Drought Modulates Root–Microbe Interactions and Functional Gene Expression in Plateau Wetland Herbaceous Plants
Abstract
1. Introduction
2. Results
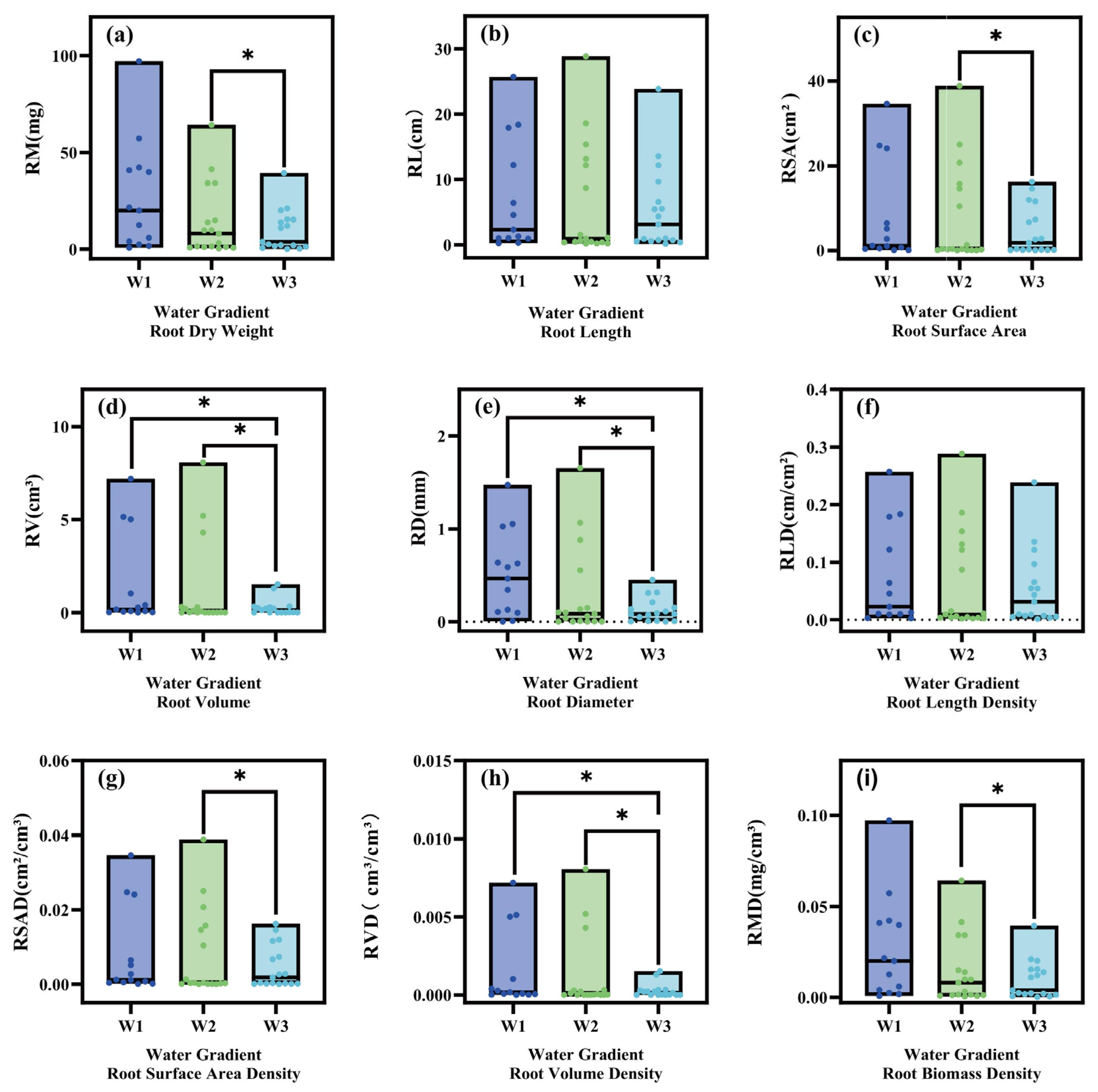
2.1. Effects of Different Moisture Statuses on the Root Traits of Herbaceous Plants
2.1.1. Differential Analysis of the Plant Root Traits of Herbaceous Plants at the Community Scale
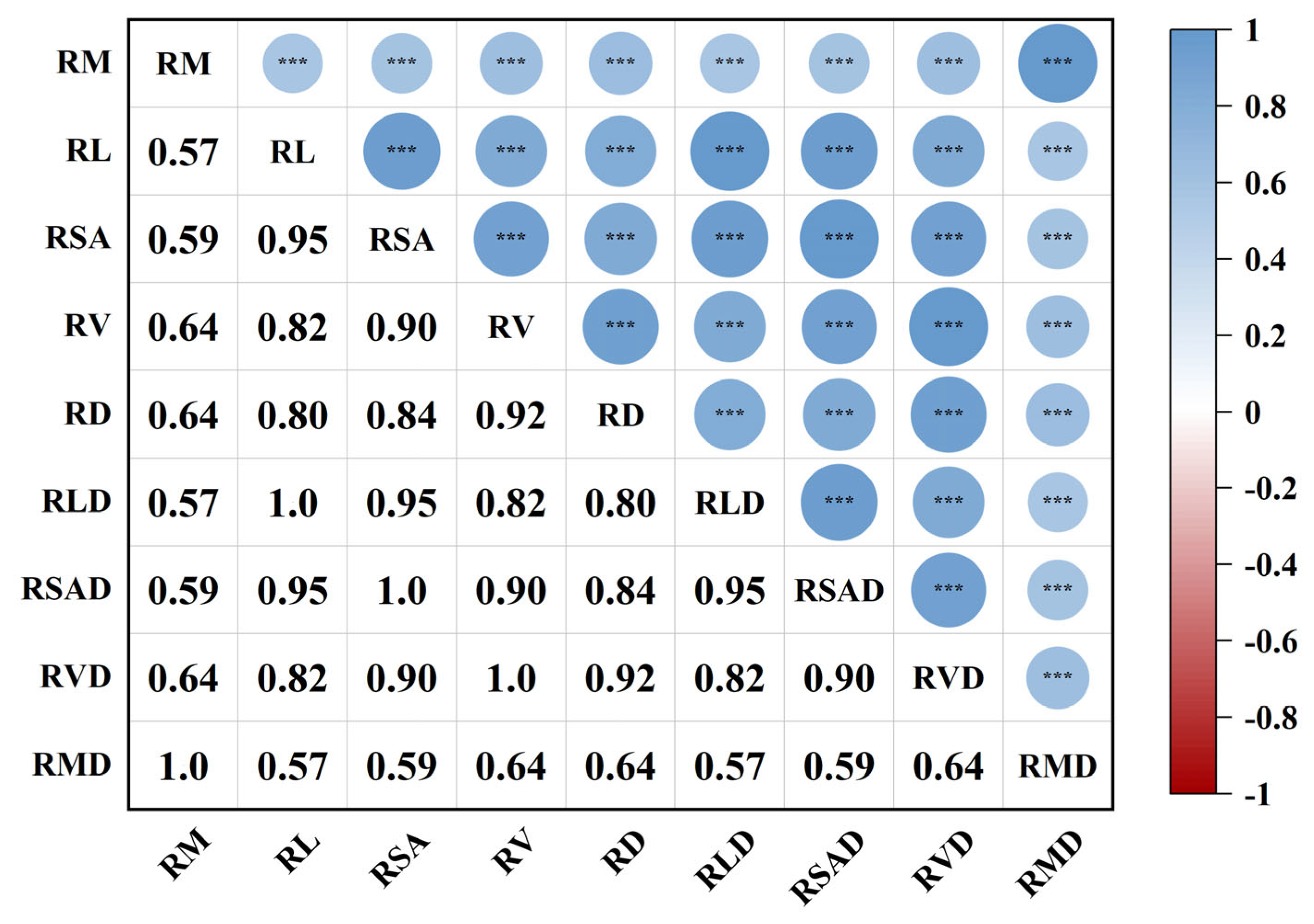
2.1.2. Synergies and Trade-Offs in Herbaceous Plant Root Traits
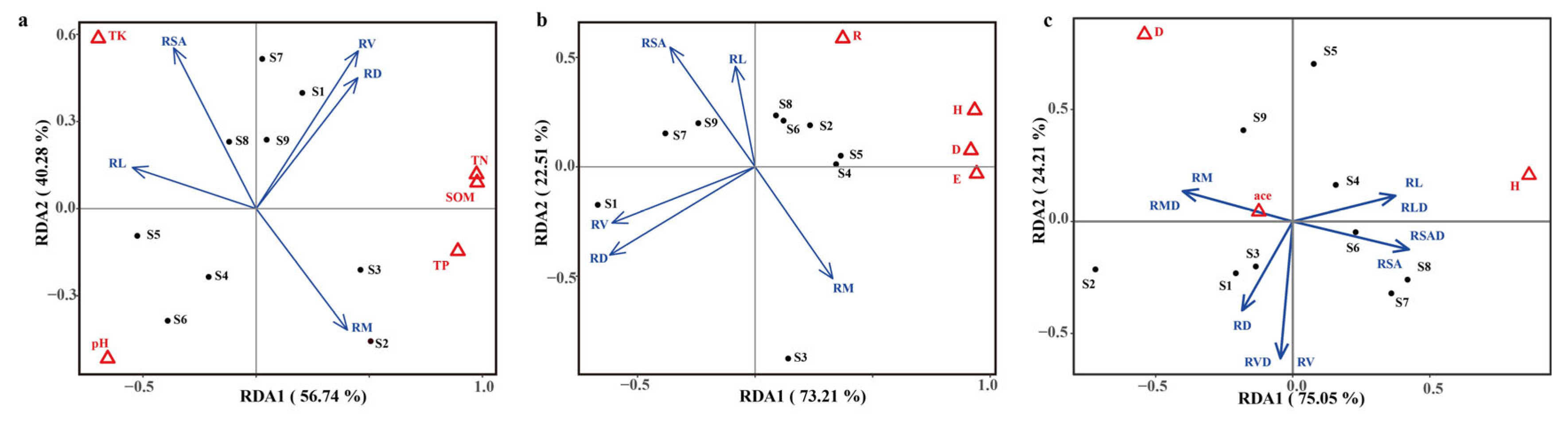
2.1.3. Factors Influencing the Plant Root Traits in Herbaceous Plants
2.2. Effects of Different Moisture Statuses on Ectorhizosphere Soil Microbial Communities
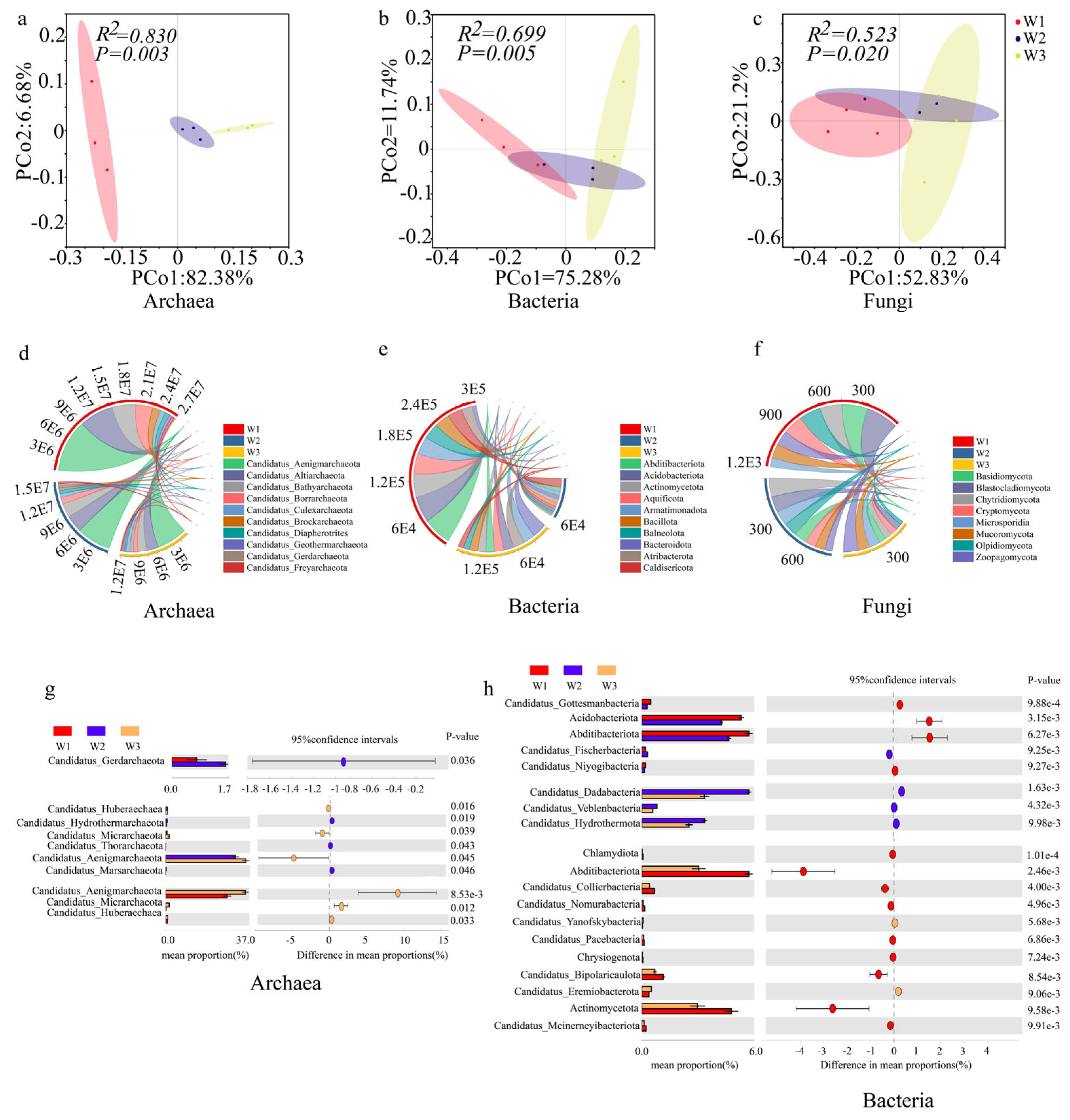
2.2.1. Analysis of Ectorhizosphere Soil Microbial Community Diversities
2.2.2. Analysis of Ectorhizosphere Soil Microbial Community Structure and Its Key Phyla
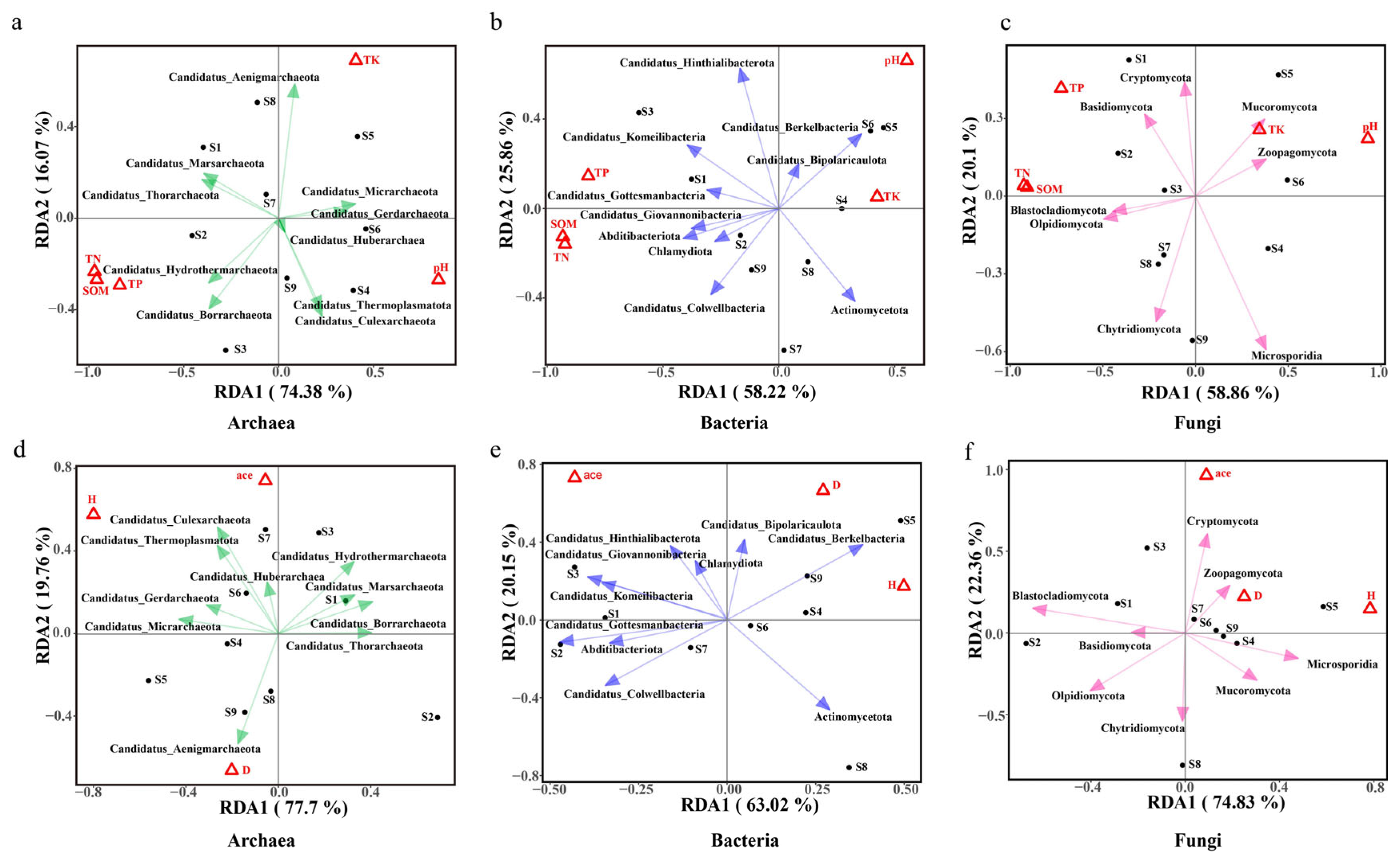
2.2.3. Relationships Among Ectorhizosphere Soil Microbial Community Compositions and Environmental Factors
2.3. Interactions Among Herbaceous Plant Root Traits and Microbial Communities
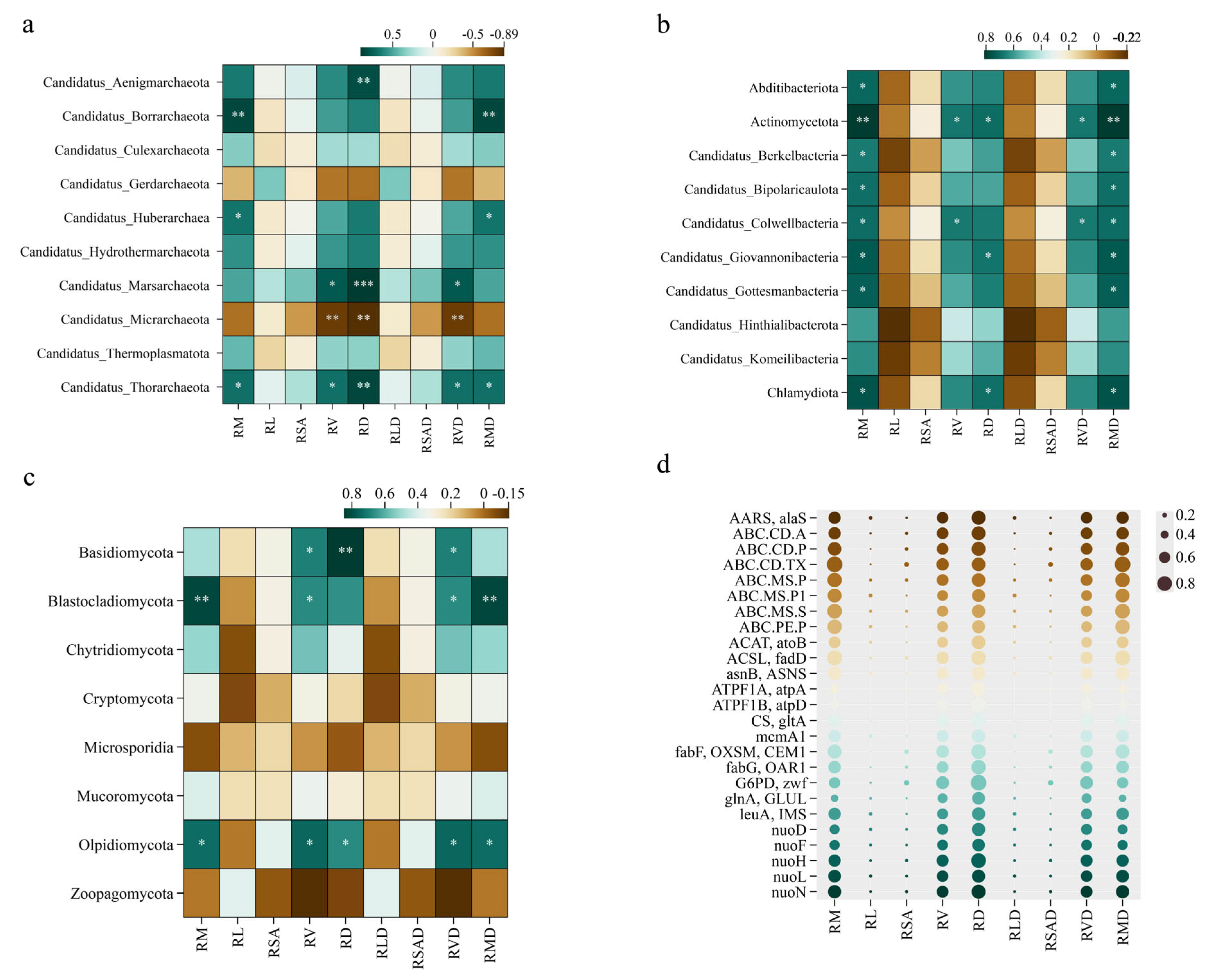
2.3.1. Correlations of Plant Root Traits with the Relative Abundances of Species at the Microbial Keystone Phylum Level
2.3.2. Relationships Among Plant Root Traits and Functional Microbial Genes
3. Discussion
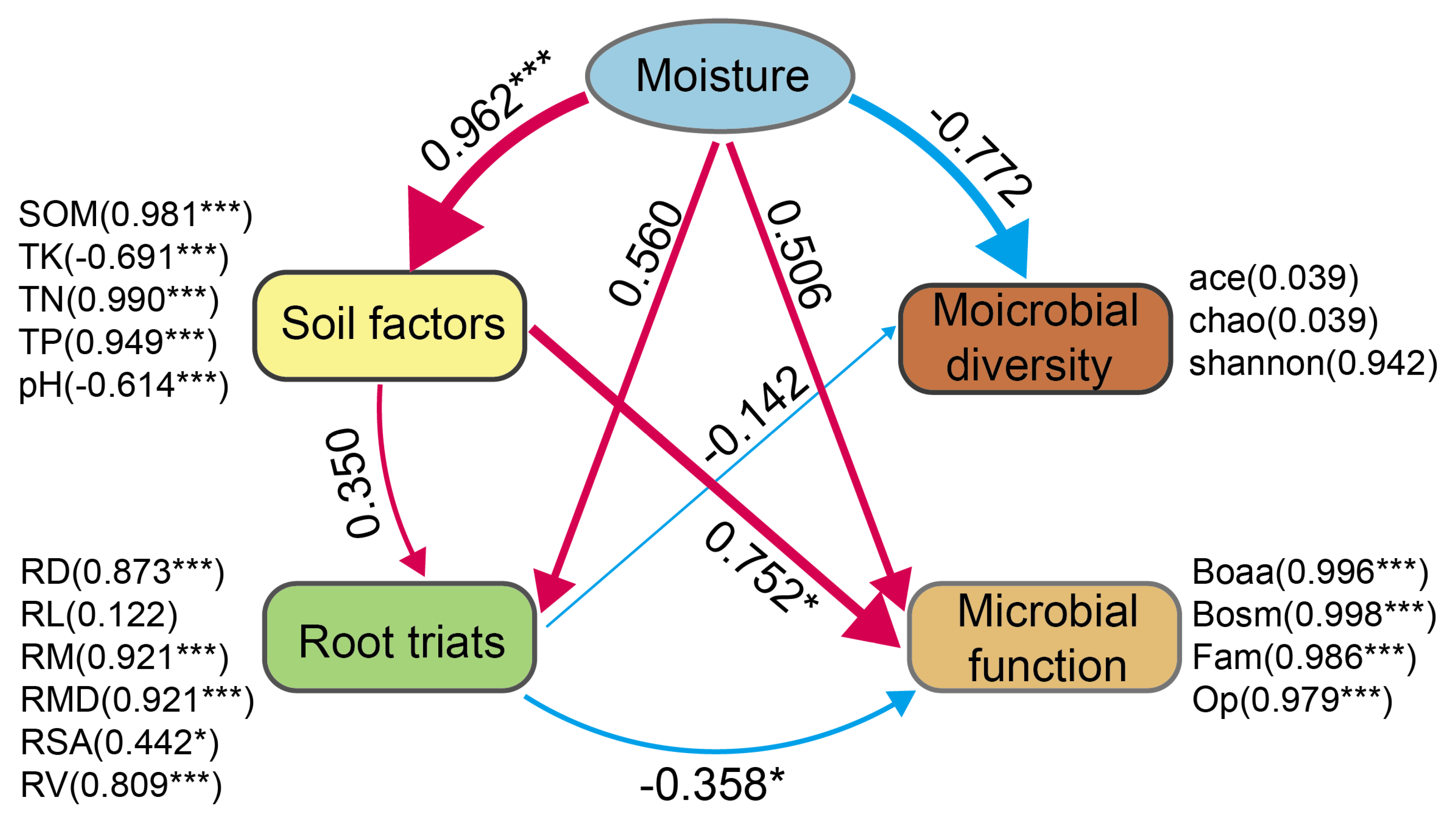
3.1. Response of Plant Root Traits to Moisture Status
3.2. Ecological Functions of Soil Microbial Communities
3.3. Relationships Among Plant Root Traits and Ectorhizosphere Soil Microbial Compositions
3.4. Relationships Among Root Functions and Ectorhizosphere Soil Microbial Functions
3.5. Potential Applications of Microbial Functions in Wetland Ecological Restoration
4. Materials and Methods
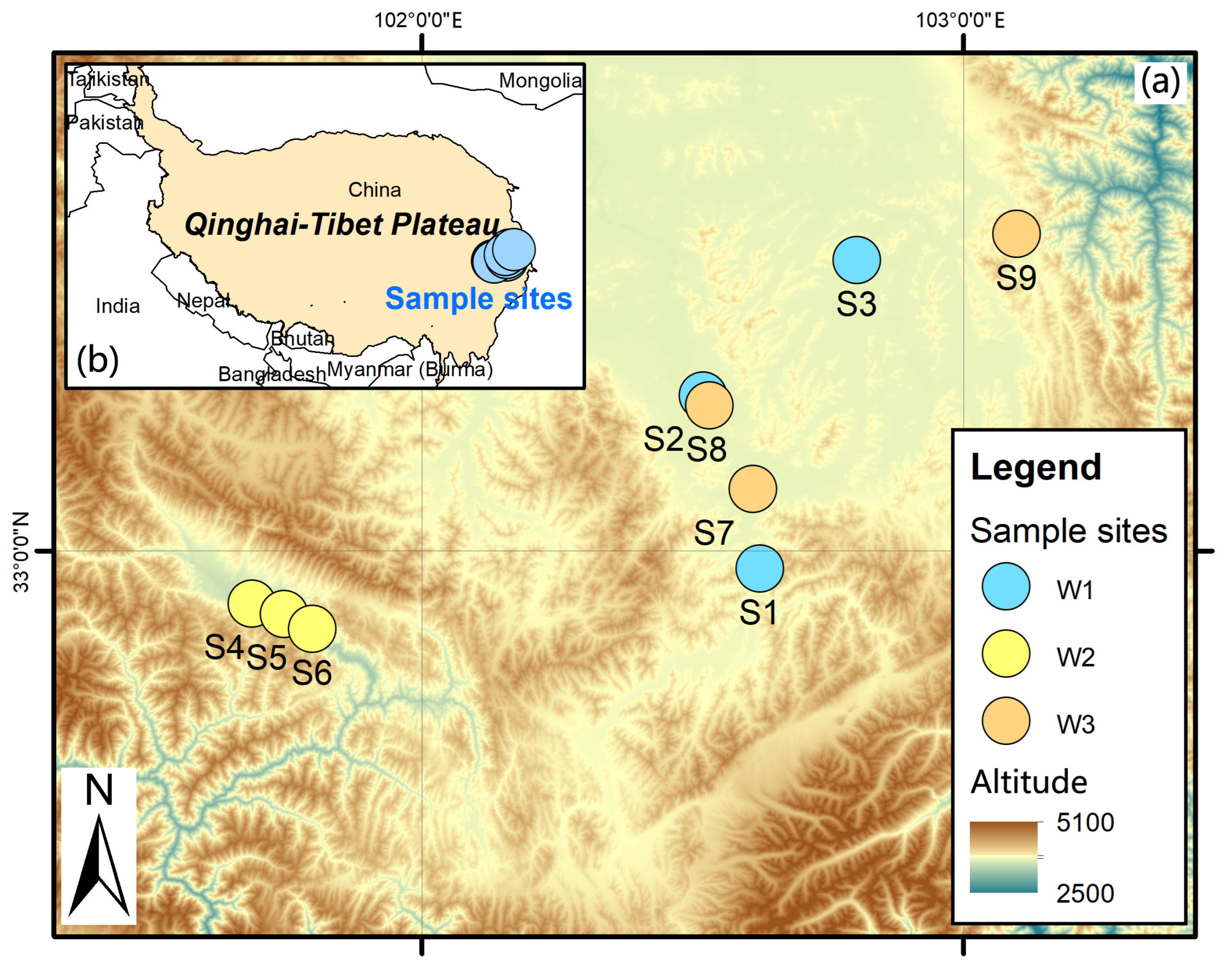
4.1. Study Area
4.2. Sample Collection and Analysis
4.2.1. Sample Collection Method
4.2.2. Determination of Soil Physical and Chemical Properties
4.2.3. Determination of Root Traits in Herbaceous Plants
4.2.4. Measurement of Herbaceous Plant Diversity and Microbial Diversity
4.2.5. Macro-Genome Sequencing Methods for Analyzing Soil Microbial Communities
4.3. Data Analysis and Graphing
5. Conclusions
6. Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Lyu, X. Research Directions in Wetland Function and Wetland Science. Wetl. Sci. 2003, 5, 7–11. [Google Scholar]
- Sandi, S.G.; Rodriguez, J.F.; Saintilan, N.; Wen, L.; Kuczera, G.; Riccardi, G.; Saco, P.M. Resilience to drought of dryland wetlands threatened by climate change. Sci. Rep. 2020, 10, 13232. [Google Scholar] [CrossRef]
- Liu, C.; Siri, M.; Li, H.; Ren, C.; Huang, J.; Feng, C.; Liu, K. Drought is threatening plant growth and soil nutrients of grassland ecosystems: A meta-analysis. Ecol. Evol. 2023, 13, e10092. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Liu, L.; Liu, F.; Zhang, H. Recent changes in wetlands on the Tibetan Plateau: A review. J. Geogr. Sci. 2015, 25, 879–896. [Google Scholar] [CrossRef]
- Kang, D.; Chen, Y.; Feng, S.; Liu, Q.; Zou, S. Microbial community diversity and assembly processes in the aridification of wetlands on the Qinghai-Tibet Plateau. iScience 2025, 28, 112494. [Google Scholar]
- Ouyang, L.; Ba, S.; La, D.; Liu, X.; Liu, G.; Liu, W.; Ding, B. Analysis of Plant and Soil Bacterial Diversity in Wetlands on the Tibetan Plateau and Their Influencing Factors. Acta Bot. Sin. 2023, 41, 44–52. [Google Scholar]
- Jiang, W.; Xiong, M.; Zou, S.; Kang, D. Microbial genes for degrading plant-derived carbon are a key factor affecting soil respiration and temperature sensitivity in plateau peatlands. Pedosphere 2024, 34, 1026–1037. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Zhao, Z. Vegetation community structure and soil nutrient changes in degraded alpine wetlands on the Qinghai-Tibet Plateau. J. Grassl. Sci. 2020, 29, 9–18. [Google Scholar]
- Xiong, M.; Jiang, W.; Zou, S.; Kang, D.; Yan, X. Microbial carbohydrate-active enzymes influence soil carbon by regulating the of plant- and fungal-derived biomass decomposition in plateau peat wetlands under differing water conditions. Front. Microbiol. 2023, 14, 1266016. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, R.; Yin, H.; He, Y.; Tang, Z.; Song, S. Mechanisms of plant responses to water stress. Mod. Agric. Res. 2024, 30, 64–68. [Google Scholar]
- He, P.-C.; Qing, Y.E. Plant Functional Traits: From Individual Plant to Global Scale. J. Trop. Subtrop. Bot. 2019, 27, 523–533. [Google Scholar]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Fry, E.L.; Eldridge, D.J.; de Vries, F.T.; Manning, P.; Hamonts, K.; Kattge, J.; Boenisch, G.; Singh, B.K.; Bardgett, R.D. Plant attributes explain the distribution of soil microbial communities in two contrasting regions of the globe. New Phytol. 2018, 219, 574–587. [Google Scholar] [CrossRef]
- Chen, D.; Pan, Q.; Bai, Y.; Hu, S.; Huang, J.; Wang, Q.; Naeem, S.; Elser, J.J.; Wu, J.; Han, X.; et al. Effects of plant functional group loss on soil biota and net ecosystem exchange: A plant removal experiment in the Mongolian grassland. J. Ecol. 2016, 104, 734–743. [Google Scholar] [CrossRef]
- Bever, J.D. Soil community feedback and the coexistence of competitors: Conceptual frameworks and empirical tests. New Phytol. 2003, 157, 465–473. [Google Scholar] [CrossRef] [PubMed]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bakker, R.; Verwaal, J.; Scheublin, T.R.; Rutten, M.; Van Logtestijn, R.; Staehelin, C. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol. Ecol. 2006, 56, 178–187. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.; Veen, G.F. Where, when and how plant–soil feedback matters in a changing world. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.T.; Mack, K.M.L.; Bever, J.D. Plant-soil feedbacks as drivers of succession: Evidence from remnant and restored tallgrass prairies. Ecosphere 2015, 6, art158. [Google Scholar] [CrossRef]
- Bever, J.D.; Westover, K.M.; Antonovics, J. Incorporating the Soil Community into Plant Population Dynamics: The Utility of the Feedback Approach. J. Ecol. 1997, 85, 561–573. [Google Scholar] [CrossRef]
- McNear, D. The rhizosphere-roots, soil and everything in between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Liang, Q.; Liao, H.; Yan, X. Quantitative analysis of plant root morphology. Acta Bot. Sin. 2007, 24, 695–702. [Google Scholar]
- Guo, Y.; Yao, J.; Dong, Y.; Yan, J.; Yang, N.; Feng, Y.; Wei, X.; Liang, W. Root distribution characteristics of pure stands and mixed forests of Pinus tabulaeformis and Robinia pseudoacacia. J. Appl. Ecol. 2023, 34, 2881–2888. [Google Scholar]
- Wang, X. Physiological Responses of Four Types of Grasses to Drought Stress. Master’s Thesis, Fujian Agricultural and Forestry University, Fuzhou, China, 2015. [Google Scholar]
- Cheng, J.; Zhang, Y. Biomass allocation strategies of two herbaceous plants in arid regions under water stress. Arid. Zone Res. 2012, 29, 432–439. [Google Scholar]
- Liu, J.; Li, X.; Yao, M. Research Progress on the Construction of Plant Rhizosphere Microbial Communities. Acta Microbiol. Sin. 2021, 61, 231–248. [Google Scholar]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, Y.; Ao, Y.; Shen, J.; Jin, K. Root-soil interactions under soil compaction stress. J. Nutr. Fertil. Sci. 2021, 27, 531–543. [Google Scholar]
- Xi, N.; De Long, J.R.; Davison, J.; Kardol, P.; Forero, L.E.; Zobel, M.; Semchenko, M. Plant–soil microbial interactions as modulators of species coexistence and productivity. Trends Ecol. Evol. 2025, 40, 673–686. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Liu, H.; Liu, D.; Wang, W.; Liang, C.; Recknagel, F. Nutrient absorption kinetics of three emergent aquatic plants: Phragmites australis, Typha angustifolia, and Juncus effusus. Acta Ecol. Sin. 2014, 34, 2238–2245. [Google Scholar]
- Wu, S.; Wang, G.; Yang, L.; Liu, S. Research Progress on the Impact of Drought on the Structure and Function of Forest Ecosystems. World For. Res. 2024, 37, 37–45. [Google Scholar]
- Li, F.; Pei, N.; Shi, Z.; Luo, S.; Tang, Y.; Liu, X.; Chen, Z.; Sun, B. Secondary Forest and Artificial Forest Root Biomass, Morphological Characteristics, Nutrients, and Their Relationship with Soil Nutrients. J. Ecol. Environ. 2019, 28, 2349–2355. [Google Scholar]
- Zhe, H.; Luo, Y.; Zhang, H.; Sun, F. Study on the Relationship between Root Biomass of Saplings of Alnus mandshurica, Quercus mongolica, and Ulmus parvifolia in Daqing Gou and Soil Nutrients and pH Value. Arid. Zone Resour. Environ. 2011, 25, 183–189. [Google Scholar]
- Wang, C.; Long, R.; Ding, L. The influence of functional group diversity and composition of different grassland types on plant community productivity in alpine meadows. Biodivers. Sci. 2004, 12, 403–409. [Google Scholar]
- Liu, Y.; Li, F.; Sun, Q.; Xie, Y. Advances in the study of soil microorganisms in wetland ecosystems. J. Appl. Environ. Biol. 2013, 19, 547–552. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Wang, Y.; Bai, Z.; Zhuang, G. Analysis of fungal community structure in the soil of Zoige Alpine Wetland. Acta Ecol. Sin. 2009, 29, 260–266. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, Y.; Kang, X.; Dong, X.; Li, W.; Chen, H.; Wang, Y. Effects of drought on the archaeal community in soil of the Zoige wetlands of the Qinghai–Tibetan plateau. Eur. J. Soil Biol. 2012, 52, 84–90. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, L.; Weng, B.; Ying, C.; Huang, Y. Ecological effects of soil drought stress on soil microorganisms in round-leaved cassia. J. Xiamen Univ. (Nat. Sci. Ed.) 2005, 44, 66–68. [Google Scholar]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Zhao, J.; Li, G.; Lai, X.; Li, J.; Wang, H.; Yang, D. Response of fungal community structure in Baikal needle grassland soil to nitrogen and water addition. Acta Ecol. Sin. 2018, 38, 195–205. [Google Scholar]
- Wang, Z.; Wang, W.; Ma, Z.; Chen, K.; Ben, H.; Min, K.; Qiao, Y. Effects of different grass species growth on soil nutrients and microbial communities in the Gansu Artemisia annua community. J. Grassl. Sci. 2023, 31, 3750–3758. [Google Scholar]
- Zhang, G.; Bai, J.; Jia, J.; Wang, W.; Wang, D.; Zhao, Q.; Wang, C.; Chen, G. Soil microbial communities regulate the threshold effect of salinity stress on SOM decomposition in coastal salt marshes. Fundam. Res. 2023, 3, 868–879. [Google Scholar] [CrossRef]
- Jiao, J.; Fu, X.; Zhang, S.; Liu, W.; Zhou, J.; Wu, X.; Lin, X.; Tian, Y.; Tang, G.; Li, P.; et al. Analysis of the physicochemical properties and microbial community structure of rhizosphere soil in Sichuan pepper trees of different ages. J. Northwest For. Univ. 2023, 38, 156–165. [Google Scholar]
- Yan, F.; Zhao, X.; Shao, L.; Wang, X.; Liang, Y.; Chen, Y. Effects of Different Vegetation Restoration Types on Soil Microbial Community Structure in the Restoration Area of the Jibei Quarry. Environ. Sci. 2025, 46, 1213–1224. [Google Scholar]
- Tong, Y.; Zhang, C.; Yu, Y.; Cao, Q.; Yang, Z.; Zhang, X.; Wang, M.; Dong, Q. Response of soil microbial characteristics in perennial high-altitude cultivated grasslands to short-term nitrogen addition. Environ. Sci. 2024, 45, 7316–7325. [Google Scholar]
- Herms, C.H.; Hennessy, R.C.; Bak, F.; Dresbøll, D.B.; Nicolaisen, M.H. Back to our roots: Exploring the role of root morphology as a mediator of beneficial plant-microbe interactions. Environ. Microbiol. 2022, 24, 3264–3272. [Google Scholar] [CrossRef]
- Jousset, A.; Schulz, W.; Scheu, S.; Eisenhauer, N. Intraspecific genotypic richness and relatedness predict the invasibility of microbial communities. ISME J. 2011, 5, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottovā, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2012, 109, 1159–1164. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, L.; Zhang, R.; Fan, S. Advances in Research on the Mechanisms of Interactions Between Plant Rhizosphere Exudates and Soil Microorganisms. Chin. Agric. Sci. Bull. 2020, 36, 26–35. [Google Scholar]
- Tomazelli, D.; Peron, R.A.d.S.; Mendes, S.D.C.; Pinto, C.E.; Baldissera, T.C.; Baretta, D.; Mendes, L.W.; Goss-Souza, D.; Klauberg-Filho, O. Plant diversity and root traits shape rhizosphere microbial communities in natural grasslands and cultivated pastures. Rhizosphere 2024, 29, 100864. [Google Scholar] [CrossRef]
- Li, Y.-X.; Rao, Y.-Z.; Qi, Y.-L.; Qu, Y.-N.; Chen, Y.-T.; Jiao, J.-Y.; Shu, W.-S.; Jiang, H.; Hedlund, B.P.; Hua, Z.-S.; et al. Deciphering Symbiotic Interactions of “Candidatus Aenigmarchaeota” with Inferred Horizontal Gene Transfers and Co-occurrence Networks. mSystems 2021, 6, e0060621. [Google Scholar] [CrossRef]
- Cui, X.; Lin, X.; Li, J.; Zhang, H.; Han, Y. Diversity, functional characteristics, and applications of stress-tolerant actinomycetes in environmental remediation. Acta Microbiol. Sin. 2023, 63, 1930–1943. [Google Scholar]
- Wang, S.; Chang, S.; Li, X.; Zhang, Y. Soil fungal diversity and community structure in the Tianshan Forest Region. Acta Ecol. Sin. 2021, 41, 124–134. [Google Scholar]
- Wu, L.K.; Lin, X.M.; Lin, W.X. Research progress and prospects on plant-soil-microbial interactions mediated by root exudates. Acta Bot. Sin. 2014, 38, 298–310. [Google Scholar]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van Der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Song, Z.H.; Tian, P.; Pei, T. Overview of the role of soil microorganisms in soil improvement and remediation. Grassl. Sci. 2024, 41, 2622–2636. [Google Scholar]
- Kang, D.; Li, Y.; Ma, L.; Zou, S. Landslide scales affect soil organic carbon accumulation by influencing microbial decomposition of plant-derived carbon after earthquakes. Ecol. Indic. 2023, 155, 110949. [Google Scholar] [CrossRef]
- Pantigoso, H.A.; Ossowicki, A.; Stringlis, I.A.; Carrión, V.J. Hub metabolites at the root–microbiome interface: Unlocking plant drought resilience. Trends Plant Sci. 2025. [Google Scholar] [CrossRef]
- Shah, B.A.; Kasarlawar, S.T.; Phale, P.S. Glucose-6-Phosphate Dehydrogenase, ZwfA, a Dual Cofactor-Specific Isozyme Is Predominantly Involved in the Glucose Metabolism of Pseudomonas bharatica CSV86T. Microbiol. Spectr. 2022, 10, e03818-22. [Google Scholar] [CrossRef]
- Abedini, D.; Jaupitre, S.; Bouwmeester, H.; Dong, L. Metabolic interactions in beneficial microbe recruitment by plants. Curr. Opin. Biotechnol. 2021, 70, 241–247. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef]
- Suarez-Fernandez, M.; Ferreira, J.J.; Campa, A. Impact of Farming System on Soil Microbial Communities Associated with Common Bean in a Region of Northern Spain. Plants 2025, 14, 1359. [Google Scholar] [CrossRef]
- Aleksieienko, I.; Fernandes Hertel, M.; Reilhan, J.; De Castro, M.; Légeret, B.; Caixeta Oliveira, H.; Reiter, I.M.; Santaella, C. Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings. Plants 2025, 14, 1659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Jia, Z.; An, W.; Zhao, G.; Su, Q.; Li, Y. Research Progress and Hot Topics Analysis on the Adaptive Mechanisms of Plant Root Systems Under Drought Stress. Jiangsu Agric. Sci. 2023, 51, 34–40. [Google Scholar]
- Ren, H.; Lu, H. The issue of appropriate trees for appropriate locations in ecosystem restoration. J. Trop. Subtrop. Bot. 2025, 33, 100–106. [Google Scholar]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guo, H.; Duan, G.; Wang, Z.; Fan, G.; Li, J. Research Progress on the Role and Mechanism of Arbuscular Mycorrhizal Fungi in Enhancing Plant Stress Resistance and Soil Improvement. Chin. Powder Technol. 2024, 30, 164–172. [Google Scholar]
- Liang, S.; Li, H.; Wu, H.; Yan, B.; Song, A. Microorganisms in coastal wetland sediments: A review on microbial community structure, functional gene, and environmental potential. Front. Microbiol. 2023, 14, 1163896. [Google Scholar] [CrossRef]
- Ma, J.; Gao, C.; Yi, X.; Wu, Y.; Li, J.; Zeng, X.; Cai, J. Analysis of soil microbial characteristics in different habitats during the ecological restoration process of Haifeng Wetland, Guangdong Province. Environ. Sci. 2023, 44, 2908–2917. [Google Scholar]
- Ma, H.; Zhao, Y.; Yang, K.; Wang, Y.; Zhang, C.; Ji, M. Application oriented bioaugmentation processes: Mechanism, performance improvement and scale-up. Bioresour. Technol. 2022, 344, 126192. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Chen, K.; Cao, W.; Meng, L.; Yang, B.; Xu, M.; Xing, Y.; Li, P.; Freilich, S.; Chen, C.; et al. Engineering natural microbiomes toward enhanced bioremediation by microbiome modeling. Nat. Commun. 2024, 15, 4694. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Garbeva, P.; Raaijmakers, J.M.; Medema, M.H. Strategies for tailoring functional microbial synthetic communities. ISME J. 2024, 18, wrae049. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Cong, W.-F.; Bezemer, T.M. Legacies at work: Plant–soil–microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 15. [Google Scholar] [CrossRef]
- Yang, D.; Hu, L.; Song, X.; Wang, C. Effects of rainfall variability on litter mass and decomposition of different plant functional groups in alpine meadows. Acta Bot. Sin. 2021, 45, 1314–1328. [Google Scholar]
- Chen, Y.; Xie, Z. Effects of storage conditions on soil biochemical indicators and the application of stored soil samples. Acta Pedol. Sin. 2018, 55, 783–796. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Yan, G.; Xing, Y.; Han, S.; Zhang, J.; Wang, Q.; Mu, C. Long-time precipitation reduction and nitrogen deposition increase alter soil nitrogen dynamic by influencing soil bacterial communities and functional groups. Pedosphere 2020, 30, 363–377. [Google Scholar] [CrossRef]
- Komy, Z.R. Comparative Study of Titrimetric and Gravimetric Methods for the Determination of Organic Carbon in Soils. Int. J. Environ. Anal. Chem. 1995, 60, 41–47. [Google Scholar] [CrossRef]
- Fu, B.; Liu, S.; Chen, L.; Lü, Y.; Qiu, J. Soil quality regime in relation to land cover and slope position across a highly modified slope landscape. Ecol. Res. 2004, 19, 111–118. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. The Influence of Root Systems of Typical Herbaceous Plants in the Loess Plateau on Soil Infiltration and Spatial Differences. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2021. [Google Scholar]
- Zeng, Y.; Mai Reziya, ·W.; Abdulheli, ·Y.; Aikebai, ·N.M.; Liang, Y.; Liu, Y. Plant Diversity and Community Stability in Different Habitats Along the Upper Reaches of the Tarim River, Xinjiang. J. Ecol. 2025, 1–10. [Google Scholar]
- Modi, A.; Vai, S.; Caramelli, D.; Lari, M. The Illumina Sequencing Protocol and the NovaSeq 6000 System. In Bacterial Pangenomics: Methods and Protocols; Mengoni, A., Bacci, G., Fondi, M., Eds.; Springer US: New York, NY, USA, 2021; pp. 15–42. [Google Scholar]
| Microbial Group | Treatment | Chao1 | Shannon‒Wiener Index | Simpson Index |
|---|---|---|---|---|
| archaea | W1 | 32 | 2.32 | 0.16 |
| W2 | 32 | 2.32 | 0.17 | |
| W3 | 32 | 2.24 | 0.20 | |
| bacteria | W1 | 159 | 4.21 | 0.20 |
| W2 | 160 | 4.23 | 0.20 | |
| W3 | 160 | 4.19 | 0.20 | |
| Fungi | W1 | 8 | 2.02 | 0.14 |
| W2 | 8 | 2.04 | 0.13 | |
| W3 | 8 | 2.00 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Feng, S.; Liu, Q.; Kang, D.; Zou, S. Drought Modulates Root–Microbe Interactions and Functional Gene Expression in Plateau Wetland Herbaceous Plants. Plants 2025, 14, 2413. https://doi.org/10.3390/plants14152413
Chen Y, Feng S, Liu Q, Kang D, Zou S. Drought Modulates Root–Microbe Interactions and Functional Gene Expression in Plateau Wetland Herbaceous Plants. Plants. 2025; 14(15):2413. https://doi.org/10.3390/plants14152413
Chicago/Turabian StyleChen, Yuanyuan, Shishi Feng, Qianmin Liu, Di Kang, and Shuzhen Zou. 2025. "Drought Modulates Root–Microbe Interactions and Functional Gene Expression in Plateau Wetland Herbaceous Plants" Plants 14, no. 15: 2413. https://doi.org/10.3390/plants14152413
APA StyleChen, Y., Feng, S., Liu, Q., Kang, D., & Zou, S. (2025). Drought Modulates Root–Microbe Interactions and Functional Gene Expression in Plateau Wetland Herbaceous Plants. Plants, 14(15), 2413. https://doi.org/10.3390/plants14152413






