Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves
Abstract
1. Introduction
2. Results
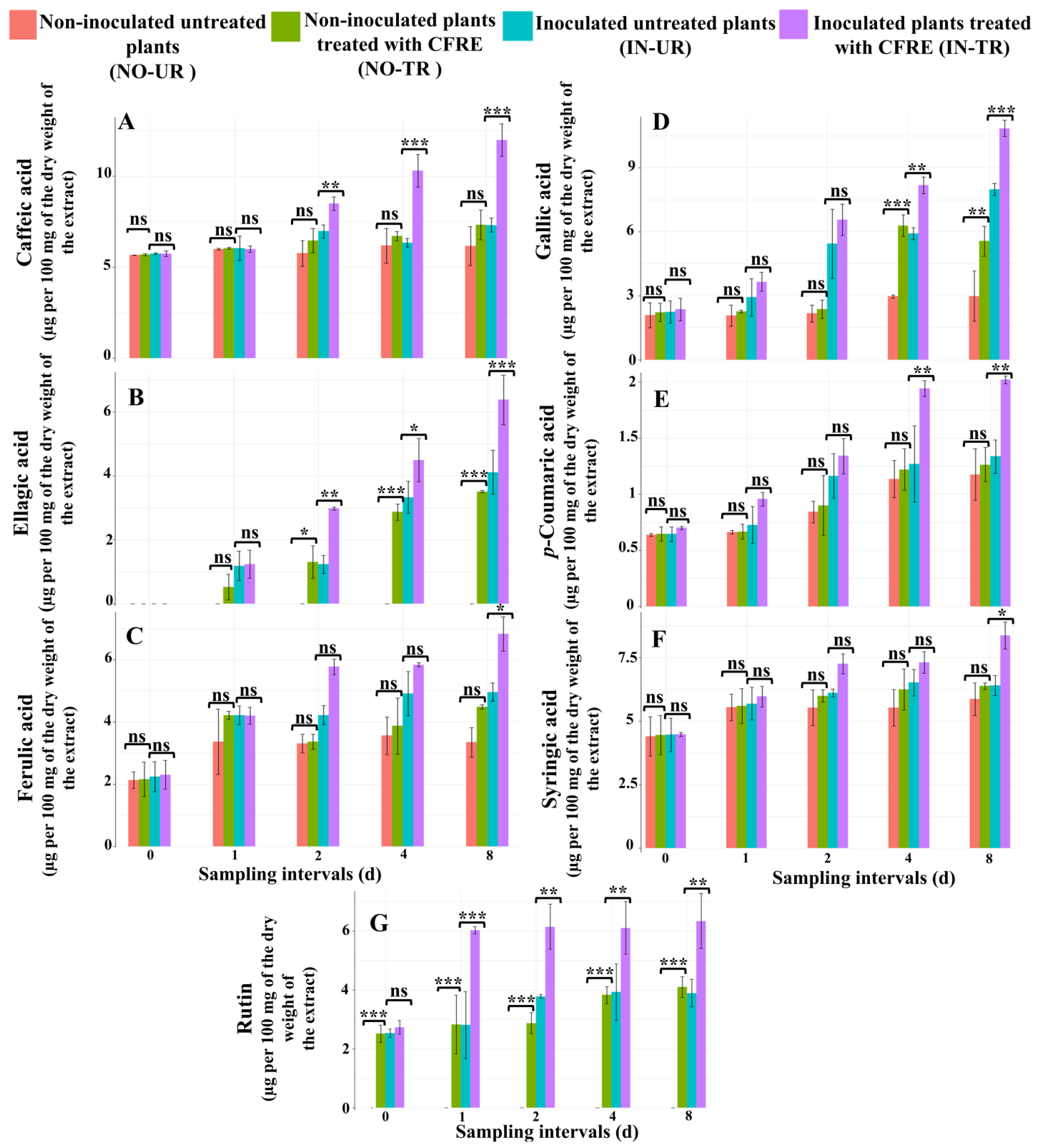
2.1. Chemical Analysis of Phytoalexins in Cucumber Leaves Induced by CFRE
2.1.1. Analysis of the Concentration of Phenolic Acid Compounds in Cucumber Leaves
Caffeic Acid
Ellagic Acid
Ferulic Acid
Gallic Acid
p-Coumaric Acid
Syringic Acid
2.1.2. Analysis of the Concentration of Flavonoid Compounds in Cucumber Leaves
Luteolin, Quercetin, and Rutin
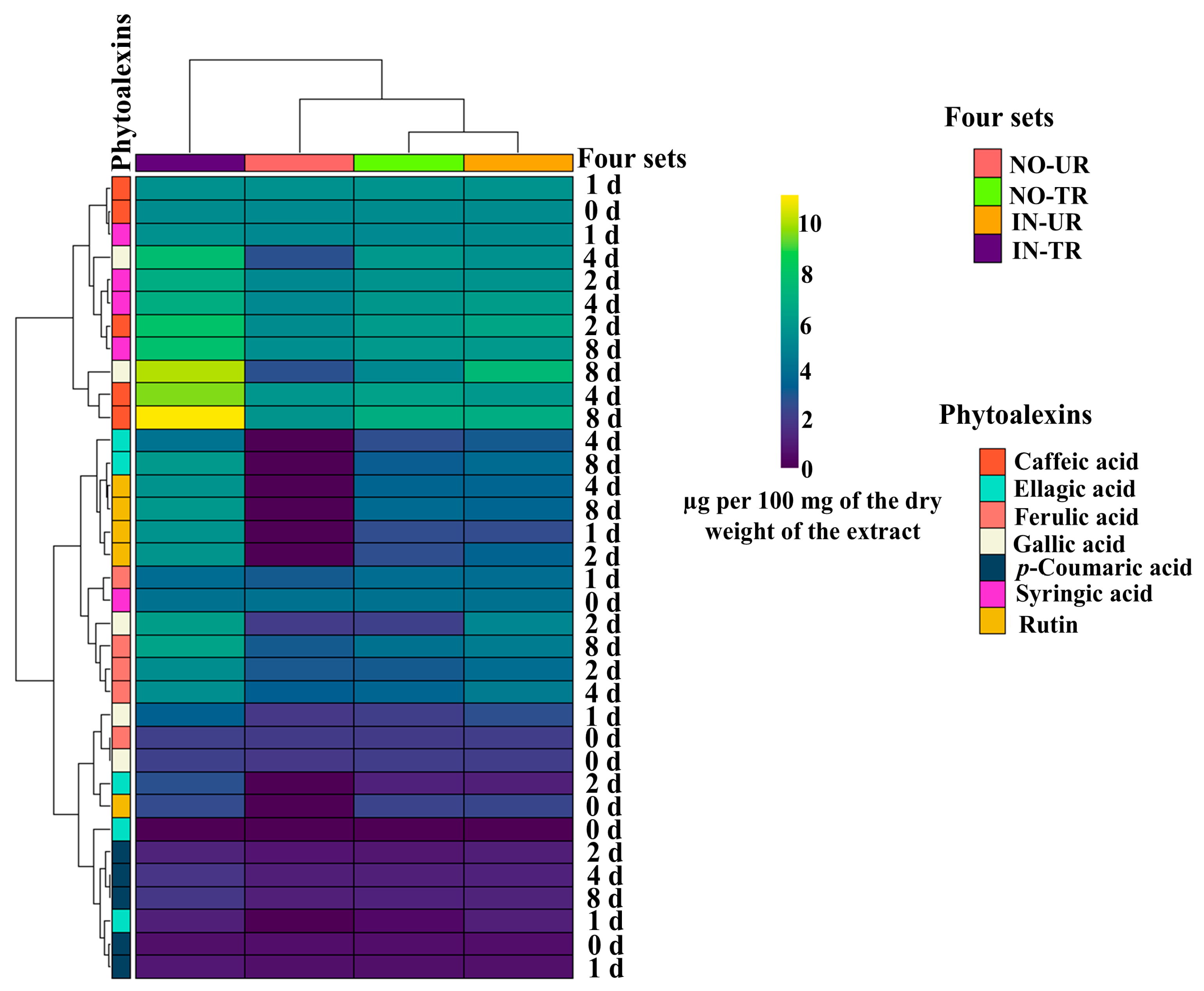
2.2. Heatmap of Phytoalexins in Cucumber Leaves Induced by CFRE
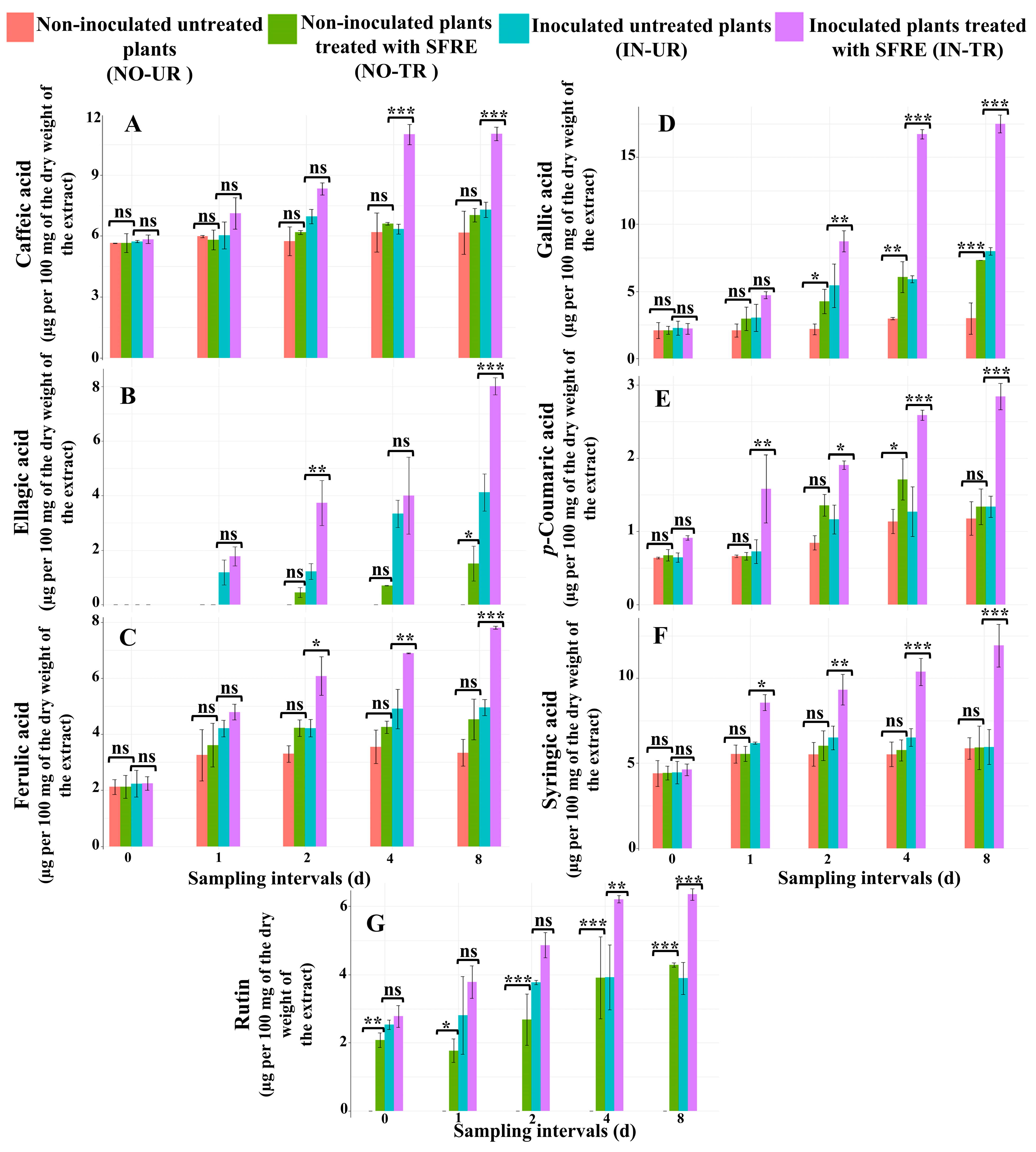
2.3. Chemical Analysis of Phytoalexins in Cucumber Leaves Induced by SFRE
2.3.1. Analysis of the Concentration of Phenolic Acid Compounds in Cucumber Leaves
Caffeic Acid
Ellagic Acid
Ferulic Acid
Gallic Acid
p-Coumaric Acid
Syringic Acid
2.3.2. Analysis of the Concentration of Flavonoid Compounds in Cucumber Leaves
Luteolin, Quercetin, and Rutin
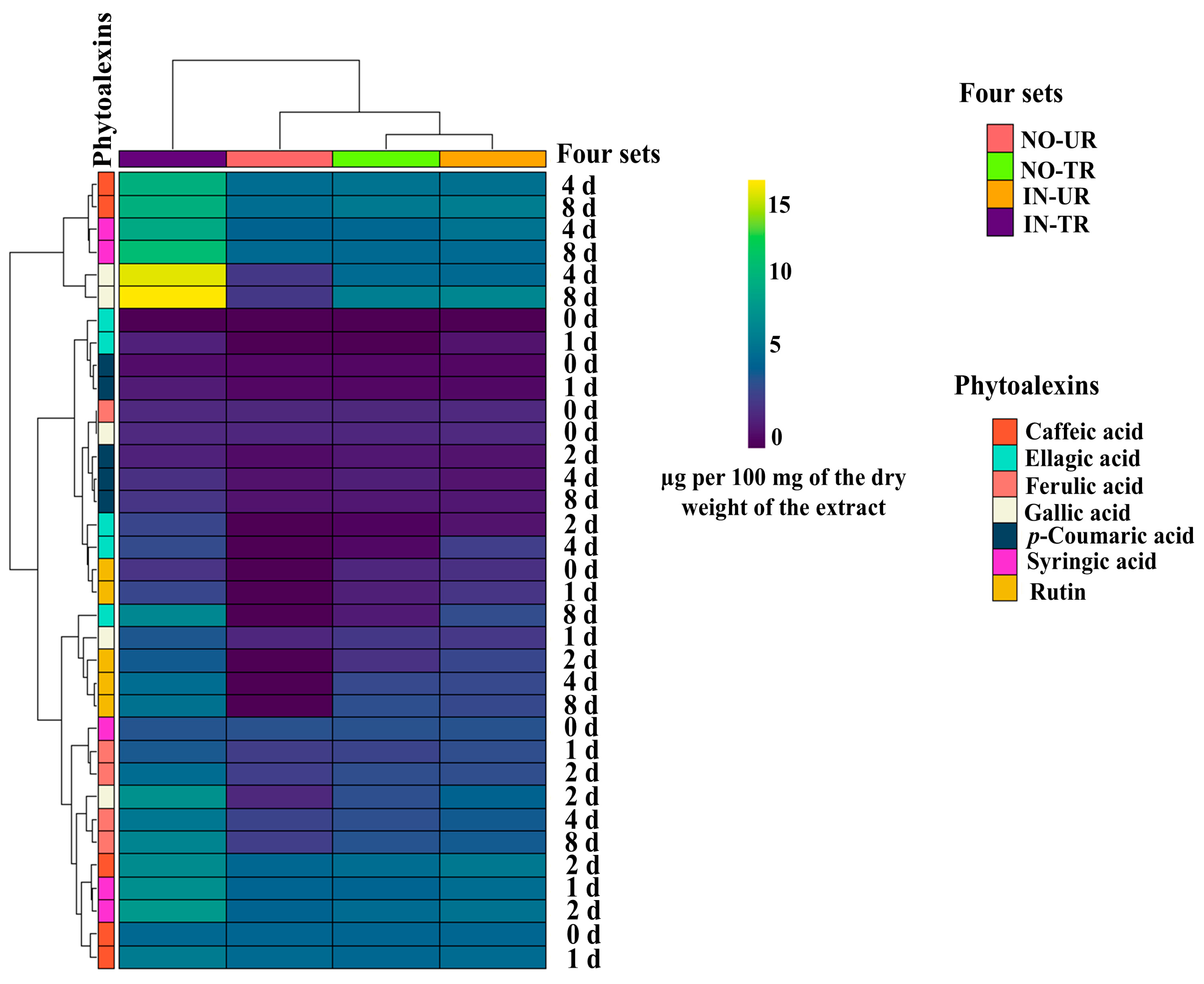
2.4. Heatmap of Phytoalexins in Cucumber Leaves Induced by SFRE
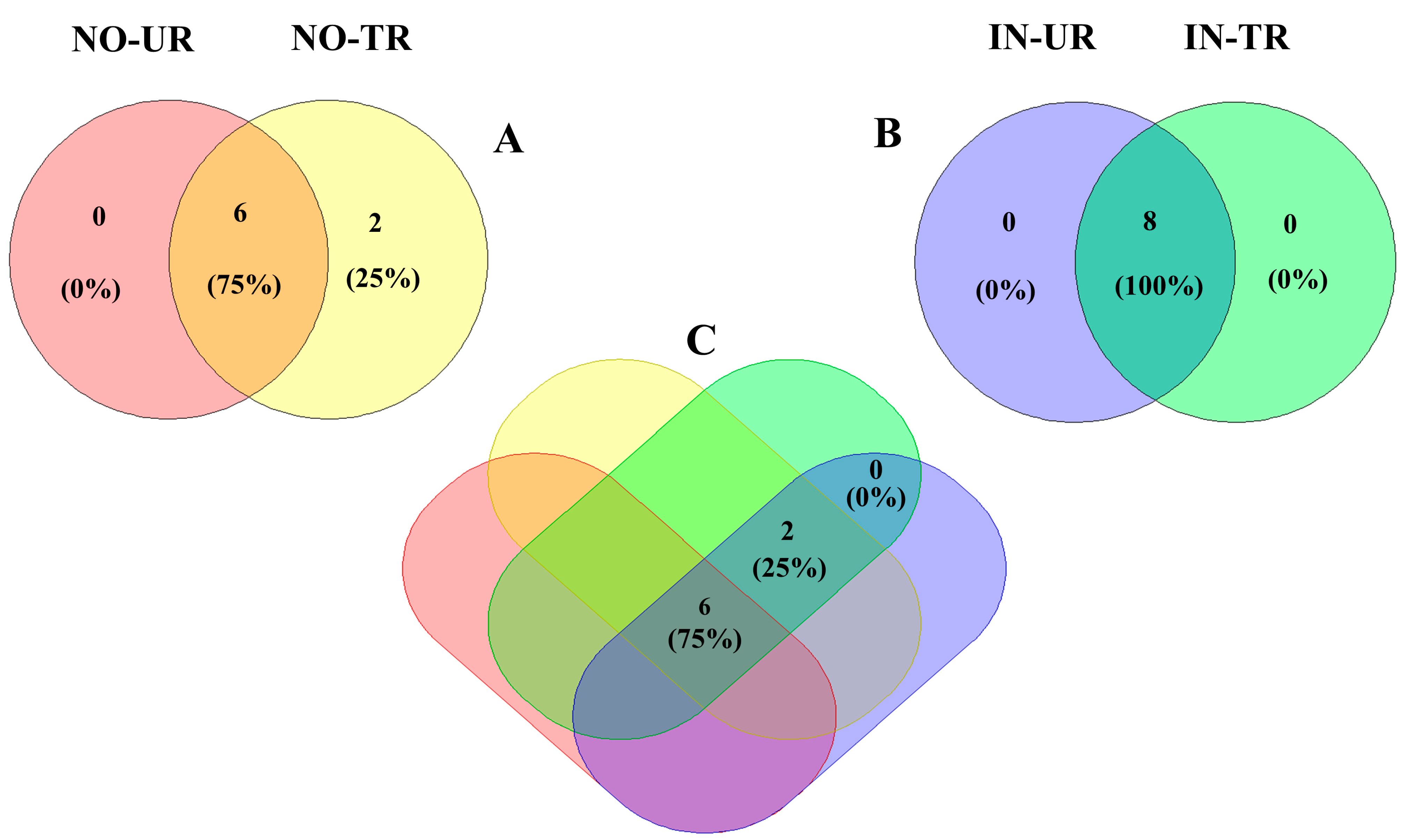
2.5. Venn Diagram of Phytoalexins Induced by CFRE and SFRE Treatment in Cucumber Leaves
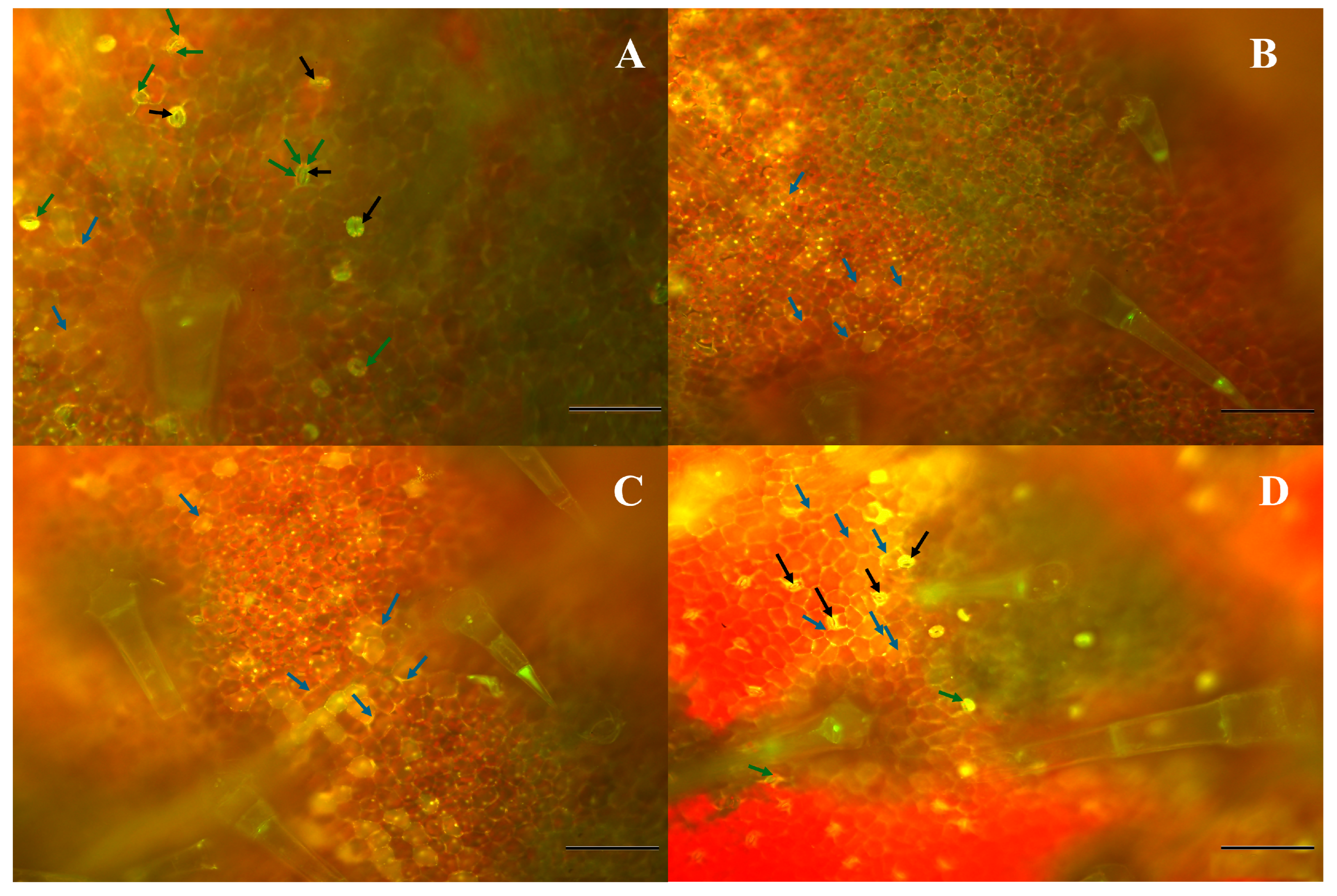
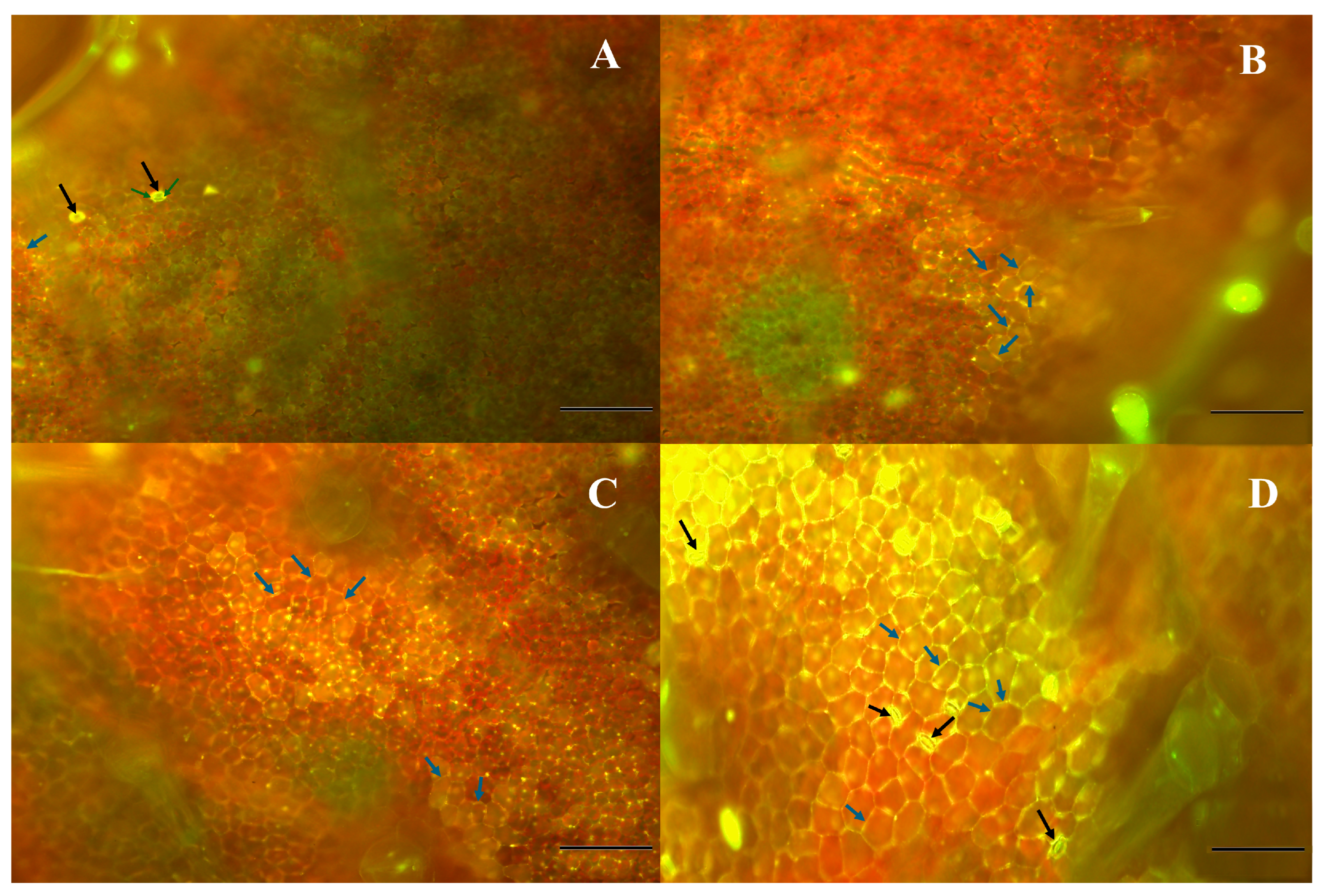
2.6. Fluorescence Microscopy
3. Discussion
4. Materials and Methods
4.1. Extraction of Celery and Spinach Flavonoid-Rich Extract (CFRE, SFRE)
4.2. Effect of CFRE and SFRE Treatment on the Accumulation of Phytoalexins in Podosphaera Fusca-Infected Cucumber Leaves
4.2.1. Plant Material and Fungal Inoculation
4.2.2. Experimental Design
4.3. Chemical Analysis of Phytoalexins in Cucumber Leaves
4.4. Fluorescence Microscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFRE | Celery flavonoid-rich extract |
| SFRE | Spinach flavonoid-rich extract |
| IN-TR | Seedlings inoculated with P. fusca, sprayed with 4 mg mL−1 of either CFRE or SFRE in distilled water |
| NO-TR | Non-inoculated seedlings sprayed with 4 mg mL−1 of either CFRE or SFRE |
| IN-UT | Inoculated seedlings sprayed with distilled water |
| NO-UT | Non-inoculated seedlings sprayed with distilled water |
| PR | Pathogenicity-related |
| PTI | PAMP-triggered immunity |
| ROS | Reactive oxygen species |
References
- Yue, C.; Du, C.; Wang, X.; Tan, Y.; Liu, X.; Fan, H. Powdery mildew-induced changes in phyllosphere microbial community dynamics of cucumber. FEMS Microbiol. Ecol. 2024, 100, fiae050. [Google Scholar] [CrossRef]
- Fukino, N.; Yoshioka, Y.; Sugiyama, M.; Sakata, Y.; Matsumoto, S. Identification and validation of powdery mildew (Podosphaera xanthii)-resistant loci in recombinant inbred lines of cucumber (Cucumis sativus L.). Mol. Breed. 2013, 32, 267–277. [Google Scholar] [CrossRef]
- Romero-Contreras, Y.J.; Gonzalez-Serrano, F.; Formey, D.; Aragon, W.; Chacón, F.I.; Torres, M.; Cevallos, M.A.; Rafael Dib, J.; Rebollar, E.A.; Serrano, M. Amphibian skin bacteria display antifungal activity and induce plant defense mechanisms against Botrytis cinerea. Front. Plant Sci. 2024, 15, 1392637. [Google Scholar] [CrossRef]
- Nie, J.; Yuan, Q.; Zhang, W.; Pan, J. Genetics, resistance mechanism, and breeding of powdery mildew resistance in cucumbers (Cucumis sativus L.). Hortic. Plant J. 2023, 9, 603–615. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.G.; Agosteo, G.E.; Abdelfattah, A.; Romeo, F.V.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Evaluation of a pomegranate peel extract (PGE) as alternative mean to control olive anthracnose. Phytopathology 2017, 107, 1462–1467. [Google Scholar] [CrossRef]
- Khaleil, M.M.; Alnoman, M.M.; Elrazik, E.S.A.; Zagloul, H.; Khalil, A.M.A. Essential oil of Foeniculum vulgare Mill. As a green fungicide and defense-inducing agent against fusarium root rot disease in Vicia faba L. Biology 2021, 10, 696. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.; Scibetta, S.; Strano, M.; Cacciola, S.; Belgacem, I.; Agosteo, G.; Schena, L. Pre- and postharvest applications of a pomegranate peel extract to control citrus fruit decay during storage and shelf life. Plant Dis. 2021, 105, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Saltos-Rezabala, L.A.; Silveira, P.R.D.; Tavares, D.G.; Moreira, S.I.; Magalhães, T.A.; Botelho, D.M.D.S.; Alves, E. Thyme essential oil reduces disease severity and induces resistance against Alternaria linariae in tomato plants. Horticulturae 2022, 8, 919. [Google Scholar] [CrossRef]
- Soleimani, H.; Ghanadian, M.; Mostowfizadeh-Ghalamfarsa, R. Spinach flavonoid-rich extract: Unleashing plant defense mechanisms against cucumber powdery mildew. Sustain. Chem. Pharm. 2024, 41, 101740. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Zhou, W.; Zhang, P.; Fan, Y.J. Application of osthol induces a resistance response against powdery mildew in pumpkin leaves. Int. J. Mol. Sci. 2007, 8, 1001–1012. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Sivakumar, D.; Beukes, M.; Korsten, L. Expression of pathogenesis-related (PR) genes in avocados fumigated with thyme oil vapours and control of anthracnose. Food Biochem. 2016, 194, 938–943. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Rapisarda, P.; Schena, L. Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant Pathol. 2017, 66, 633–640. [Google Scholar] [CrossRef]
- Banani, H.; Olivieri, L.; Santoro, K.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Thyme and savory essential oil efficacy and induction of resistance against Botrytis cinerea through priming of defense responses in apple. Foods 2018, 7, 11. [Google Scholar] [CrossRef]
- La Spada, F.; Aloi, F.; Coniglione, M.; Pane, A.; Cacciola, S.O. Natural biostimulants elicit plant immune system in an integrated management strategy of the postharvest green mold of orange fruits incited by Penicillium digitatum. Front. Plant Sci. 2021, 12, 684722. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, M.; Karami, A.; Cacciola, S.O. Defense mechanisms induced by celery seed essential oil against powdery mildew incited by Podosphaera fusca in cucumber. J. Fungi 2024, 10, 17. [Google Scholar] [CrossRef]
- Fofana, B.; McNally, D.J.; Labbé, C.; Boulanger, R.; Benhamou, N.; Séguin, A.; Bélanger, R.R. Milsana-induced resistance in powdery mildew-infected cucumber plants correlates with the induction of chalcone synthase and chalcone isomerase. Physiol. Mol. Plant Pathol. 2002, 61, 121132. [Google Scholar] [CrossRef]
- Zhu, F.; Cao, M.Y.; Zhang, Q.P.; Mohan, R.; Schar, J.; Mitchell, M.; Chen, H.; Liu, F.; Wang, D.; Fu, Z.Q. Join the green team: Inducers of plant immunity in the plant disease sustainable control toolbox. J. Adv. Res. 2024, 57, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.P.; Kirk, T.K.; Sherwood, R.T. Lignification as a mechanism of disease resistance. Annu. Rev. Phytopathol. 1980, 18, 259–288. [Google Scholar] [CrossRef]
- Chen, A.; Liu, T.; Wang, Z.; Chen, X. Plant root suberin: A layer of defence against biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 1056008. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of phenylpropanoids and flavonoids in plant resistance to pests and diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Camagna, M.; Ojika, M.; Takemoto, D. Detoxification of the solanaceous phytoalexins rishitin, lubimin, oxylubimin and solavetivone via a cytochrome P450 oxygenase. Plant Signal Behav. 2020, 15, 1707348. [Google Scholar] [CrossRef]
- Maserti, B.E.; Michelozzi, M.; Cencetti, G.; Riolo, P.; La Spada, F.; Aloi, F.; Pane, A.; Bartolini, P.; Pecori, F.; De Andrade Silva, E.M.; et al. Leaf volatile organic compounds profiles from two citrus genotypes differing in susceptibility to Phytophthora citrophthora infection. Physiol. Mol. Plant Pathol. 2024, 133, 102319. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Daayf, F.; Ongena, M.; Boulanger, R.; Elhadrami, I.; B’elanger, R. Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis. J. Chem. Ecol. 2000, 26, 1579–1593. [Google Scholar] [CrossRef]
- Soleimani, H.; Mostowfizadeh-Ghalamfarsa, R.; Ghanadian, S.M. Celery flavonoid-rich extract significantly reduces cucumber powdery mildew severity and enhances plant defense responses. Sci. Rep. 2025, 15, 10589. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Dev, M.; Dev, V.; Singh, K.; Pant, P.; Rawat, S. Role of phytoalexins in plant disease resistance. In Biorationals and Biopesticides: Pest Management; Kumar, R., de Oliveira, M., de Aguiar Andrade, E., Suyal, D., Soni, R., Eds.; De Gruyter: Berlin, Germany, 2024; pp. 127–140. [Google Scholar] [CrossRef]
- Gao, Y.J.; Fu, W.J.; Liu, J.; Chen, Y.J.; Dai, G.H. Morphological changes of Podosphaera xanthii and induced biochemical defenses of cucumber after treated by (+)-(S)-ar-turmerone. Physiol. Mol. Plant Pathol. 2020, 112, 101524. [Google Scholar] [CrossRef]
- Margaritopoulou, T.; Toufexi, E.; Kizis, D.; Balayiannis, G.; Anagnostopoulos, C.; Theocharis, A.; Rempelos, L.; Troyanos, Y.; Leifert, C.; Markellou, E. Reynoutria sachalinensis extract elicits SA-dependent defense responses in courgette genotypes against powdery mildew caused by Podosphaera xanthii. Sci. Rep. 2020, 10, 3354. [Google Scholar] [CrossRef]
- Li, S.; Pi, J.; Zhu, H.; Yang, L.; Zhang, X.; Ding, W. Caffeic acid in tobacco root exudate defends tobacco plants from infection by Ralstonia solanacearum. Front. Plant Sci. 2021, 12, 690586. [Google Scholar] [CrossRef]
- Kaya, O.; Bozkurt, A.; Karakus, S.; Daler, S.; Yilmaz, T. Essential oil compounds modulate nutritional quality and stress response in Botrytis cinerea-infected grape (Vitis vinifera L. cv. ‘Karaerik’). Physiol. Mol. Plant Pathol. 2024, 133, 102346. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid derivatives and their role in plants’ health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef]
- Szatmári, Á.; Zvara, Á.; Móricz, Á.M.; Besenyei, E.; Szabó, E.; Ott, P.G.; Puskás, L.G.; Bozsó, Z. Pattern triggered immunity (PTI) in tobacco: Isolation of activated genes suggests role of the phenylpropanoid pathway in inhibition of bacterial pathogens. PLoS ONE 2014, 9, e102869. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.A.; Daim, L.D.J.; Ithnin, N.; Ooi, T.E.K.; Md-Noh, N.; Mohamed, M.; Mohd-Yusof, H.; Appleton, D.R.; Kulaveerasingam, H. Expression of phenylpropanoid and flavonoid pathway genes in oil palm roots during infection by Ganoderma boninense. Plant Gene 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenbock, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Bani, M.; Pérez-De-Luque, A.; Rubiales, D.; Rispail, N. Physical and chemical barriers in root tissues contribute to quantitative resistance to Fusarium oxysporum f. sp. pisi in pea. Front. Plant Sci. 2018, 9, 199. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.M.; dos Santos Nascimento, L.B.; Casanova, L.M.; Leal-Costa, M.V.; Costa, S.S.; Tavares, E.S. Differential distribution of flavonoids and phenolic acids in leaves of Kalanchoe delagoensis Ecklon & Zeyher (Crassulaceae). Microsc. Microanal. 2020, 26, 1061–1068. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Arendrup, M.C.; Arikan, S.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P.; et al. EUCAST DEFINITIVE DOCUMENT E.DEF 9.1: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. Clin. Microb. Infec. 2008, 14, 982–984. [Google Scholar] [CrossRef] [PubMed]
- Esfandiary, E.; Karimipour, M.; Mardani, M.; Ghanadian, M.; Alaei, H.A.; Mohammadnejad, D.; Esmaeili, A. Neuroprotective effects of Rosa damascena extract on learning and memory in a rat model of amyloid-β-induced Alzheimer’s disease. Adv. Biomed. Res. 2015, 4, 131. [Google Scholar] [CrossRef]
- Shojaie, B.; Mostajeran, A.; Ghanadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turk. J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani, H.; Gharibi, S.; Cacciola, S.O.; Mostowfizadeh-Ghalamfarsa, R. Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves. Plants 2025, 14, 2414. https://doi.org/10.3390/plants14152414
Soleimani H, Gharibi S, Cacciola SO, Mostowfizadeh-Ghalamfarsa R. Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves. Plants. 2025; 14(15):2414. https://doi.org/10.3390/plants14152414
Chicago/Turabian StyleSoleimani, Hajar, Shima Gharibi, Santa Olga Cacciola, and Reza Mostowfizadeh-Ghalamfarsa. 2025. "Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves" Plants 14, no. 15: 2414. https://doi.org/10.3390/plants14152414
APA StyleSoleimani, H., Gharibi, S., Cacciola, S. O., & Mostowfizadeh-Ghalamfarsa, R. (2025). Celery and Spinach Flavonoid-Rich Extracts Enhance Phytoalexin Production in Powdery Mildew-Infected Cucumber Leaves. Plants, 14(15), 2414. https://doi.org/10.3390/plants14152414







