ZnO Nanoparticles: Advancing Agricultural Sustainability
Abstract
1. Introduction
2. Micronutrients: Plant Growth and Development
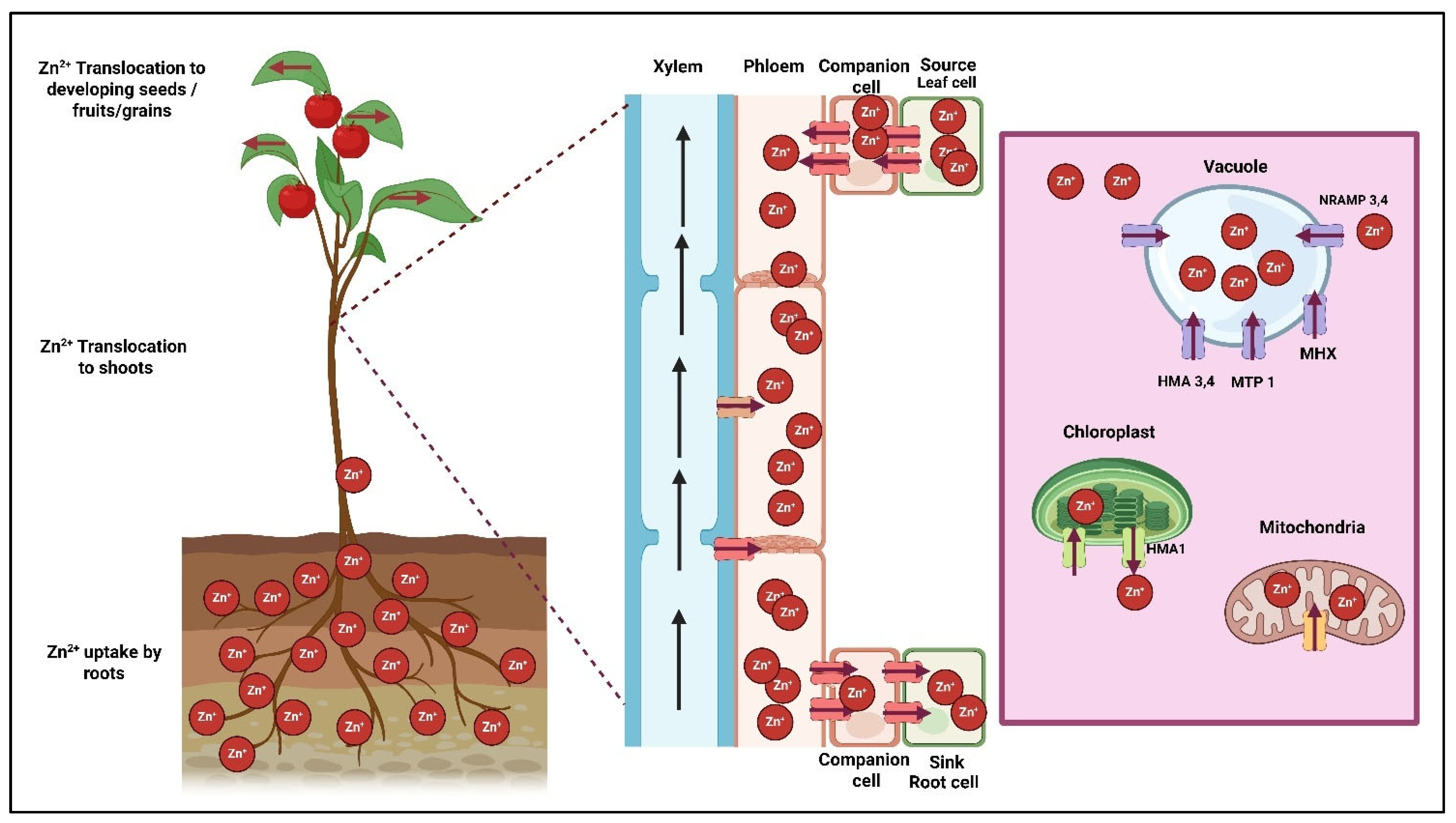
2.1. Zinc as Biocatalyst
2.2. Zinc in Hormone Regulation
2.3. Zinc for Abiotic and Biotic Stress
2.3.1. Abiotic Stress Regulation
2.3.2. Biotic Stress
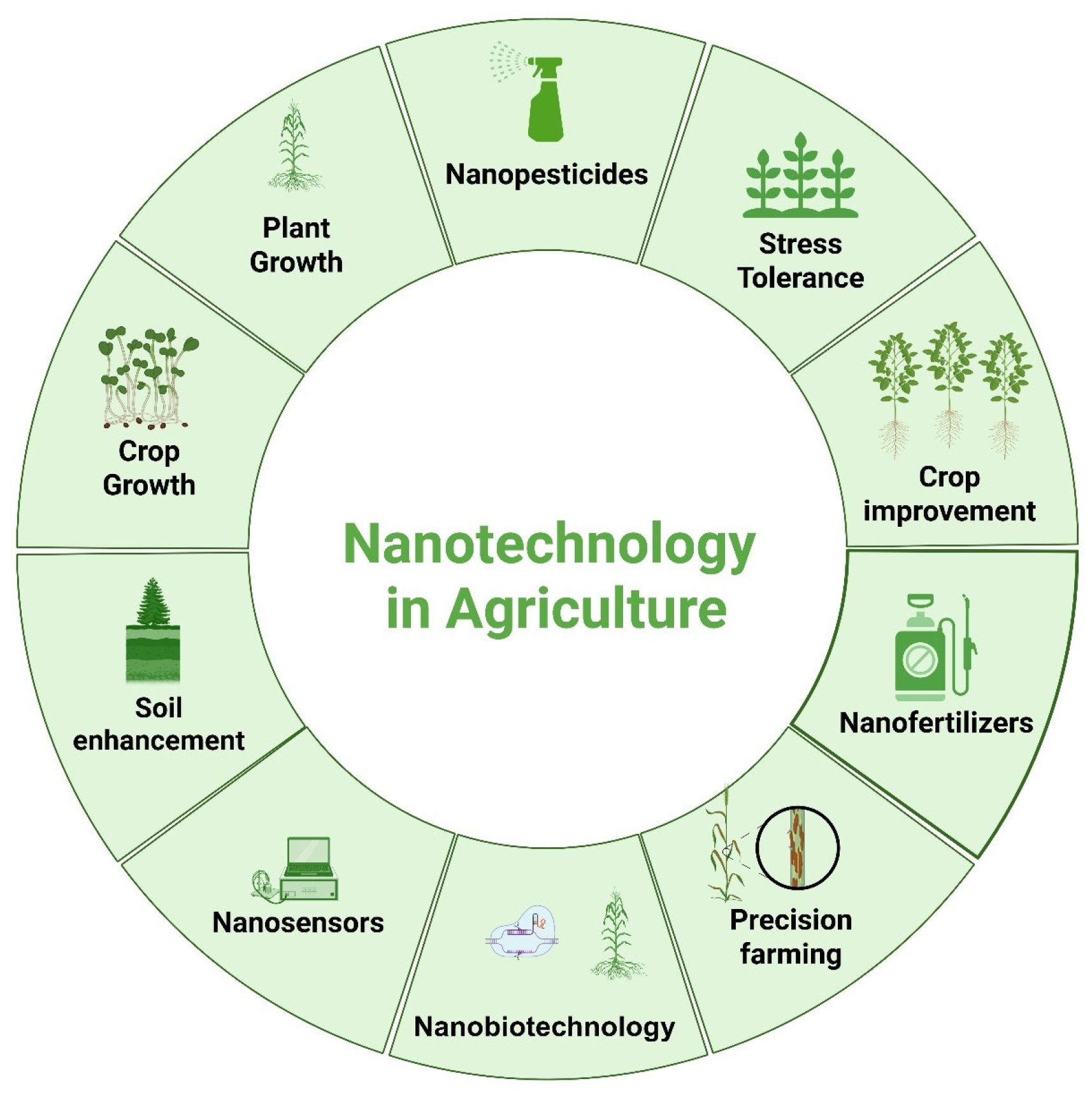
3. Nanotechnology
4. Mode of Applications of Zn Oxide (ZnO) Nanoparticles in Crop Protection
4.1. Seed Priming
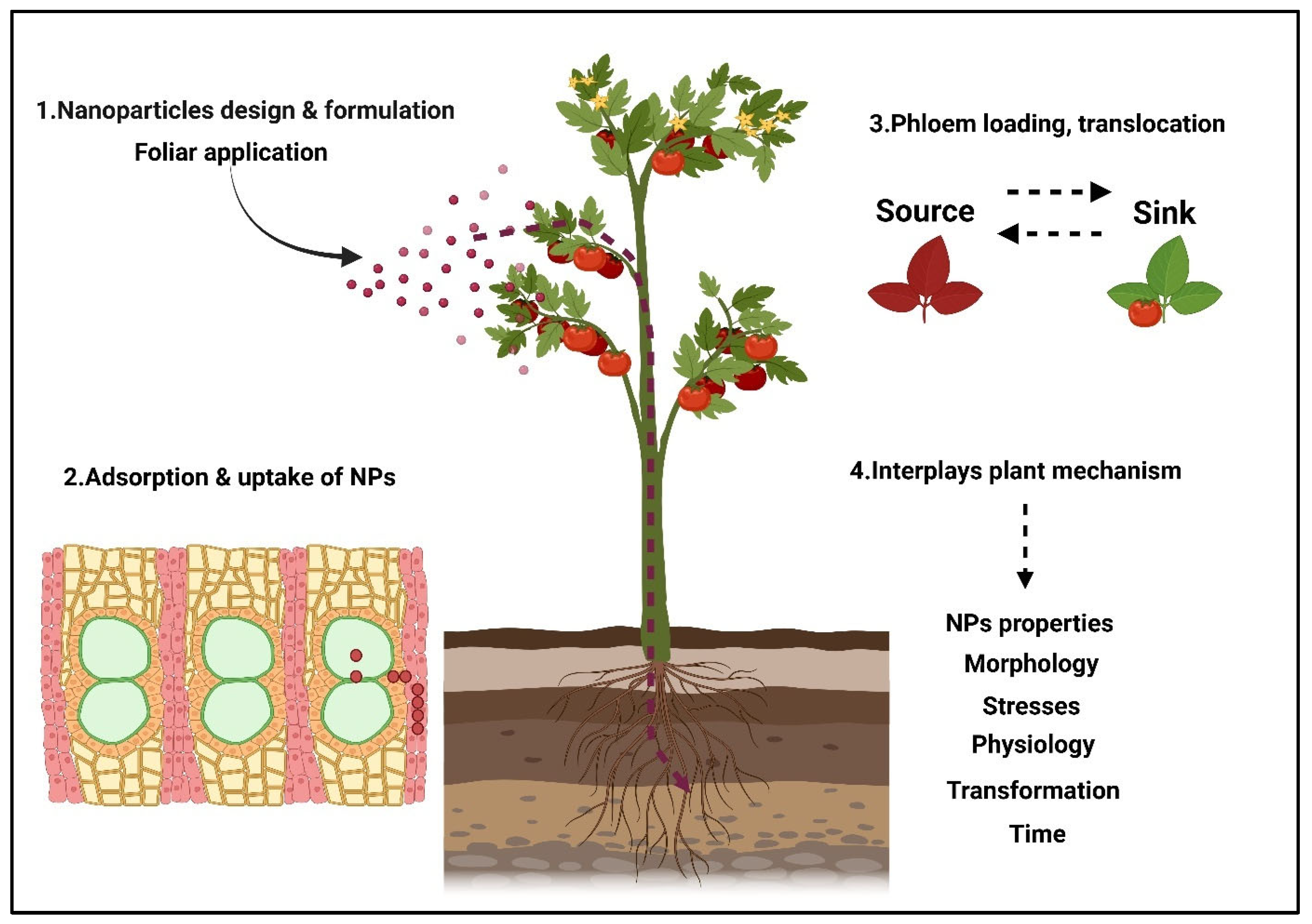
4.2. Foliar Sprays
5. Applications of Zinc Nanoparticles on Various Growth Parameters
5.1. Plant Growth and Development
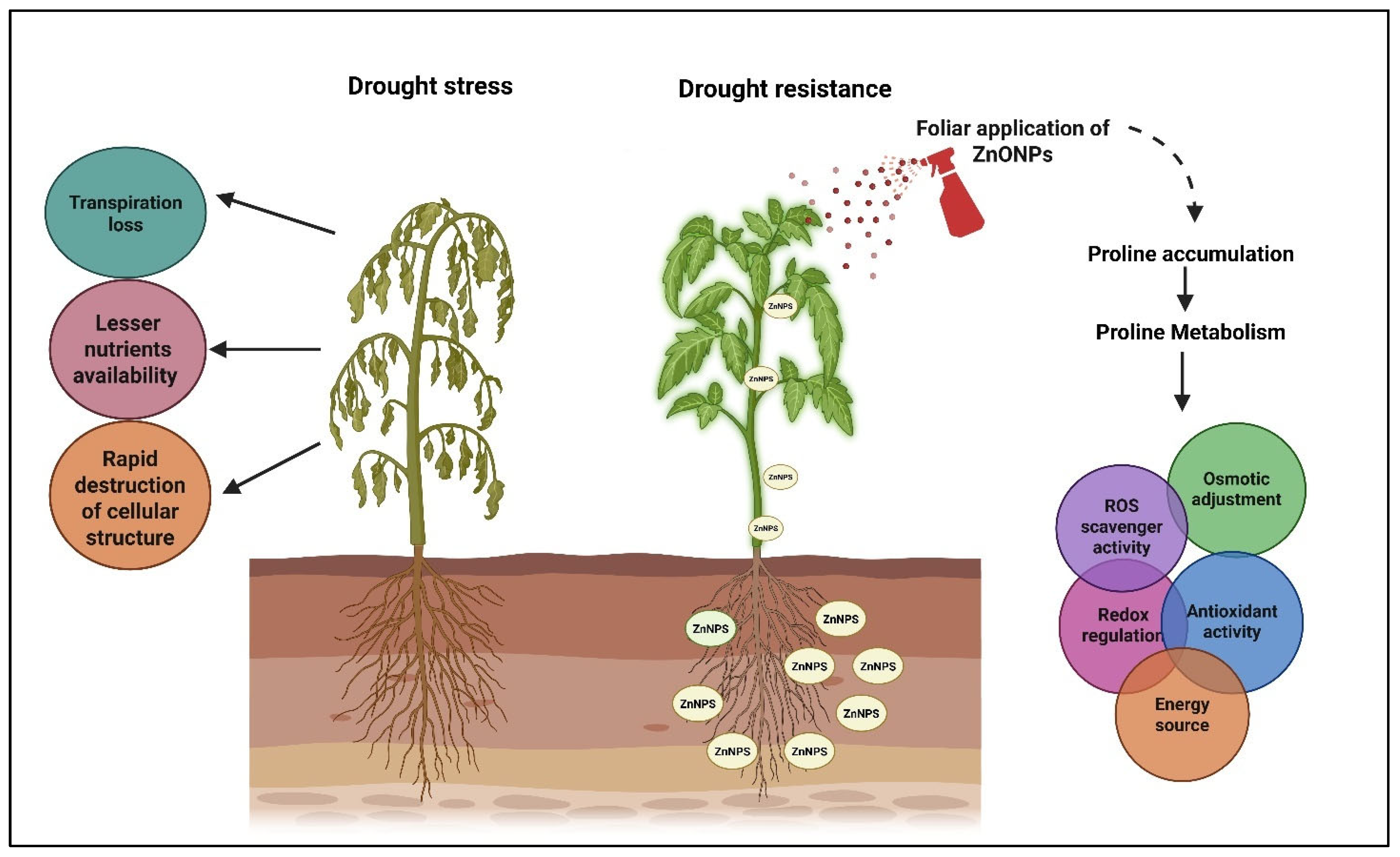
5.2. Nano Zinc Role in Abiotic Stress
5.3. Applications of Nano-Zinc in Biotic Stress
5.3.1. Fungus
5.3.2. Bacterial
5.3.3. Virus
5.3.4. Antimycotic and Mycotoxin Inhibiting Affinity
6. Applications of ZnONPs in Precision Agriculture for Efficient Food Production
Soil Remediation
7. Safety Measures for Active Delivery of ZnNPs in the Environment
8. Ecological Impacts
Toxicity Toward Non-Target Organisms
9. Future Perspectives and Research Opportunities
9.1. Advancements in Ecofriendly Synthesis
9.2. Future Scope and Research Opportunities
10. Conclusions
- Seed priming: ZnONPs have been studied for their positive effects in enhancing seed germination, showing improved seed vigor and viability. However, the concentration of ZnONPs is crucial in the seed priming process; lower dosages tend to show greater activity, while higher dosages may negatively affect seedling development.
- The present review highlights the importance of Zn as a master metallic catalyst, playing a key role in many biochemical reactions. It also emphasizes the role of Zn in the production of various phytohormones, which are essential for plant growth and development. The structural importance of Zn is particularly critical in plant resistance mechanisms, as many Zn-domain plant proteins are involved in expressing resistance to both abiotic and biotic stresses.
- The present review also emphasizes the entry of foliar-applied ZnONPs into the plant system and their response mechanisms in mitigating various abiotic stresses such as drought, salinity, ROS production, and the activation of stress-related enzymes. Their role in overcoming these stresses is clearly outlined.
- Role and importance of ZnONPs in mitigating biotic stress. The mode of action of these NPs against fungi, bacteria, and viruses has been studied, along with their actinomytic properties and effectiveness in countering myotoxicity, which are now better understood.
- It is anticipated that this review could further streamline research on innovative methodological alternatives for the delivery of ZnONPs, aiming for efficient application with minimal environmental risks.
Author Contributions
Funding
Conflicts of Interest
References
- Jat, M.; Saharawat, Y.; Gupta, R. Conservation agriculture in cereal systems of South Asia: Nutrient management perspectives. Karnataka J. Agric. Sci. 2011, 24, 100–105. [Google Scholar]
- Porter, J.; Xie, L.; Challinor, A.J.; Howden, M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects; Cambridge University Press: Cambridge, UK, 2014; pp. 485–533. [Google Scholar]
- Dahlberg, K. Beyond the Green Revolution: The Ecology and Politics of Global Agricultural Development; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 1996, 188, S86–S98. [Google Scholar] [CrossRef]
- Mohanty, L.K.; Singh, N.; Raj, P.; Prakash, A.; Tiwari, A.K.; Singh, V.; Sachan, P. Nurturing crops, enhancing soil health, and sustaining agricultural prosperity worldwide through agronomy. J. Exp. Agric. Int. 2024, 46, 46–67. [Google Scholar] [CrossRef]
- Kumar, S. <voice> Soil, soul and society. SANSAI Environ. J. Glob. Community 2012, 6, 5–13. [Google Scholar]
- Montgomery, D.R. Growing a Revolution: Bringing Our Soil Back to Life; WW Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- Stocking, M.A. Tropical soils and food security: The next 50 years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro-and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Reddy, M.P.; Sen, A.; Swamy, C.T.; Chelladurai, P.; Sivadurga, K.; Pandey, A.; Srivastava, R.; Kaur, A.; Purewal, S.S. Chemistry of Macro-and Micronutrients. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; CRC Press: Boca Raton, FL, USA, 2025; p. 96. [Google Scholar]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K. Impact of micronutrients in mitigation of abiotic stresses in soils and plants—A progressive step toward crop security and nutritional quality. Adv. Agron. 2022, 173, 1–78. [Google Scholar]
- Shafi, A.; Zahoor, I. Abiotic and biotic stress-induced alterations in the micronutrient status of plants. In Plant Micronutrients: Deficiency and Toxicity Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 285–309. [Google Scholar]
- Pandey, S.N. Biomolecular functions of micronutrients toward abiotic stress tolerance in plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 153–170. [Google Scholar]
- Fazeli-Nasab, B.; Piri, R.; Rahmani, A.F. Assessment of the role of rhizosphere in soil and its relationship with microorganisms and element absorption. In Plant Protection: From Chemicals to Biologicals; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022; p. 225. [Google Scholar]
- Pandey, S.N. Role of micronutrients in biochemical responses of crops under abiotic stresses. In Sustainable Agriculture in the Era of Climate Change; Springer International Publishing: Cham, Switzerland, 2020; pp. 93–112. [Google Scholar]
- Faye, O.; Baschieri, A.; Falkingham, J.; Muindi, K. Hunger and food insecurity in Nairobi’s slums: An assessment using IRT models. J. Urban Health 2011, 88, 235–255. [Google Scholar] [CrossRef]
- Thompson, B.; Cohen, M.J.; Meerman, J. World food insecurity and malnutrition: Scope, trends, causes and consequences. In The Impact of Climate Change and Bioenergy on Nutrition; Springer Science & Business Media: Berlin, Germany, 2012; pp. 21–41. [Google Scholar]
- Heaton, S. Organic Farming, Food Quality and Human Health: A review of the Evidence; Soil Association: Bristol, UK, 2001. [Google Scholar]
- Kumar, D.; Tripathi, D.K.; Chauhan, D.K. Phytoremediation potential and nutrient status of Barringtonia acutangula Gaerth. Tree seedlings grown under different chromium (CrVI) treatments. Biol. Trace Elem. Res. 2014, 157, 164–174. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, V.P.; Tripathi, D.K.; Prasad, S.M.; Chauhan, D.K. Effect of Arsenic on Growth, Arsenic Uptake, Distribution of Nutrient Elements and Thiols in Seedlings of Wrightia arborea (Dennst.) Mabb. Int. J. Phytoremediation 2015, 17, 128–134. [Google Scholar] [CrossRef]
- Liu, S.-L.; Yang, R.-J.; Ma, M.-D.; Dan, F.; Zhao, Y.; Jiang, P.; Wang, M.-H. Effects of exogenous NO on the growth, mineral nutrient content, antioxidant system, and ATPase activities of Trifolium repens L. plants under cadmium stress. Acta Physiol. Plant. 2015, 37, 1721. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of nutrients in plant growth and development. In Contaminants in Agriculture: Sources, Impacts and Management; Springer Nature: Berlin, Germany, 2020; pp. 43–59. [Google Scholar]
- Farhana; Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO nanoparticle-mediated seed priming induces biochemical and antioxidant changes in chickpea to alleviate Fusarium wilt. J. Fungi 2022, 8, 753. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Américo-Pinheiro, J.H.P.; Vo, D.-V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Qasim, M.; Akhtar, W.; Haseeb, M.; Sajjad, H.; Rasheed, M. Potential role of nanoparticles in plants protection. Life Sci. J. 2022, 19, 31–38. [Google Scholar]
- Muhammad, F.; Raza, M.A.S.; Iqbal, R.; Zulfiqar, F.; Aslam, M.U.; Yong, J.W.H.; Altaf, M.A.; Zulfiqar, B.; Amin, J.; Ibrahim, M.A. Ameliorating drought effects in wheat using an exclusive or co-applied rhizobacteria and ZnO nanoparticles. Biology 2022, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.; Mashwani, Z.-u.-R.; Poczai, P. Improvement of plant responses by nanobiofertilizer: A step towards sustainable agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Application of nanoparticles for enhanced UV-B stress tolerance in plants. Plant Nano Biol. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Chitena, L. Investigation of the Efficacy of a ZnO-Starch Nanocomposite Packaging to Prolong the Shelf Life of Fragaria ananassa (Strawberries); Botswana International University of Science and Technology: Palapye, Botswana, 2022. [Google Scholar]
- Donia, D.T.; Carbone, M. Seed priming with zinc oxide nanoparticles to enhance crop tolerance to environmental stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Hanif, S.; Javed, R.; Cheema, M.; Kiani, M.Z.; Farooq, S.; Zia, M. Harnessing the potential of zinc oxide nanoparticles and their derivatives as nanofertilizers: Trends and perspectives. Plant Nano Biol. 2024, 10, 100110. [Google Scholar] [CrossRef]
- Desai, S.; Singh, M.; Chavan, A.; Wagh, N.S.; Lakkakula, J. Micro-and nanoencapsulation techniques in agriculture. In Agricultural Nanobiotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–323. [Google Scholar]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Liyakat, K.S.S.; Liyakat, K.K.S. Nanosensors in Agriculture Field: A Study. Int. J. Appl. Nanotechnol. 2024, 10, 12–22. [Google Scholar]
- Kumar, P.; Chugh, P.; Ali, S.S.; Chawla, W.; Sushmita, S.; Kumar, R.; Raval, A.V.; Shamim, S.; Bhatia, A.; Kumar, R. Trends of nanobiosensors in modern agriculture systems. Appl. Biochem. Biotechnol. 2025, 197, 667–690. [Google Scholar] [CrossRef]
- Singh, B.; Natesan, S.K.A.; Singh, B.; Usha, K. Improving zinc efficiency of cereals under zinc deficiency. Curr. Sci. 2005, 88, 36–44. [Google Scholar]
- Samreen, T.; Shah, H.U.; Ullah, S.; Javid, M. Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mungbeans plant (Vigna radiata). Arab. J. Chem. 2017, 10, S1802–S1807. [Google Scholar] [CrossRef]
- Fei, X.; Fu, X.-Z.; Wang, N.-Q.; Xi, J.-L.; Huang, Y.; Wei, Z.; Ling, L.-L.; Peng, L.-Z. Physiological changes and expression characteristics of ZIP family genes under zinc deficiency in navel orange (Citrus sinensis). J. Integr. Agric. 2016, 15, 803–811. [Google Scholar] [CrossRef]
- Escudero-Almanza, D.J.; Ojeda-Barrios, D.L.; Hernández-Rodríguez, O.A.; Chávez, E.S.; Ruíz-Anchondo, T.; Sida-Arreola, J.P. Carbonic Anhydrase and Zinc in Plant Physiology; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2012. [Google Scholar]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc metalloenzymes in plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Bhatla, S.C.; Lal, M.A. Recently discovered plant growth regulators. In Plant Physiology, Development and Metabolism; Springer Nature: Berlin, Germany, 2018; pp. 681–728. [Google Scholar]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef]
- Di, D.-W.; Zhang, C.; Luo, P.; An, C.-W.; Guo, G.-Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Won, C.; Shen, X.; Mashiguchi, K.; Zheng, Z.; Dai, X.; Cheng, Y.; Kasahara, H.; Kamiya, Y.; Chory, J.; Zhao, Y. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18518–18523. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis; The Arabidopsis Book/American Society of Plant Biologists: Rockville, MD, USA, 2014; Volume 12, p. e0173. [Google Scholar]
- Ahmed, A.; Khalil, M.; Abd El-Rahman, A.; Nadia, A.H. Effect of Zinc, Tryptophan and Indole Acetic Acid on Growth, Yield and Chemical Composition of Valencia Orange Trees. J. Appl. Sci. Res. 2012, 910–914. Available online: http://www.aensionline.com/jasr/jasr/2012/901-914.pdf (accessed on 23 June 2025).
- Parthasarathy, A.; Cross, P.J.; Dobson, R.C.; Adams, L.E.; Savka, M.A.; Hudson, A.O. A three-ring circus: Metabolism of the three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Harris, D.; Rashid, A.; Arif, M.; Yunas, M. Alleviating micronutrient deficiencies in alkaline soils of the North-West Frontier Province of Pakistan: On-farm seed priming with zinc in wheat and chickpea. Micronutr. South South East Asia 2005, 143, 924–931. [Google Scholar]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Shah, H. ‘On-farm’seed priming with zinc sulphate solution—A cost-effective way to increase the maize yields of resource-poor farmers. Field Crops Res. 2007, 102, 119–127. [Google Scholar] [CrossRef]
- Hera, M.H.R.; Hossain, M.; Paul, A.K. Effect of foliar zinc spray on growth and yield of heat tolerant wheat under water stress. Int. J. Biol. Environ. Eng. 2018, 1, 10–16. [Google Scholar]
- Bajji, M.; Kinet, J.-M.; Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002, 36, 61–70. [Google Scholar] [CrossRef]
- Karam, F.; Lahoud, R.; Masaad, R.; Kabalan, R.; Breidi, J.; Chalita, C.; Rouphael, Y. Evapotranspiration, seed yield and water use efficiency of drip irrigated sunflower under full and deficit irrigation conditions. Agric. Water Manag. 2007, 90, 213–223. [Google Scholar] [CrossRef]
- Bagci, S.; Ekiz, H.; Yilmaz, A.; Cakmak, I. Effects of zinc deficiency and drought on grain yield of field-grown wheat cultivars in Central Anatolia. J. Agron. Crop Sci. 2007, 193, 198–206. [Google Scholar] [CrossRef]
- Babaeian, M.; Heidari, M.; Ghanbari, A. Effect of Water Stress and Foliar Micronutrient Application on Physiological Characteristics and Nutrient Uptake in Sunflower (Helianthus annus L.). Iran. J. Crop Sci. 2010, 12, 377–391. [Google Scholar]
- Ghanepour, S.; Shakiba, M.-R.; Toorchi, M.; Oustan, S. Role of Zn nutrition in membrane stability, leaf hydration status, and growth of common bean grown under soil moisture stress. J. Biodivers. Environ. Sci. 2015, 6, 9–20. [Google Scholar]
- DaCosta, M.; Huang, B. Osmotic adjustment associated with variation in bentgrass tolerance to drought stress. J. Am. Soc. Hortic. Sci. 2006, 131, 338–344. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Cai, C.; Chen, Z.; Zhu, C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol. Plant. 2019, 165, 690–700. [Google Scholar] [CrossRef]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Huang, J.; Guo, S.-Q.; Yang, X.; Bao, Y.-M.; Tang, H.-J.; Zhang, H.-S. Overexpression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L.). FEBS Lett. 2008, 582, 1037–1043. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 2012, 235, 1141–1155. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Li, L.; Shi, K.; Zheng, Y.; Sun, X. Drought stress tolerance mediated by zinc-induced antioxidative defense and osmotic adjustment in cotton (Gossypium hirsutum). Acta Physiol. Plant. 2015, 37, 167. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmad, F.; Abid, M.; Ullah, M.A. Impact of zinc fertilization on gas exchange characteristics and water use efficiency of cotton crop under arid environment. Pak. J. Bot. 2009, 41, 2189–2197. [Google Scholar]
- Tsonev, T.; Cebola Lidon, F.J. Zinc in plants-an overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Waraich, E.A.; Ahmad, R.; Ashraf, M. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Moradi, L.; Ehsanzadeh, P. Effects of Cd on photosynthesis and growth of safflower (Carthamus tinctorius L.) genotypes. Photosynthetica 2015, 53, 506–518. [Google Scholar] [CrossRef]
- Helfenstein, J.; Pawlowski, M.L.; Hill, C.B.; Stewart, J.; Lagos-Kutz, D.; Bowen, C.R.; Frossard, E.; Hartman, G.L. Zinc deficiency alters soybean susceptibility to pathogens and pests. J. Plant Nutr. Soil Sci. 2015, 178, 896–903. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Kalim, S.; Luthra, Y.; Gandhi, S. Cowpea root rot severity and metabolic changes in relation to manganese application. J. Phytopathol. 2003, 151, 92–97. [Google Scholar] [CrossRef]
- Nelson, P.N.; Webb, M.J.; Berthelsen, S.; Curry, G.; Yinil, D.; Fidelis, C. Nutritional Status of Cocoa in Papua New Guinea; Australian Centre for International Agricultural Research: Canberra, Australia, 2011. [Google Scholar]
- Gupta, S.K.; Rai, A.K.; Kanwar, S.S.; Sharma, T.R. Comparative Analysis of Zinc Finger Proteins Involved in Plant Disease Resistance. PLoS ONE 2012, 7, e42578. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yu, Y.P.; Wang, D.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zheng, C.C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef]
- Xu, C.; He, C. The rice OsLOL2 gene encodes a zinc finger protein involved in rice growth and disease resistance. Mol. Genet. Genom. 2007, 278, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Rai, A.K.; Kanwar, S.S.; Chand, D.; Singh, N.K.; Sharma, T.R. The single functional blast resistance gene Pi54 activates a complex defence mechanism in rice. J. Exp. Bot. 2012, 63, 757–772. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Feechan, A.; Yun, B.-W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.-Q.; Xie, Z.-S.; Pallas, J.A. S-nitrosylation of AtSABP3 antagonizes the expression of plant immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef]
- Bheemidi, V.S.; Tiruckovela, M.; Chettipalli, N.D.; Yanamadala, S.V. Novel applications of nanotechnology in life sciences. J. Bioanal. Biomed. 2011, 3. [Google Scholar] [CrossRef]
- Lekkala, V.V.; Reddy, M.C.; Reddy, V.C.; Kanthirigala, S.K.; Chitta, S.; Reddy, K.R.; Lomada, D. Advancements in nanoparticles-based therapies for biomedical applications. Nano-Struct. Nano-Objects 2024, 40, 101365. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Okitsu, K.; Mizukoshi, Y.; Yamamoto, T.A.; Maeda, Y.; Nagata, Y. Sonochemical synthesis of gold nanoparticles on chitosan. Mater. Lett. 2007, 61, 3429–3431. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef]
- Ahmed, S.; Qasim, S.; Ansari, M.; Shah, A.A.; Rehman, H.U.; Shah, M.N.; Ghafoor, U.; Naqvi, S.A.H.; Hassan, M.Z.; Ur Rehman, S. Green synthesis of zinc nanoparticles and their effects on growth and yield of Pisum sativum. J. King Saud Univ. Sci. 2022, 34, 102132. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Choudhary, N.; Alswieleh, A.M.; Sharma, A.K.; Bhardwaj, A.K.; Khan, S.H.; Yadav, K.K.; Cheon, J.-K.; Jeon, B.-H. Onion peel waste mediated-green synthesis of zinc oxide nanoparticles and their phytotoxicity on mung bean and wheat plant growth. Materials 2022, 15, 2393. [Google Scholar] [CrossRef] [PubMed]
- Asmat-Campos, D.; López-Medina, E.; Montes de Oca-Vásquez, G.; Gil-Rivero, E.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Villena-Zapata, L.; Gurreonero-Fernández, J.; Rafael-Amaya, R. ZnO Nanoparticles obtained by green synthesis as an alternative to improve the germination characteristics of L. esculentum. Molecules 2022, 27, 2343. [Google Scholar] [CrossRef] [PubMed]
- Azim, Z.; Singh, N.; Khare, S.; Singh, A.; Amist, N.; Yadav, R.K. Green synthesis of zinc oxide nanoparticles using Vernonia cinerea leaf extract and evaluation as nano-nutrient on the growth and development of tomato seedling. Plant Nano Biol. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef]
- Rashid, M.U.; Shah, S.J.; Attacha, S.; Khan, L.; Saeed, J.; Shah, S.T.; Mohamed, H.I. Green synthesis and characterization of zinc oxide nanoparticles using Citrus limetta peels extract and their antibacterial activity against brown and soft rot pathogens and antioxidant potential. Waste Biomass Valorization 2024, 15, 3351–3366. [Google Scholar] [CrossRef]
- Parveen, K.; Kumar, N.; Ledwani, L. Green Synthesis of Zinc Oxide Nanoparticles Mediated from Cassia renigera Bark and Detect Its Effects on Four Varieties of Rice. ChemistrySelect 2022, 7, e202200415. [Google Scholar] [CrossRef]
- Hossain, A.; Abdallah, Y.; Ali, M.A.; Masum, M.M.I.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-fruit-based green synthesis of zinc oxide nanoparticles and titanium dioxide nanoparticles against soft rot bacterial pathogen Dickeya dadantii. Biomolecules 2019, 9, 863. [Google Scholar] [CrossRef]
- Sharma, P.; Urfan, M.; Anand, R.; Sangral, M.; Hakla, H.R.; Sharma, S.; Das, R.; Pal, S.; Bhagat, M. Green synthesis of zinc oxide nanoparticles using Eucalyptus lanceolata leaf litter: Characterization, antimicrobial and agricultural efficacy in maize. Physiol. Mol. Biol. Plants 2022, 28, 363–381. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Fonseca, J.D.d.S.; Wojciechowska, E.; Kulesza, J.; Barros, B.S. Carbon Nanomaterials in Seed Priming: Current Possibilities. ACS Omega 2024, 9, 44891–44906. [Google Scholar] [CrossRef] [PubMed]
- Monreal, C.; DeRosa, M.; Mallubhotla, S.; Bindraban, P.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Small amounts of pesticides reaching target insects. Environ. Dev. Sustain. 2012, 14, 1–2. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of agrochemicals on soil health. In Agrochemicals Detection, Treatment and Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–187. [Google Scholar]
- Al-Juthery, H.; Hardan, H.; Al-Swedi, F.G.; Obaid, M.; Al-Shami, Q. Effect of foliar nutrition of nano-fertilizers and amino acids on growth and yield of wheat. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bali, Indonesia, 27–29 May 2019; p. 012046. [Google Scholar]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Li, J.; Lombi, E.; Bland, G.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation, and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef]
- Yu, M.; Yao, J.; Liang, J.; Zeng, Z.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H. Development of functionalized abamectin poly (lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017, 7, 11271–11280. [Google Scholar] [CrossRef]
- Knoche, M.; Petracek, P. Foliar uptake of PGRs: Barriers, mechanisms, model systems, and factors. In Proceedings of the XII International Symposium on Plant Bioregulators in Fruit Production, Orlando, FL, USA, 28 July 2013; pp. 125–141. [Google Scholar]
- Holloway, P. Surface Factors Affecting the Wetting of Leaves. Pestic. Sci. 1970, 1, 156–163. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Nadiminti, P.P.; Dong, Y.D.; Sayer, C.; Hay, P.; Rookes, J.E.; Boyd, B.J.; Cahill, D.M. Nanostructured liquid crystalline particles as an alternative delivery vehicle for plant agrochemicals. ACS Appl. Mater. Interfaces 2013, 5, 1818–1826. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Palocci, C.; Valletta, A.; Chronopoulou, L.; Donati, L.; Bramosanti, M.; Brasili, E.; Baldan, B.; Pasqua, G. Endocytic pathways involved in PLGA nanoparticle uptake by grapevine cells and role of cell wall and membrane in size selection. Plant Cell Rep. 2017, 36, 1917–1928. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 2003, 26, 103–124. [Google Scholar] [CrossRef]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.; Sajanlal, P.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Al-Jubori, Y.M.S. Effect of Fertilization with Conventional, Nano NPK and Nano Chelated Zinc on Growth and Yield of Kamali and Taifi Grape Cultivars. Ph.D. Thesis, University of Mosul College of Agriculture and Forestry, Mosul, Iraq, 2023. [Google Scholar]
- Haydar, M.S.; Kundu, S.; Kundu, S.; Mandal, P.; Roy, S. Zinc oxide nano-flowers improve the growth and propagation of mulberry cuttings grown under different irrigation regimes by mitigating drought-related complications and enhancing zinc uptake. Plant Physiol. Biochem. 2023, 202, 107910. [Google Scholar] [CrossRef]
- Guha, T.; Mukherjee, A.; Kundu, R. Nano-scale zero valent iron (nZVI) priming enhances yield, alters mineral distribution and grain nutrient content of Oryza sativa L. cv. Gobindobhog: A field study. J. Plant Growth Regul. 2022, 41, 710–733. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Jayaraj, M.; Manikandan, R.; Geetha, N.; Rene, E.R.; Sharma, N.; Sahi, S. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: A physiochemical analysis. Plant Physiol. Biochem. 2017, 110, 59–69. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; José-Yacamán, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515, 60–69. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41: Nanotechnology for Plant Growth and Development; Springer Nature: Berlin, Germany, 2020; pp. 83–99. [Google Scholar]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef]
- Pejam, F.; Ardebili, Z.O.; Ladan-Moghadam, A.; Danaee, E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE 2021, 16, e0248778. [Google Scholar] [CrossRef]
- Mahdavi, S.; Karimi, R.; Valipouri Goudarzi, A. Effect of nano zinc oxide, nano zinc chelate and zinc sulfate on vineyard soil Zn-availability and grapevines (Vitis vinifera L.) yield and quality. J. Plant Nutr. 2022, 45, 1961–1976. [Google Scholar] [CrossRef]
- Waqas Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar] [CrossRef]
- Jafir, M.; Khan, A.; Ahmad, A.; Hussain, K.; ur Rehman, M.Z.; Nazeer Ahmad, S.J.; Irfan, M.; Sabir, M.A.; Khan, T.H.; Zulfiqar, U. Zinc nanoparticles for enhancing plant tolerance to abiotic stress: A bibliometric analysis and review. J. Soil Sci. Plant Nutr. 2024, 24, 1704–1719. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Makhasha, E.; Al-Obeed, R.S. Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress. Horticulturae 2024, 10, 1096. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Abd El, A.E.-w.N.; Khalifa, S.M.; Alqahtani, M.D.; Abd–Alrazik, A.M.; Abdel-Aziz, H.; Mancy, A.; Elnaggar, I.A.; Alharbi, B.M.; Hamdy, A.; Elkelish, A. Nano-enhanced growth and resilience strategies for pomegranate Cv. wonderful: Unveiling the impact of zinc and boron nanoparticles on fruit quality and abiotic stress management. J. Agric. Food Res. 2024, 15, 100908. [Google Scholar] [CrossRef]
- Hussein, M.; Abou-Baker, N. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- Ahmed, R.; Zia-ur-Rehman, M.; Sabir, M.; Usman, M.; Rizwan, M.; Ahmad, Z.; Alharby, H.F.; Al-Zahrani, H.S.; Alsamadany, H.; Aldhebiani, A.Y. Differential response of nano zinc sulphate with other conventional sources of Zn in mitigating salinity stress in rice grown on saline-sodic soil. Chemosphere 2023, 327, 138479. [Google Scholar] [CrossRef]
- Mahmood, I.; Sami, A.; Asad, S.A.; Shah, G.A.; Rana, R.M.; Raja, N.I.; Sher, A.; Mashwani, Z.-u.-R.; Qayyum, A.; Iqbal, J. Zinc-Oxide-Nanoparticles in Conjugation with Zn-Solubilizing Bacteria Improve Zn Biofortification and Nitrogen Use Efficiency in Wheat. J. Soil Sci. Plant Nutr. 2024, 24, 5565–5585. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Deng, C.; Wang, Y.; Adisa, I.O.; Zhou, J.; White, J.C. Chitosan and zinc oxide nanoparticle-enhanced tripolyphosphate modulate phosphorus leaching in soil. ACS Agric. Sci. Technol. 2023, 3, 487–498. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants—Influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaidi, W.J.; Ali, A.M.; Muhsen, T.A. Efficacy of nanoparticle zinc oxide in the resistance of fungus Rhizoctonia solani causing black scurf disease in local potatoes. Casp. J. Environ. Sci. 2023, 21, 95–103. [Google Scholar]
- González-Merino, A.M.; Hernández-Juárez, A.; Betancourt-Galindo, R.; Ochoa-Fuentes, Y.M.; Valdez-Aguilar, L.A.; Limón-Corona, M.L. Antifungal activity of zinc oxide nanoparticles in Fusarium oxysporum-Solanum lycopersicum pathosystem under controlled conditions. J. Phytopathol. 2021, 169, 533–544. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. BioMetals 2022, 35, 601–616. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Bassiouni, S.; Elkhoby, W.; Zayed, B. Effect of zinc oxide nanoparticles on brown spot disease and rice productivity under saline soil. J. Plant Prot. Pathol. 2016, 7, 171–181. [Google Scholar] [CrossRef]
- Dhiman, S.; Singh, S.; Varma, A.; Goel, A. Phytofabricated zinc oxide nanoparticles as a nanofungicide for management of Alternaria blight of Brassica. Biometals 2021, 34, 1275–1293. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Khan, M.R.; Abd_Allah, E.F.; Parveen, A. Titanium dioxide and zinc oxide nanoparticles affect some bacterial diseases, and growth and physiological changes of beetroot. Int. J. Veg. Sci. 2019, 25, 409–430. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Khan, A.; Khan, M.; Abd-Allah, E. Effects of zinc oxide nanoparticles (ZnO NPs) and some plant pathogens on the growth and nodulation of lentil (Lens culinaris Medik.). Acta Phytopathol. Et Entomol. Hung. 2018, 53, 195–211. [Google Scholar] [CrossRef]
- Soliman, M.; Lee, B.; Ozcan, A.; Rawal, T.B.; Young, M.; Mendis, H.C.; Rajasekaran, P.; Washington, T.; Pingali, S.V.; O’Neill, H. Engineered zinc oxide-based nanotherapeutics boost systemic antibacterial efficacy against phloem-restricted diseases. Environ. Sci. Nano 2022, 9, 2869–2886. [Google Scholar] [CrossRef]
- Shantharaj, D.; Naranjo, E.; Merfa, M.V.; Cobine, P.A.; Santra, S.; De La Fuente, L. Zinc oxide-based nanoformulation zinkicide mitigates the xylem-limited pathogen Xylella fastidiosa in tobacco and southern highbush blueberry. Plant Dis. 2023, 107, 1096–1106. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Fan, G.; Liu, C.; Sun, X. Preventing viral disease by ZnONPs through directly deactivating TMV and activating plant immunity in Nicotiana benthamiana. Environ. Sci. Nano 2019, 6, 3653–3669. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.-A.M.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef]
- Cai, L.; Cai, L.; Jia, H.; Liu, C.; Wang, D.; Sun, X. Foliar exposure of Fe3O4 nanoparticles on Nicotiana benthamiana: Evidence for nanoparticles uptake, plant growth promoter and defense response elicitor against plant virus. J. Hazard. Mater. 2020, 393, 122415. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K.; Kokane, S.; Kumar, P.; Ozcan, A.; Warghane, A.; Motghare, M.; Santra, S.; Sharma, A.K. Antimicrobial nano-zinc oxide-2S albumin protein formulation significantly inhibits growth of “Candidatus Liberibacter asiaticus” in planta. PLoS ONE 2018, 13, e0204702. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Johnson, E.; Myers, M.E.; Young, M.; Rajasekaran, P.; Das, S.; Santra, S. Potential of nano-formulated zinc oxide for control of citrus canker on grapefruit trees. Plant Dis. 2016, 100, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Varympopi, A.; Dimopoulou, A.; Papafotis, D.; Avramidis, P.; Sarris, I.; Karamanidou, T.; Kerou, A.K.; Vlachou, A.; Vellis, E.; Giannopoulos, A. Antibacterial activity of copper nanoparticles against Xanthomonas campestris pv. vesicatoria in tomato plants. Int. J. Mol. Sci. 2022, 23, 4080. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A.; Shoala, T.; Ali, A.A.; Alaa, M.; Abd-Elsalam, K. Chemically-Produced Copper, Zinc Nanoparticles and Chitosan-Bimetallic Nanocomposites and Their Antifungal Activity Against Three Phytopathogenic Fungi. Int. J. Agric. Technol. 2017, 13, 753–769. [Google Scholar]
- Patra, P.; Mitra, S.; Debnath, N.; Goswami, A. Biochemical-, biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: An in vivo and in vitro toxicity study. Langmuir 2012, 28, 16966–16978. [Google Scholar] [CrossRef]
- Zabrieski, Z.; Morrell, E.; Hortin, J.; Dimkpa, C.; McLean, J.; Britt, D.; Anderson, A. Pesticidal activity of metal oxide nanoparticles on plant pathogenic isolates of Pythium. Ecotoxicology 2015, 24, 1305–1314. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Britt, D.W.; Anderson, A.J. Antifungal activity of ZnO nanoparticles and their interactive effect with a biocontrol bacterium on growth antagonism of the plant pathogen Fusarium graminearum. Biometals 2013, 26, 913–924. [Google Scholar] [CrossRef]
- Elsharkawy, M.M. Antiviral Activity of Nanoparticles in Plants. In Nanotechnology in Plant Health; CRC Press: Boca Raton, FL, USA, 2024; pp. 285–299. [Google Scholar]
- Wagner, G.; Korenkov, V.; Judy, J.D.; Bertsch, P.M. Nanoparticles composed of Zn and ZnO inhibit Peronospora tabacina spore germination in vitro and P. tabacina infectivity on tobacco leaves. Nanomaterials 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.A.; Manan, R.; Aslam Mirza, M.; Rashid Khan, H.; Qayyum, S.; Ahmed, Z. Biogenic synthesis of copper oxide and zinc oxide nanoparticles and their application as antifungal agents. Int. J. Mater. Sci. Eng. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Yehia, R.S.; Ahmed, O.F. In vitro study of the antifungal efficacy of zinc oxide nanoparticles against Fusarium oxysporum and Penicilium expansum. Afr. J. Microbiol. Res 2013, 7, 1917–1923. [Google Scholar]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Malik, A.; Alwarthan, A.; Shaik, M.R. The enormity of the zinc deficiency problem and available solutions; an overview. Arab. J. Chem. 2022, 15, 103668. [Google Scholar] [CrossRef]
- Arunachalam, P.; Kannan, P.; Prabukumar, G.; Govindaraj, M. Zinc deficiency in Indian soils with special focus to enrich zinc in peanut. Afr. J. Agric. Res. 2013, 8, 6681–6688. [Google Scholar]
- Rodriguez, H.G.; Ramanjaneyulu, A.; Sarkar, N.C.; Maiti, R. Advances in Agro-Technology; Puspa Publishing House: Kolkata, India, 2013. [Google Scholar]
- Elsharkawy, M.; Derbalah, A.; Hamza, A.; El-Shaer, A. Zinc oxide nanostructures as a control strategy of bacterial speck of tomato caused by Pseudomonas syringae in Egypt. Environ. Sci. Pollut. Res. 2020, 27, 19049–19057. [Google Scholar] [CrossRef]
- Hassan, M.; Marwa, A.; El-Feky, S. Role of green synthesized ZnO nanoparticles as antifungal against postharvest gray and black mold of sweet bell pepper. J. Biotechnol. Bioeng. 2019, 3, 8–15. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Brar, P.S.; Chauhan, A.; Bhardwaj, M. Comparison of fruit and quality parameters of tomato and capsicum by using different amount of nano ZnO through foliar and seed application. J. Plant Nutr. 2024, 47, 3767–3776. [Google Scholar] [CrossRef]
- Al-Tihafi, S.; Riad, K. Effect of incubation and spraying with zinc in the growth and yield of the sweet peppers (Qurtuba cultivar) cultivated inside the plastic house under drip irrigation system. J. Babylon Univ. Sci. 2007, 14, 447–453. [Google Scholar]
- Mehmood, S.; Ahmed, W.; Rizwan, M.; Bundschuh, J.; Elnahal, A.S.; Li, W. Green synthesized zinc oxide nanoparticles for removal of carbamazepine in water and soil systems. Sep. Purif. Technol. 2024, 334, 125988. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Liu, Y.-H.; Chu, T.-T.; Young, S.-J. Improved UV-sensing of Au-decorated ZnO nanostructure MSM photodetectors. IEEE Sens. J. 2022, 22, 5644–5650. [Google Scholar] [CrossRef]
- Bhadra, J.; Ponnamma, D.; Alkareem, A.; Parangusan, H.; Ahmad, Z.; Al-Thani, N.; Daifalla, A.K.; Al-Sanari, N.A.; Mohamed, R. Development of a piezoelectric nanogenerator based on mesoporous silica/zinc oxide hybrid nanocomposite fibres. Int. J. Energy Res. 2022, 46, 8503–8515. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef]
- Özkan, Y.; Irende, İ.; Akdeniz, G.; Kabakçi, D.; Sökmen, M. Evaluation of the comparative acute toxic effects of TiO2, Ag-TiO2 and ZnO-TiO2 composite nanoparticles on honey bee (Apis mellifera). J. Int. Environ. Appl. Sci. 2014, 10, 26–36. [Google Scholar]
- Amini-Esfidvajani, M.-B.; Sadeghi, A.A.; Shawrang, P.; Chamani, M.; Aminafshar, M. Effect of nano-particles of zinc oxide and selenium on antioxidant status, aminotransferase enzymes activities and genes expression of sod-1 and vg in honey bee during the hot season. J. Trace Elem. Miner. 2022, 2, 100034. [Google Scholar] [CrossRef]
- Karim, A.S.; Abdullah, F.O.; Mohammad, N.Z.M. Green Synthesis and Evaluation of ZnO NPs and study the effect of Their toxic on Honey Bee (Apis mellifera). Baghdad Sci. J. 2024, 21, 2124. [Google Scholar] [CrossRef]
- Milivojević, T.; Glavan, G.; Božič, J.; Sepčić, K.; Mesarič, T.; Drobne, D. Neurotoxic potential of ingested ZnO nanomaterials on bees. Chemosphere 2015, 120, 547–554. [Google Scholar] [CrossRef]
- Ahmed, S.; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.; Turney, T.W. Reducing ZnO nanoparticle cytotoxicity by surface modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, J.; Yao, H.; Li, Z.; Wei, J. An efficient method to improve the dispersion and biocompatibility of ZnO nanoparticles. J. Dispers. Sci. Technol. 2025, 46, 289–296. [Google Scholar] [CrossRef]
- Kalpana, V.; Devi Rajeswari, V. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, Z.; Hou, Z.; Li, T.; Lu, X. Ecotoxicological effect of zinc oxide nanoparticles on soil microorganisms. Front. Environ. Sci. Eng. 2015, 9, 912–918. [Google Scholar] [CrossRef]
- Ge, Y.; Schimel, J.P.; Holden, P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011, 45, 1659–1664. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Tsai, Y.-C.; Hsiung, C.-E.; Lin, Y.-H.; Shih, Y.-h. Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J. Hazard. Mater. 2017, 322, 348–356. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants–a soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Lahive, E.; Matzke, M.; Svendsen, C.; Spurgeon, D.; Pouran, H.; Zhang, H.; Lawlor, A.; Pereira, M.G.; Lofts, S. Soil properties influence the toxicity and availability of Zn from ZnO nanoparticles to earthworms. Environ. Pollut. 2023, 319, 120907. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Lee, I. Effects of Zn and ZnO nanoparticles and Zn2+ on soil enzyme activity and bioaccumulation of Zn in Cucumis sativus. Chem. Ecol. 2011, 27, 49–55. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K.; Sharma, A.K. Myconanotechnology in agriculture: A perspective. World J. Microbiol. Biotechnol. 2013, 29, 191–207. [Google Scholar] [CrossRef]
- Fayaz, A.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.; Venketesan, R. Nanomedicine Nanotechnology. Biol. Med. 2010, 6, 103–109. [Google Scholar]
- Hemath Naveen, K.; Kumar, G.; Karthik, L.; Bhaskara Rao, K. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch. Appl. Sci. Res. 2010, 2, 161–167. [Google Scholar]
- Jaidev, L.; Narasimha, G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf. B Biointerfaces 2010, 81, 430–433. [Google Scholar] [CrossRef]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostructure Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Lekkala, V.V.; Sirigireddy, B.; Reddy, M.C.; Lomada, D. Synthesis and Characterization of Silver and Zinc Nanoparticles From Vitex altissima: Comparative Analysis of Anti-Oxidant, Anti-Inflammatory, Antibacterial, and Anti-Biofilm Activities. Chem. Biodivers. 2025, 22, e202402166. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
| S. No | Application Area | Example/Study | References |
|---|---|---|---|
| 1 | Fertilizer Efficiency Enhancement | Increased growth and yield in wheat and maize due to improved root development. | [25,26] |
| 2 | Pest and Disease Management | Formulation of ZnONPs in sprays for fungal and bacterial infection protection and insect repulsion. | [27] |
| 3 | Soil Health Improvement | Improved soil fertility in zinc-deficient soils and better nutrient availability. | [28,29] |
| 4 | UV Protection for Crops | Application of ZnONPs on tomato and strawberry plants to reduce UV stress. | [30,31] |
| 5 | Seed Germination and Growth Promotion | Faster germination and more robust seedlings in wheat treated with ZnONPs. | [32] |
| 6 | Water Management | Development of ZnONPs-based materials for consistent water supply in crops during dry periods. | [33] |
| 7 | Nano-Encapsulation of Agrochemicals | Nano-encapsulation of herbicides and insecticides using ZnONPs for enhanced efficacy. | [34] |
| 8 | Biotic and Abiotic Stress Tolerance | Application of ZnONPs in soybean for drought resistance and in rice for salinity tolerance. | [35] |
| 9 | Nano-biosensors for Precision Agriculture | Development of nano-biosensors using ZnONPs for optimizing agricultural inputs in precision farming. | [36,37] |
| S. No | Synthesized Plant Species | Crop Species | Changes Observed | Reference |
|---|---|---|---|---|
| 1 | Syzygium aromaticum L. | Pisum sativum L. | Increase the growth and yield as well as productivity of staple crops. | [86] |
| 2 | Bio-inspired chitosan and Zn nanoparticles | Solanum lycopersicum L. | Suppressed Xoo infections in rice. | [87] |
| 3 | Onion Peel Extract | Vigna radiate and Triticum aestivum | Enhances the seedling growth and germination percentage and fresh as well as dry weight. | [88] |
| 4 | Coriandrum sativum | Lycopersicon esculentum | Show an excellent promotion of enzymatic and metabolic activity to achieve cell elongation. | [89] |
| 5 | Vernonia cinerea | Tomato seedling | Boost up the growth and development. | [90] |
| 6 | Eucalyptus globules | Alternaria leaf blotch—in Apple | ZnNPs damage the surface of the fungal hyphae, thereby discharging cellular materials, ensuing in the contraction of hyphae. | [91] |
| 7 | Citrus limetta Peels Extract | Solanum tuberosum L. | Show potential in vitro and in vivo antibacterial activity reduces the fungal infections. | [92] |
| 8 | Cassia renigera Bark | Xanthomonas oryzae | Improvement in germination rate, moisture rate, and growth rate (shoot/root length, number of leaves). | [93] |
| 9 | Citrus limon | Dickeya dadantii | Inhibited growth, swimming motility, biofilm formation, and maceration of sweet potato tubers and likely combat the bacterial pathogen via multiple mechanisms. | [94] |
| 10 | Eucalyptus lanceolata leaf litter | Zea mays L. | Increase grain yield compared with the controls. | [95] |
| S. No | Nanoparticles | Crop Species | Observed Changes | Reference |
|---|---|---|---|---|
| 1 | ZnO (0, 1000, and 3000 ppm) | Trigonella foenum-graecum | Reversed salinity-induced consequences | [138] |
| 2 | Zn-, B-, Si-, and Zeolite NPs | Solanum tuberosum L., Diamont cultivar | Increase (Relative Water content) RWC, Proline, Chlorophyll | [139] |
| 3 | ZnO (50, 100, and 150 mg/L) and Si (150 and 300 mg/L) | Mangifera indica L. | Application of both NPs enhanced leaf NPK content | [140] |
| 4 | ZnO (0, 25, 50, and 100 mg/L) | T. aestivum L. | Foliar spray enhanced chlorophyll content, and the activities of (Superoxide dismutase) SOD and (Peroxidase) POXs | [141] |
| 5 | ZnONPs (50 and 100 ppm) | Solanum melongena L. | Improved in uptake of macro- and micronutrients also increased (Relative Water content) RWC in leaves | [142] |
| 6 | ZnO (25 mg/L) | Leucaena leucocephal | Improved pigments and soluble proteins, reduced peroxidation | [120] |
| 7 | ZnO (0, 50, and 100 mg L−1) | G. max | Improved root and shoot growth | [143] |
| S. No | Zinc NPs | Pathogen Studied | Impact | Reference |
|---|---|---|---|---|
| Bacterial | ||||
| 1 | Zinkicide | X. alfalfae subsp. citrumelonis | 7/8-fold lower MIC | [158] |
| 2 | ZnONPs | Xanthomonas axonopodis pv. phaseoli | Reduction in disease severity on pathogen challenge | [151] |
| 3 | ZnONPs | Xanthomonas oryzae pv. oryzae | Antimicrobial agent for bacterial leaf blight of rice | [159] |
| 4 | Cu-Zn hybrid NPs | Xanthomonas perforans (Cu tolerant GEV485) | Complete inhibition of growth till 24 h of incubation | [160] |
| Fungal | ||||
| 5 | ZnONPs | Alternaria alternata | Mean inhibition rate (EC50) range 235 and 848 g/mL higher efficacy compared to ZnSO4 | [161] |
| 6 | ZnONPs/CS-Zn-CuNPs | Alternaria alternata, B. cinerea, R. solani | Highest mycelial inhibition by chitosan mixed Zn-Cu nanocomposite | [162] |
| 7 | ZnONPs | Aspergillus niger | Dose-dependent decrease in radial growth diameter | [163] |
| 8 | ZnO and CuO NPs | Pythium ultimum, Pythium aphanidermatum | Inhibition of growth at low concentrations-morphological changes in the hyphae | [164] |
| 9 | ZnONPs | Fusarium graminearum | Dose-dependent inhibition of fungal growth | [165] |
| Virus | ||||
| 10 | ZnONPs | TMV | Deactivating TMV and activating plant immunity in Nicotiana benthamiana | [154] |
| 11 | ZnONPs | Tomato Bushy Stunt Virus | Activating RNA interference in plants to guard antiviral protection | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravishankar, L.V.; Puranik, N.; Lekkala, V.V.V.; Lomada, D.; Reddy, M.C.; Maurya, A.K. ZnO Nanoparticles: Advancing Agricultural Sustainability. Plants 2025, 14, 2430. https://doi.org/10.3390/plants14152430
Ravishankar LV, Puranik N, Lekkala VVV, Lomada D, Reddy MC, Maurya AK. ZnO Nanoparticles: Advancing Agricultural Sustainability. Plants. 2025; 14(15):2430. https://doi.org/10.3390/plants14152430
Chicago/Turabian StyleRavishankar, Lekkala Venkata, Nidhi Puranik, VijayaDurga V. V. Lekkala, Dakshayani Lomada, Madhava C. Reddy, and Amit Kumar Maurya. 2025. "ZnO Nanoparticles: Advancing Agricultural Sustainability" Plants 14, no. 15: 2430. https://doi.org/10.3390/plants14152430
APA StyleRavishankar, L. V., Puranik, N., Lekkala, V. V. V., Lomada, D., Reddy, M. C., & Maurya, A. K. (2025). ZnO Nanoparticles: Advancing Agricultural Sustainability. Plants, 14(15), 2430. https://doi.org/10.3390/plants14152430






