. J. Integr. Agric. 2017, 16, 959–966. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, H.; Li, S.; Zhang, Z.; Liao, Y.; Lu, Y.; Zhou, G.; Gao, S.; Nie, J.; Cao, W. Co-utilizing milk vetch, rice straw, and lime reduces the Cd accumulation of rice grain in two paddy soils in south China. Sci. Total Environ. 2022, 806, 150622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Dong, Y.B.; Jiao, Y.; Wang, B.X.; Wang, C.Y.; Song, M.X.; Xiong, Z.Q. Nitrogen management regulates nitrogen fixation efficiency of milk vetch and rice productivity under milk vetch-rice rotation system. J. Plant Nutr. Fertil. 2024, 30, 1–11. [Google Scholar] [CrossRef]
- Chen, W.X.; LI, G.S.; Qi, Y.L.; Wang, E.T.; Yuan, H.L.; Li, J.L. Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int. J. Syst. Bacteriol. 1991, 41, 275–280. [Google Scholar] [CrossRef][Green Version]
- Murooka, Y.; Xu, Y.; Sanada, K.; Araki, M.; Morinaga, T.; Yokota, A. Formation of root nodules by Rhizobium huakuii biovar. renge bv. nov. on Astragalus sinicus cv. Japan. J. Ferment. Bioeng. 1993, 76, 38–44. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shang, Y.M.; Wang, E.T.; Chen, W.F.; de Lajudie, P.; Li, B.Y.; Guo, C.; Yang, X.; Zheng, J.Q.; Liu, C.Z. Mesorhizobium jarvisii sv. astragali as predominant microsymbiont for Astragalus sinicus L. in acidic soils, Xinyang, China. Plant Soil 2018, 433, 201–212. [Google Scholar] [CrossRef]
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent development and new insight of diversification and symbiosis specificity of legume rhizobia: Mechanism and application. J. Appl. Microbiol. 2021, 131, 553–563. [Google Scholar] [CrossRef]
- Conrad, P.; de Pablo, J. Computer simulation of the cryoprotectant disaccharide α,α-trehalose in aqueous solution. J. Phys. Chem. A 1999, 103, 4049–4055. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Effect of cooling rate, freeze-drying, and storage on survival of free and immobilized Lactobacillus casei ATCC 393. LWT Food Sci. Technol. 2016, 69, 468–473. [Google Scholar] [CrossRef]
- Li, B.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl. Microbiol. Biotechnol. 2011, 92, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Pyar, H.; Peh, K. Effect of cryoprotective agents on survival and stability of Lactobacillus acidophilus cultured in food-grade medium. Int. J. Dairy Technol. 2011, 64, 578–584. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Li, Y.G.; Zhou, J.C. The symbiosis phenotype and expression patterns of five nodule-specific genes of Astragalus sinicus under ammonium and salt stress conditions. Plant Cell Rep. 2007, 26, 1421–1430. [Google Scholar] [CrossRef]
- Chang, D.N.; Ma, X.T.; Zhou, G.P.; Gao, S.J.; Liu, R.; Cao, W.D. Symbiotic compatibility of different rhizobia strains with important Chinese milk vetch (Astragalus sinicus) cultivars. Acta Prat. Sin. 2022, 31, 171–180. [Google Scholar] [CrossRef]
- Chen, H.K.; Li, F.D.; Cao, Y.Z. Characteristics, Distribution, Ecology, and Utilization of Astragalus sinicus-Rhizobia Symbiosis. In The Nitrogen Fixation and Its Research in China; Hong, G.F., Ed.; Springer: Berlin, Heidelberg, 1992; pp. 439–455. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Shi, H. Rhizobium symbiosis leads to increased drought tolerance in Chinese milk vetch (Astragalus sinicus L.). Agronomy 2022, 12, 725. [Google Scholar] [CrossRef]
- Malek, W.; Inaba, M.; Ono, H.; Kaneko, Y.; Murooka, Y. Competition for Astragalus sinicus root nodule infection between its native microsymbiont Rhizobium huakuii bv. renge B3 and Rhizobium sp. ACMP18 strain, specific for Astragalus cicer. Appl. Microbiol. Biotechnol. 1998, 50, 261–265. [Google Scholar] [CrossRef]
- Yang, L.; Nie, J.; Xu, C.; Cao, W.D. Biological nitrogen fixation of Chinese milk vetch (Astragalus sinicus L.) as affected by exogenous carbon and nitrogen input. Symbiosis 2021, 85, 69–77. [Google Scholar] [CrossRef]
- Yoshioka, K.; Maruyama, Y. Characterization and symbiotic nitrogen fixation of rhizobium that modulates Chinese milk vetch (Astragalus sinicus L.). Soil Sci. Plant Nutr. 1999, 36, 83–90. [Google Scholar] [CrossRef]
- Bian, Q.; Cheng, K.; Chen, L.; Jiang, Y.J.; Li, D.M.; Xie, Z.B.; Wang, X.Y.; Sun, B. Organic amendments increased Chinese milk vetch symbiotic nitrogen fixation by enriching Mesorhizobium in rhizosphere. Environ. Res. 2024, 252, 118923. [Google Scholar] [CrossRef]
- Chang, D.N.; Gao, S.J.; Zhou, G.P.; Deng, S.H.; Jia, J.Z.; Wang, E.T.; Cao, W.D. The chromosome-level genome assembly of Astragalus sinicus and comparative genomic analyses provide new resources and insights for understanding legume-rhizobial interactions. Plant Commun. 2022, 3, 100263. [Google Scholar] [CrossRef] [PubMed]
- Denison, R.F. Chapter 9. A Darwinian Perspective on Improving Nitrogen-Fixation Efficiency of Legume Crops and Forages. In Crop Physiology, 2nd ed.; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 207–222. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V.; Hunegnaw, D.K. Legume-rhizobium specificity effect on nodulation, biomass production and partitioning of faba bean (Vicia faba L.). Sci. Rep. 2021, 11, 3678. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhou, T.; Penttinen, P.; Zou, L.; Wang, K.; Cui, Y.Q.; Heng, N.N.; Xu, K.W. Symbiotic matching, taxonomic position, and field assessment of symbiotically efficient rhizobia isolated from soybean root nodules in Sichuan, China. Biol. Fertil. Soils 2015, 51, 707–718. [Google Scholar] [CrossRef]
- Nuswantara, S.; Fujie, M.; Yamada, T.; Malek, W.; Inaba, M.; Kaneko, Y.; Murooka, Y. Phylogenetic position of Mesorhizobium huakuii subsp. rengei, a symbiont of Astragalus sinicus cv. Japan. J. Biosci. Bioeng. 1999, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Guo, X.W.; Terefework, Z.; Paulin, L.; Cao, Y.Z.; Hu, F.R.; Lindstrom, K.; Li, F.D. Genetic diversity among rhizobial isolates from field-grown Astragalus sinicus of Southern China. Syst. Appl. Microbiol. 1999, 22, 312–320. [Google Scholar] [CrossRef]
- Mhada, M.; Zvinavashe, A.; Hazzoumi, Z.; Zeroual, Y.; Marelli, B.; Kouisni, L. Bioformulation of silk-based coating to preserve and deliver Rhizobium tropici to Phaseolus vulgaris under saline environments. Front. Plant Sci. 2022, 12, 700273. [Google Scholar] [CrossRef]
- Jofre, A.; Aymerich, T.; Garriga, M. Impact of different cryoprotectants on the survival of freeze-dried Lactobacillus rhamnosus and Lactobacillus casei/paracasei during long-term storage. Benef. Microbes 2015, 6, 381–386. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice inhibition for cryopreservation: Materials, strategies, and challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta 2021, 1865, 129749. [Google Scholar] [CrossRef]
- Liu, X.B.; He, J.; Liao, C.L. Optimization of cryoprotectants to harvest high active cells of a Scenedesmus-lysing Enterobacter sp. during long-term preservation. Algal Res. 2016, 13, 298–302. [Google Scholar] [CrossRef]
- Wdowiak-Wróbel, S.; Małek, W.; Palusińska-Szysz, M. Low temperature adaptation and the effects of cryoprotectants on mesorhizobia strains. J. Basic Microbiol. 2016, 56, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Romyasamit, C.; Saengsuwan, P.; Boonserm, P.; Thamjarongwong, B.; Singkhamanan, K. Optimization of cryoprotectants for freeze-dried potential probiotic Enterococcus faecalis and evaluation of its storage stability. Dry. Technol. 2021, 40, 2283–2292. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, J.; Seok, W.Y.; Kim, G.S.; Choi, B.; Shin, M.; Lee, J.H.; Kim, Y.; Yang, J.; Jung, Y.H. Changes in the metabolome of probiotics during the stationary phase increase resistance to lyophilization. Food Biosci. 2023, 53, 102499. [Google Scholar] [CrossRef]
- Racharaks, R.; Peccia, J. Cryopreservation of Synechococcus elongatus UTEX 2973. J. Appl. Phycol. 2019, 31, 2267–2276. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef]
- Raza, A.; Bhardwaj, S.; Rahman, M.A.; García-Caparrós, P.; Habib, M.; Saeed, F.; Charagh, S.; Foyer, C.H.; Siddique, K.H.M.; Varshney, R.K. Trehalose: A sugar molecule involved in temperature stress management in plants. Crop J. 2024, 12, 1–16. [Google Scholar] [CrossRef]
- Stefanello, R.F.; Machado, A.A.R.; Cavalheiro, C.P.; Santos, M.L.B.; Nabeshima, E.H.; Copetti, M.V.; Fries, L.L.M. Trehalose as a cryoprotectant in freeze-dried wheat sourdough production. LWT Food Sci. Technol. 2018, 89, 510–517. [Google Scholar] [CrossRef]
- Hsiao, H.C.; Lian, W.C.; Chou, C.C. Effect of packaging conditions and temperature on viability of microencapsulated bifidobacteria during storage. J. Sci. Food Agric. 2004, 84, 134–139. [Google Scholar] [CrossRef]
- Shu, G.; Wang, Z.; Chen, L.; Wan, H.; Chen, H. Characterization of freeze-dried Lactobacillus acidophilus in goat milk powder and tablet: Optimization of the composite cryoprotectants and evaluation of storage stability at different temperature. LWT Food Sci. Technol. 2018, 90, 70–76. [Google Scholar] [CrossRef]
- Khoramnia, A.; Abdullah, N.; Liew, S.L.; Sieo, C.C.; Ramasamy, K.; Ho, Y.W. Enhancement of viability of a probiotic Lactobacillus strain for poultry during freeze-drying and storage using the response surface methodology. Anim. Sci. J. 2010, 82, 127–135. [Google Scholar] [CrossRef]
- Esbelin, J.; Santos, T.; Hébraud, M. Desiccation: An environmental and food industry stress that bacteria commonly face. Food Microbiol. 2018, 69, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.B.; Choi, S.H.; Kim, S.W. Some observations in freeze-drying of recombinant bioluminescent Escherichia coli for toxicity monitoring. J. Biotechnol. 2011, 8288, 95–105. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Abdukerim, R.; Li, L.; Li, J.H.; Xiang, S.; Shi, Y.X.; Xie, X.W.; Chai, A.L.; Fan, T.F.; Li, B.J. Coating seeds with biocontrol bacteria-loaded sodium alginate/pectin hydrogel enhances the survival of bacteria and control efficacy against soil-borne vegetable diseases. Int. J. Biol. Macromol. 2024, 279, 135317. [Google Scholar] [CrossRef]
- Church, B.M.; Geary, B.; Griffitts, J.; Drake, C.L.; Ruebelmann, K.; Nelson, S.V.; Madsen, M.D. Development of a rhizobium seed coating to establish Lupine species on degraded rangelands. Plants 2024, 13, 2101. [Google Scholar] [CrossRef]
- Gong, M.; He, J.X.; Kong, M.; Huo, Q.Y.; Jiang, Y.W.; Song, J.Q.; Han, W.; Lv, G.h. A microencapsulation approach to design microbial seed coatings to boost wheat seed germination and seedling growth under salt stress. Front. Plant Sci. 2023, 14, 1283590. [Google Scholar] [CrossRef] [PubMed]
- Deaker, R.; Roughley, R.J.; Kennedy, I.R. Legume seed inoculation technology—A review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]
- McIntyre, H.J.; Davies, H.; Hore, T.A.; Miller, S.H.; Dufour, J.P.; Ronson, C.W. Trehalose biosynthesis in Rhizobium leguminosarum bv. trifolii and its role in desiccation tolerance. Appl. Environ. Microbiol. 2007, 73, 3984–3992. [Google Scholar] [CrossRef]
- Fadiji, A.; Xiong, C.; Egidi, E.; Singh, B. Formulation challenges associated with microbial biofertilizers in sustainable agriculture and paths forward. J. Sustain. Agric. Environ. 2024, 3, e70006. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yu, T.; Lou, K.; Mao, P.H.; Wang, E.T.; Chen, W.F.; Chen, W.X. Genotypic alteration and competitive nodulation of Mesorhizobium muleiense against exotic chickpea rhizobia in alkaline soils. Syst. Appl. Microbiol. 2014, 37, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kang, U.G.; Kang, J.S.; Choi, S.L.; Lee, S.S.; Kim, J.S.; Park, S.H. Effectiveness of pelleting for inoculating plant growth-promoting rhizobacteria on small seeds. Korean J. Soil Sci. Fertil. 2019, 52, 271–283. [Google Scholar] [CrossRef]
- Zheng, W.T. Genetic Diversity of Mesorhizobium spp. Nodulating with Astragalus sinicus L. and Its Correlations with Environmental Factors. Master’s Thesis, China Agricultural University, Beijing, China, 2012. [Google Scholar]
- Zhang, X.X.; Zhou, J.C.; Zhang, Z.M.; Chen, H.K.; Li, F.D. Behavior of plasmid pJB5JI in Rhizobium huakuii under free-living and symbiotic conditions. Curr. Microbiol. 1995, 31, 97–101. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jiao, Y.S.; Liu, L.X.; Wang, D.; Tian, C.F.; Wang, E.T.; Wang, L.; Chen, W.X.; Wu, S.Y.; Guo, B.L.; et al. Nonspecific symbiosis between Sophora flavescens and different rhizobia. Mol. Plant-Microbe Interact. 2018, 31, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Somasegaran, P.; Hoben, H.J. Handbook for Rhizobia: Methods in Legume-Rhizobium Technology; Springer: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Legesse, S. Isolation, identification and authentication of root nodule bacteria (rhizobia) in promoting sustainable agricultural productivity: A review. Dev. Ctry. Stud. 2016, 1, 87–93. [Google Scholar]
- Kimiti, J.M.; Odee, D.W. Integrated soil fertility management enhances population and effectiveness of indigenous cowpea rhizobia in semi-arid eastern Kenya. Appl. Soil Ecol. 2010, 45, 304–309. [Google Scholar] [CrossRef]
- Gao, H.L.; Ji, Y.R.; Chen, W.F. Selenite resistance and biotransformation to SeNPs in Sinorhizobium meliloti 1021 and the synthetic promotion on alfalfa growth. Microbiol. Res. 2024, 280, 127568. [Google Scholar] [CrossRef]
- Gao, S.J.; Zhang, Y.T.; Cao, W.D. Green manuring orchards and tea gardens: Concept, effects, and application. J. Plant Nutr. Fert. 2025, 31, 588–596. [Google Scholar] [CrossRef]
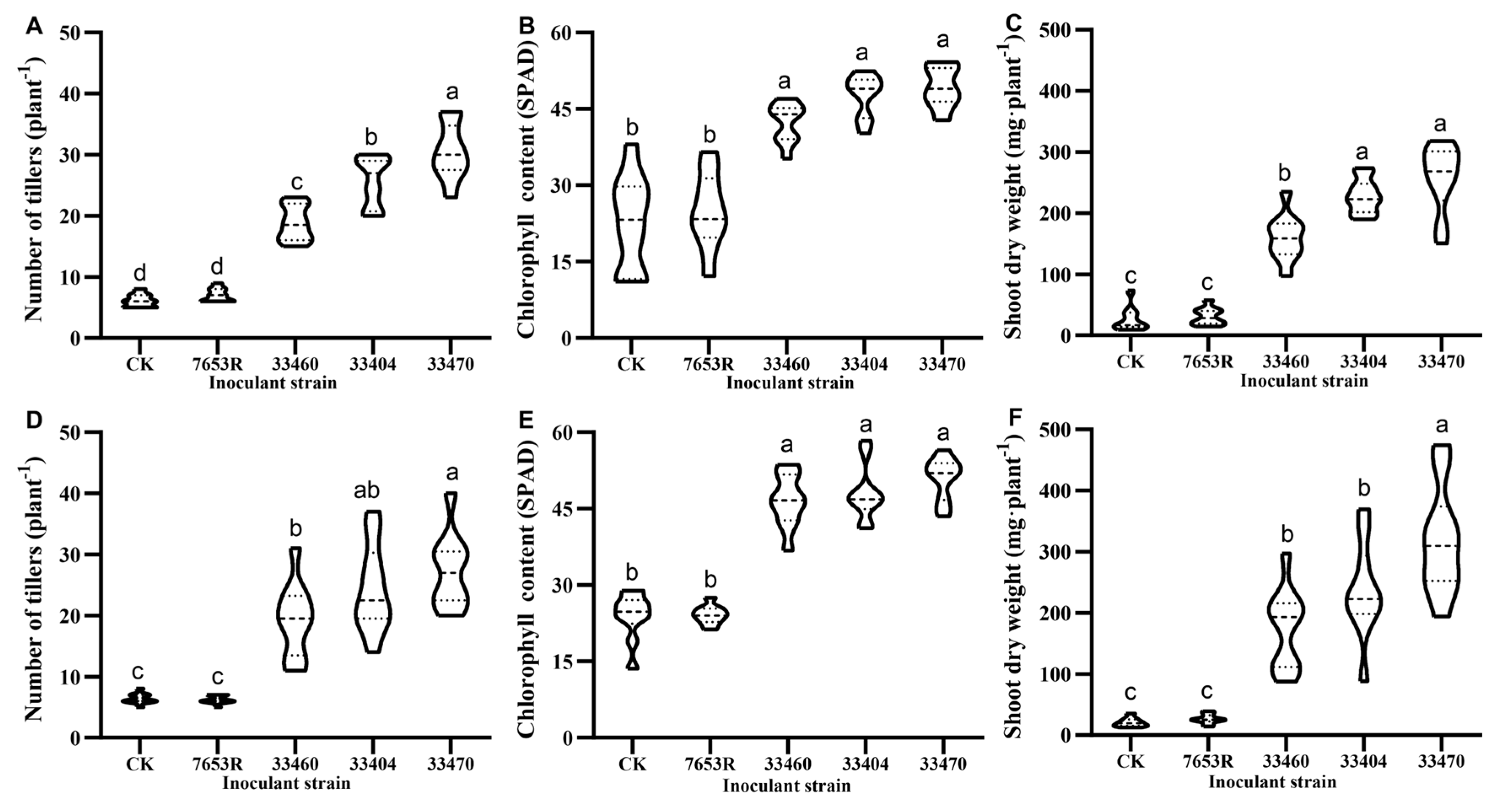
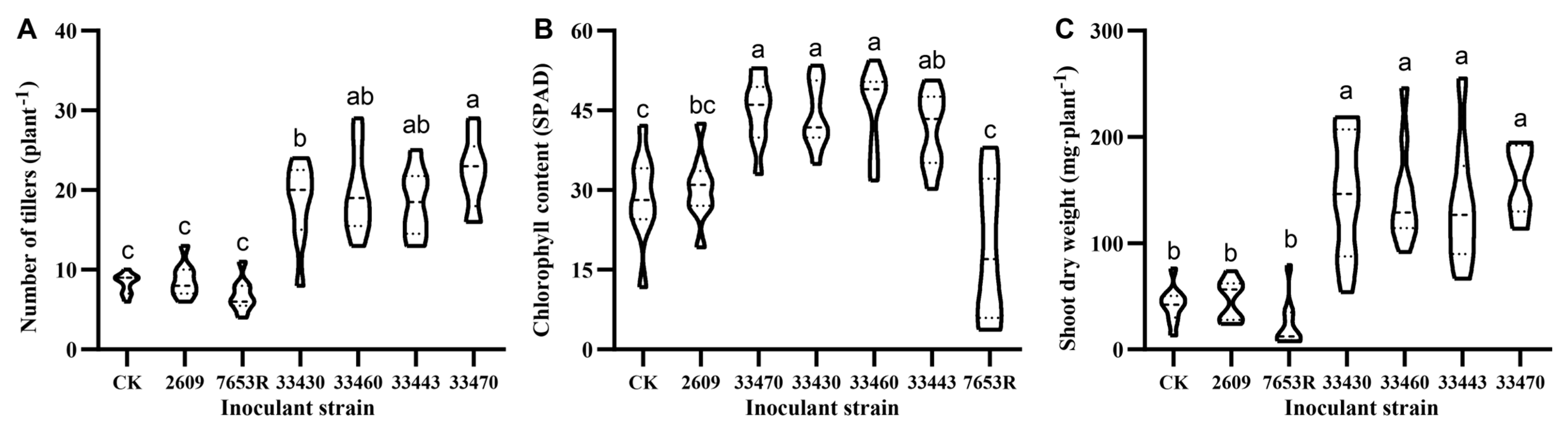

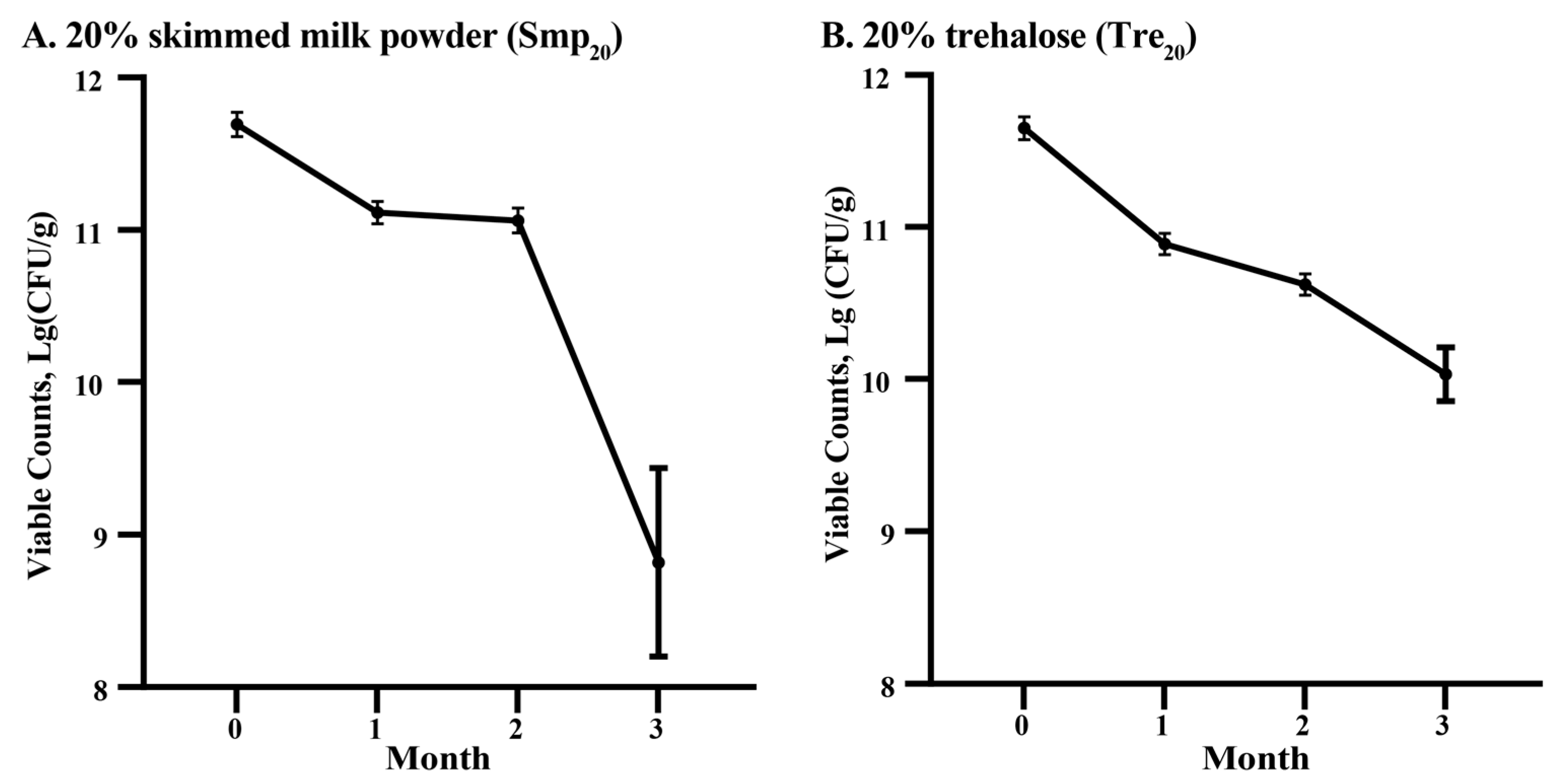

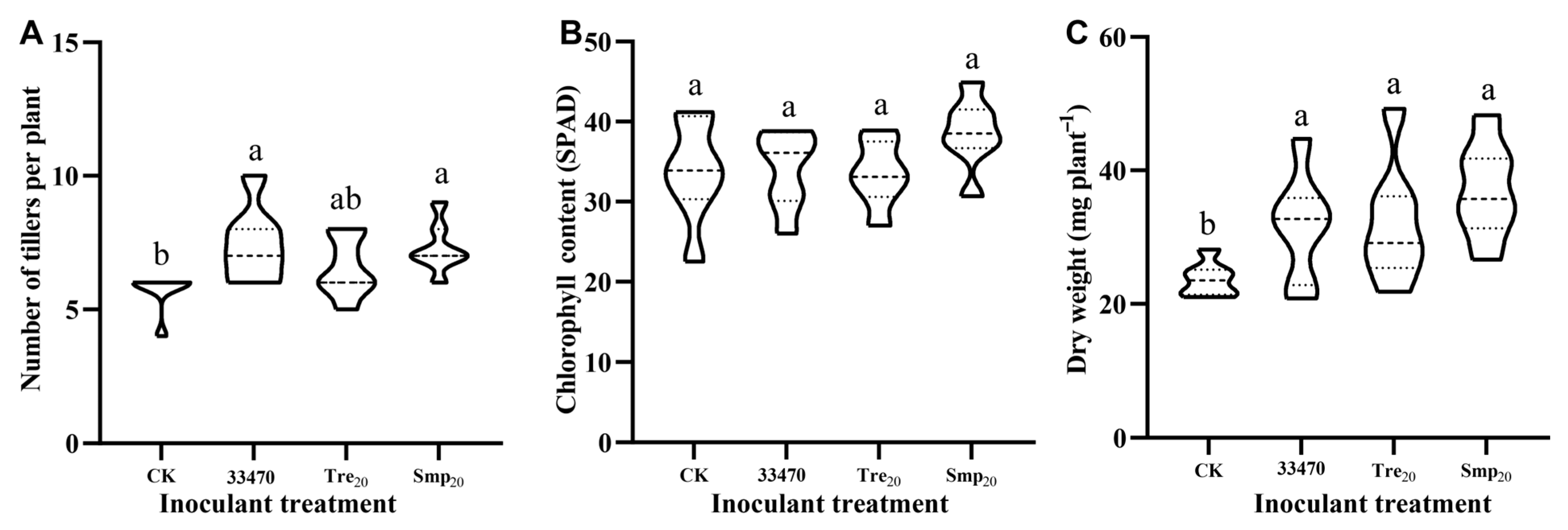


| Cryoprotectants | Present (%) | lg (CFU/g) | Cryoprotectants | Present (%) | lg (CFU/g) |
|---|---|---|---|---|---|
| Trehalose | 15 | 11.04 ± 0.04 | Sorbitol | 3 | / |
| 20 | 11.55 ± 0.02 | 5 | 11.73 ± 0.02 | ||
| 25 | 11.27 ± 0.05 | 7 | 11.67 ± 0.04 | ||
| Gelatin | 1 | 9.80 ± 0.08 | Betaine | 1 | 9.76 ± 0.14 |
| 2 | / | 2 | 11.62 ± 0.02 | ||
| 3 | 11.74 ± 0.03 | 3 | 10.03 ± 0.09 | ||
| Skimmed milk powder | 15 | 11.51 ± 0.06 | Polyvinylpyrrolidone K30 | 7 | 10.94 ± 0.02 |
| 20 | 11.58 ± 0.07 | 10 | 9.75 ± 0.07 | ||
| 25 | 11.31 ± 0.01 | 13 | / |
| Protectant | Mass Fraction (w/v, %) | ||
|---|---|---|---|
| Trehalose | 15 | 20 | 25 |
| Skimmed milk powder | 15 | 20 | 25 |
| Gelatin | 1 | 2 | 3 |
| Betaine | 1 | 2 | 3 |
| Sorbitol | 3 | 5 | 7 |
| Polyvinylpyrrolidone K30 | 7 | 10 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, D.-Y.; Chen, W.-F.; Yang, G.-P.; Li, Y.-G.; Zhang, J.-J. Screening and Application of Highly Efficient Rhizobia for Leguminous Green Manure Astragalus sinicus in Lyophilized Inoculants and Seed Coating. Plants 2025, 14, 2431. https://doi.org/10.3390/plants14152431
Xue D-Y, Chen W-F, Yang G-P, Li Y-G, Zhang J-J. Screening and Application of Highly Efficient Rhizobia for Leguminous Green Manure Astragalus sinicus in Lyophilized Inoculants and Seed Coating. Plants. 2025; 14(15):2431. https://doi.org/10.3390/plants14152431
Chicago/Turabian StyleXue, Ding-Yuan, Wen-Feng Chen, Guo-Ping Yang, You-Guo Li, and Jun-Jie Zhang. 2025. "Screening and Application of Highly Efficient Rhizobia for Leguminous Green Manure Astragalus sinicus in Lyophilized Inoculants and Seed Coating" Plants 14, no. 15: 2431. https://doi.org/10.3390/plants14152431
APA StyleXue, D.-Y., Chen, W.-F., Yang, G.-P., Li, Y.-G., & Zhang, J.-J. (2025). Screening and Application of Highly Efficient Rhizobia for Leguminous Green Manure Astragalus sinicus in Lyophilized Inoculants and Seed Coating. Plants, 14(15), 2431. https://doi.org/10.3390/plants14152431







