Polyphenol-Rich Extract of Chrysanthemum × morifolium (Ramat) Hemsl. (Hangbaiju) Prevents Obesity and Lipid Accumulation Through Restoring Intestinal Microecological Balance
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of HE Chemical Profiles
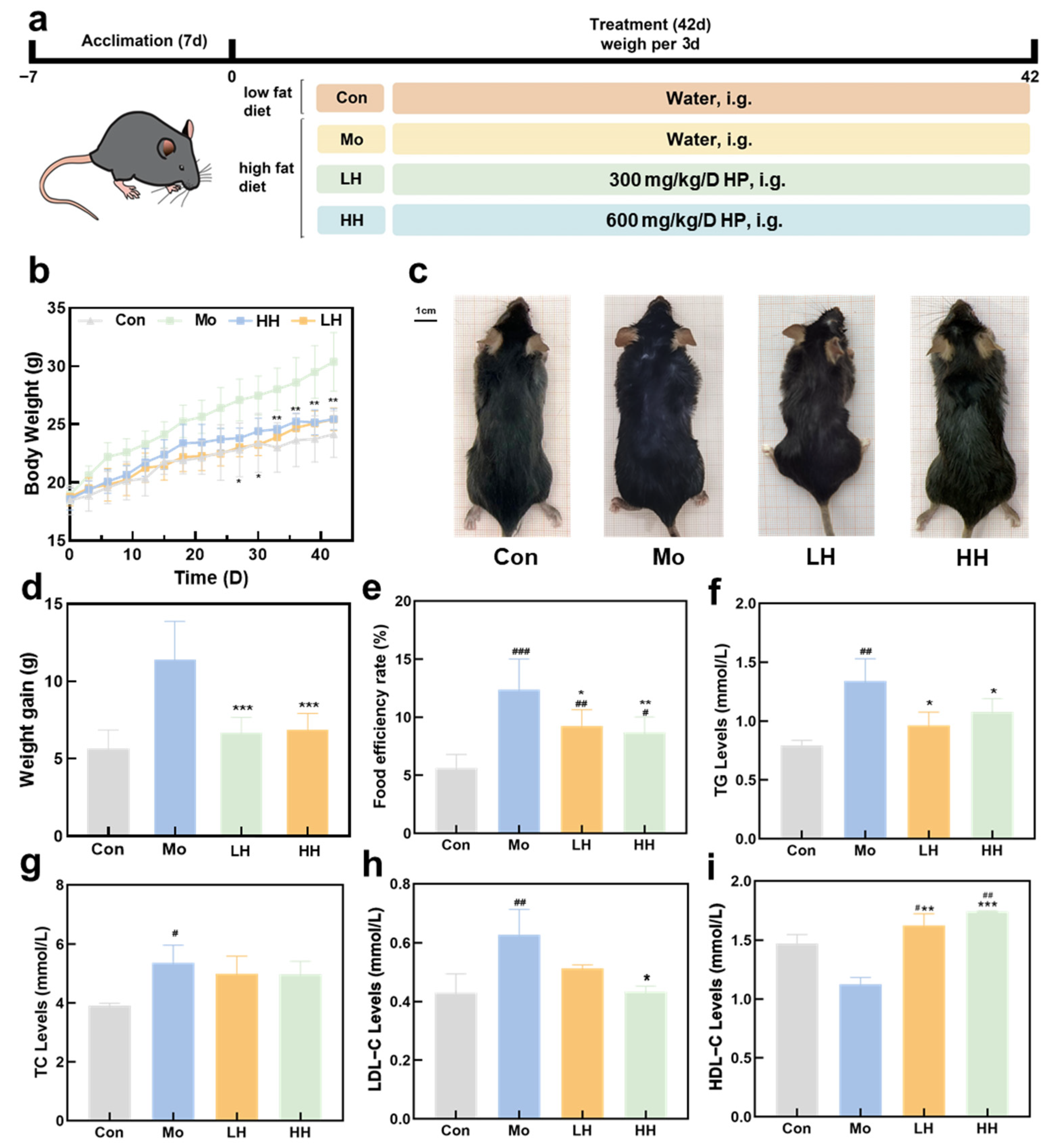
2.2. HE Alleviates HFD-Induced Obesity and Hyperglycemia
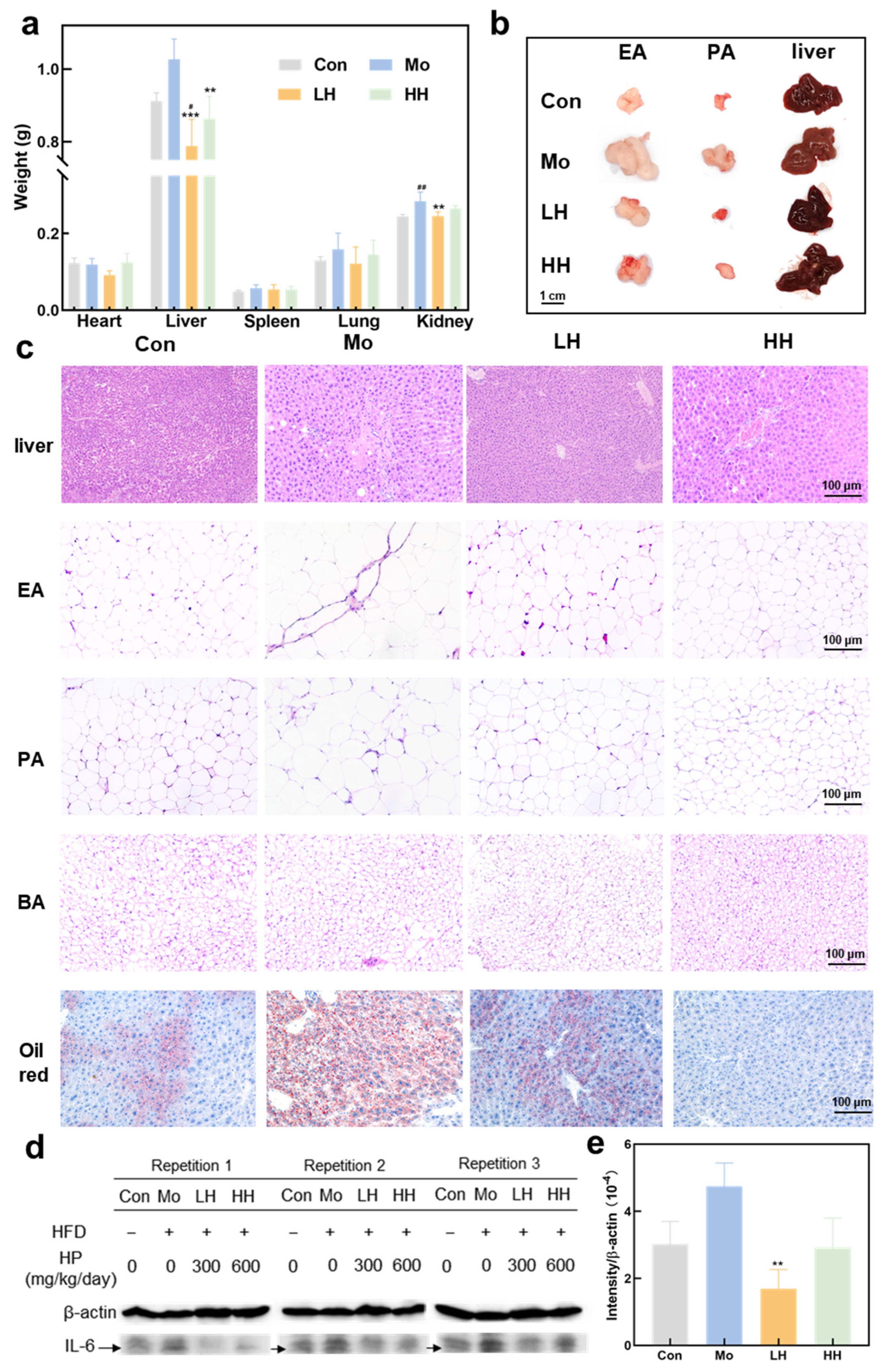
2.3. HE Ameliorates Adipose Tissue Enlargement and Attenuates Lipid Deposition and Inflammation in the Liver
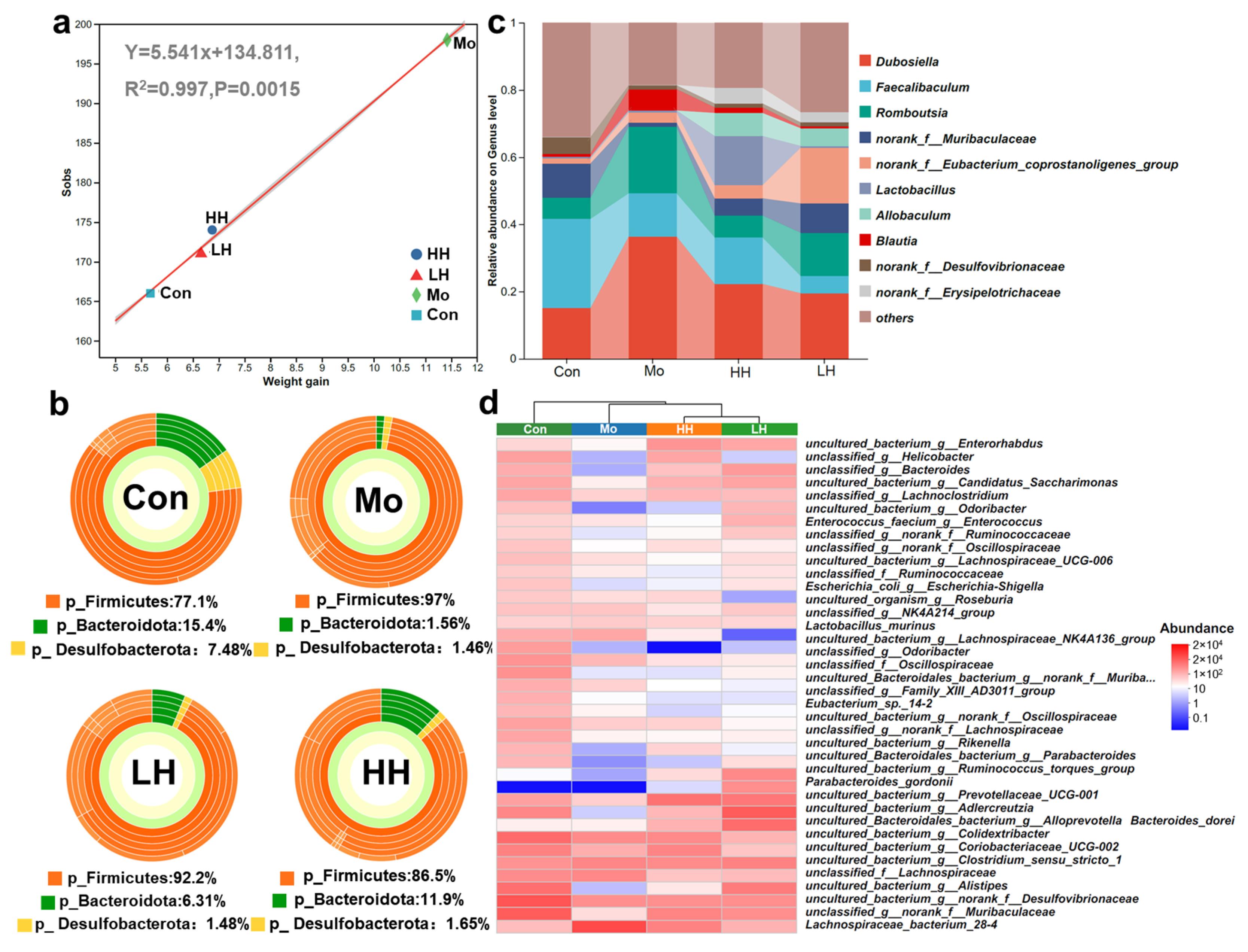
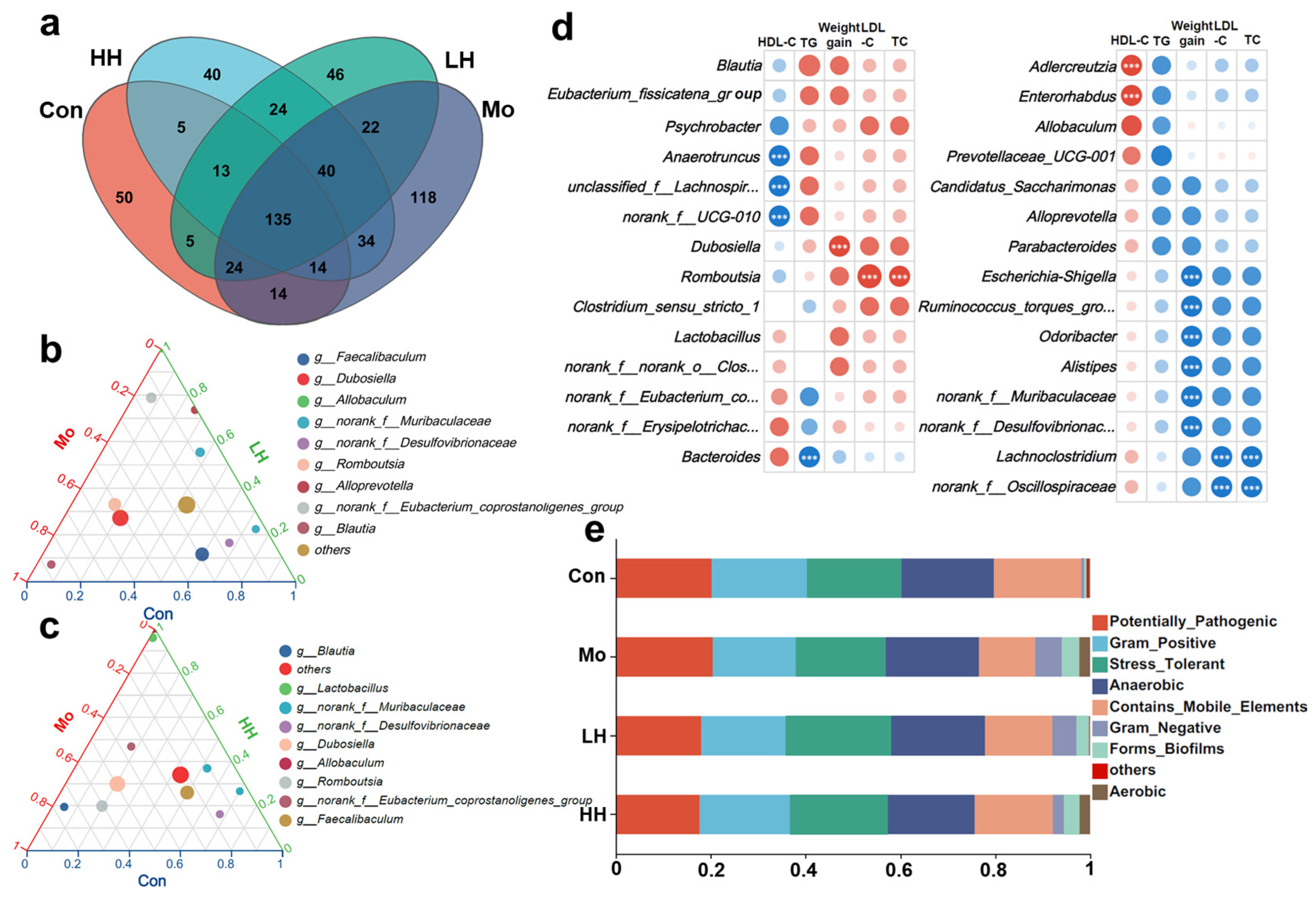
2.4. HE Altered Intestinal Microbiological Compositions of HFD Mice
2.5. HE Treatment Intervened in the Serum Lipid Profiles and Weight Growth Through Regulating Intestinal Microbiota
3. Materials and Methods
3.1. Materials
3.2. Preparation and Purification of HE
3.3. The Characterization of HE Chemical Profiles
3.3.1. UPLC–MS Analysis
3.3.2. Identification of UPLC–MS Analysis
3.3.3. HPLC Analysis
3.4. Experimental Management of Animals
3.5. Histological Analysis
3.6. Biochemical Measurement
3.7. Western Blot Analysis
3.8. Gut Microbiota Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, 23. [Google Scholar] [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.-P. Metabolic syndrome. Nat. Rev. Dis. Prim. 2024, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 January 2025).
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef] [PubMed]
- Webb, V.L.; Wadden, T.A. Intensive Lifestyle Intervention for Obesity: Principles, Practices, and Results. Gastroenterology 2017, 152, 1752–1764. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Gut microbiota in overweight and obesity: Crosstalk with adipose tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- Baser, O.; Samayoa, G.; Rodchenko, K.; Isenman, L.; Baser, E.; Yapar, N. The association between weight loss medications and cardiovascular complications. Obesity 2024, 32, 1401–1409. [Google Scholar] [CrossRef]
- Armstrong, S.C.; Bolling, C.F.; Michalsky, M.P.; Reichard, K.W.; Haemer, M.A.; Muth, N.D.; Rausch, J.C.; Rogers, V.W.; Heiss, K.F.; Besner, G.E. Pediatric metabolic and bariatric surgery: Evidence, barriers, and best practices. Pediatrics 2019, 144, e20193223. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lu, J.; Peng, C.; Lin, L. Natural constituents from food sources as therapeutic agents for obesity and metabolic diseases targeting adipose tissue inflammation. Crit. Rev. Food Sci. Nutr. 2020, 61, 1947–1965. [Google Scholar] [CrossRef]
- Li, S.Z.; Zeng, S.L.; Liu, E.H. Anti-obesity natural products and gut microbiota. Food Res. Int. 2022, 151, 110819. [Google Scholar] [CrossRef]
- Sandner, G.; König, A.; Wallner, M.; Weghuber, J. Functional foods—Dietary or herbal products on obesity: Application of selected bioactive compounds to target lipid metabolism. Curr. Opin. Food Sci. 2020, 34, 9–20. [Google Scholar] [CrossRef]
- Ouyang, H.; Fan, Y.; Wei, S.; Chang, Y.; He, J. Study on the Chemical Profile of Chrysanthemum (Chrysanthemum morifolium) and the Evaluation of the Similarities and Differences between Different Cultivars. Chem. Biodivers. 2022, 19, e202200252. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Nie, J.; Wang, S.; Zhang, Y.; Li, C.; Shao, S.; Shao, S.; Rogers, K.M.; Yuan, Y. Geographical origin authentication of edible Chrysanthemum morifolium Ramat. (Hangbaiju) Using Stable Isotopes. Separations 2023, 10, 287. [Google Scholar] [CrossRef]
- Yao, Z.; Miao, J.; Xu, W.; Lu, Q. The complete chloroplast genome sequence of a cultivar of Chrysanthemum, Chrysanthemum × morifolium ‘Hangbaiju’(Asteraceae). Mitochondrial DNA Part B 2024, 9, 403–407. [Google Scholar] [CrossRef]
- Chen, M.Z.; Wang, K.L.; Zhang, Y.N.; Zhang, M.D.; Ma, Y.J.; Sun, H.F.; Jin, Z.X.; Zheng, H.; Jiang, H.; Yu, P.; et al. New insights into the biological activities of Chrysanthemum morifolium: Natural flavonoids alleviate diabetes by targeting α-glucosidase and the PTP-1B signaling pathway. Eur. J. Med. Chem. 2019, 178, 108–115. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, P.Y.; Luo, Y.H.; Gao, B.Y.; Sun, J.H.; Lu, W.Y.; Liu, J.; Chen, P.; Zhang, Y.Q.; Yu, L.L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef]
- Yasuda, M.T.; Fujita, K.; Hosoya, T.; Imai, S.; Shimoi, K. Absorption and Metabolism of Luteolin and Its Glycosides from the Extract of Chrysanthemum morifolium Flowers in Rats and Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 7693–7699. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Dong, G.; Zhang, X.; Liu, Y.; Sun, W.; Liu, A. Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat flowers: A potentially rich source of bioactive compounds. Food Chem. 2021, 344, 128733. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, X.; He, Z.; Xie, Y.; Niu, Z.; Zhang, W.; Wang, A.; Zhang, J.; Si, C.; Li, F. Apigenin-7-O-glucoside alleviates DSS-induced colitis by improving intestinal barrier function and modulating gut microbiota. J. Funct. Foods 2023, 104, 105499. [Google Scholar] [CrossRef]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 95. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin alleviates obesity-associated metabolic syndrome by regulating the composition of the gut microbiome. Front. Microbiol. 2022, 12, 805827. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Chen, L.; Sun, G. Hypolipidemic Effects and Preliminary Mechanism of Chrysanthemum Flavonoids, Its Main Components Luteolin and Luteoloside in Hyperlipidemia Rats. Antioxidants 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.C.; Kim, W.C.; Ryu, R.; Wu, Z.; Seo, J.-S.; Choi, M.-S.; Liu, K.-H. Plasma lipidomics reveals insights into anti-obesity effect of Chrysanthemum morifolium ramat leaves and its constituent luteolin in high-fat diet-induced dyslipidemic mice. Nutrients 2020, 12, 2973. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Lee, M.-S.; Chang, E.; Kim, Y. Chrysanthemum morifolium Flower Extract Ameliorates Obesity-Induced Inflammation and Increases the Muscle Mitochondria Content and AMPK/SIRT1 Activities in Obese Rats. Nutrients 2021, 13, 3660. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Hu, C.; Wang, Z.; Xu, Q.; Pan, R.; Li, X.; Christian, M.; Zhu, P.; Zhao, J. Chrysanthemum extract mitigates high-fat diet-induced inflammation, intestinal barrier damage and gut microbiota disorder. Food Funct. 2025, 16, 1347–1359. [Google Scholar] [CrossRef]
- Liu, D.; Zhan, J.; Wang, S.; Chen, L.; Zhu, Q.; Nie, R.; Zhou, X.; Zheng, W.; Luo, X.; Wang, B. Chrysanthemum morifolium attenuates metabolic and alcohol-associated liver disease via gut microbiota and PPARα/γ activation. Phytomedicine 2024, 130, 155774. [Google Scholar] [CrossRef]
- Kim, W.J.; Yu, H.S.; Bae, W.Y.; Ko, K.Y.; Chang, K.H.; Lee, N.K.; Paik, H.D. Chrysanthemum indicum suppresses adipogenesis by inhibiting mitotic clonal expansion in 3T3-L1 preadipocytes. J. Food Biochem. 2021, 45, e13896. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Alvarado-Corella, D.; Vargas-Huertas, F.; Sánchez-Kopper, A. HRMS Characterization, antioxidant and cytotoxic activities of polyphenols in Malus domestica cultivars from Costa Rica. Molecules 2021, 26, 7367. [Google Scholar] [CrossRef]
- Huang, H.; Han, M.-H.; Gu, Q.; Wang, J.-D.; Zhao, H.; Zhai, B.-W.; Nie, S.-M.; Liu, Z.-G.; Fu, Y.-J. Identification of pancreatic lipase inhibitors from Eucommia ulmoides tea by affinity-ultrafiltration combined UPLC-Orbitrap MS and in vitro validation. Food Chem. 2023, 426, 136630. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, H.; Xin, M.; Liu, Y.; Tong, J.; Liang, J.; Sun, L. Comparative Analysis of the Chemical Constituents and Hypoglycemic Activities of Two Chinese Juhua Teas (Chrysanthemum morifolium Ramat. and Coreopsis tinctoria Nutt.). Chem. Biodivers. 2025, 22, e202500008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jia, L.; Wang, X.; Liu, T.; Qin, W.; Ma, H.; Lv, Y.; Hu, J.; Guo, Q.; Tan, S. Anti-aging formula protects skin from oxidative stress-induced senescence through the inhibition of CXCR2 expression. J. Ethnopharmacol. 2024, 318, 116996. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, W.; Zhang, Y.; Ni, L.; Lin, Y.; Zhang, X.; Huang, M. Qualitative and quantitative analysis of phenolic compounds by UPLC-MS/MS and biological activities of Pholidota chinensis Lindl. J. Pharm. Biomed. Anal. 2020, 187, 113350. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.; Balachandran, I. LC/MS characterization of phenolic antioxidants of Brindle berry (Garcinia gummi-gutta (L.) Robson). Nat. Prod. Product. Res. 2017, 31, 1191–1194. [Google Scholar] [CrossRef]
- Kang, S.A.; Choi, J.H.; Baek, K.-W.; Lee, D.I.; Jeong, M.-J.; Yu, H.S. Trichinella spiralis infection ameliorated diet-induced obesity model in mice. Int. J. Parasitol. 2021, 51, 63–71. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Jin, H.; Han, H.; Song, G.; Oh, H.-J.; Lee, B.-Y. Anti-obesity effects of GABA in C57BL/6J mice with high-fat diet-induced obesity and 3T3-L1 adipocytes. Int. J. Mol. Sci. 2024, 25, 995. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef]
- Lin, S.-X.; Yang, C.; Jiang, R.-S.; Wu, C.; Lang, D.-Q.; Wang, Y.-L.; Li, X.-Y.; Jiang, C.-P.; Liu, Q.; Shen, C.-Y. Flavonoid extracts of Citrus aurantium L. var. amara Engl. Promote browning of white adipose tissue in high-fat diet-induced mice. J. Ethnopharmacol. 2024, 324, 117749. [Google Scholar] [CrossRef]
- Liang, W.; Lindeman, J.H.; Menke, A.L.; Koonen, D.P.; Morrison, M.; Havekes, L.M.; van den Hoek, A.M.; Kleemann, R. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1β-induced chronic inflammation. Lab. Investig. 2014, 94, 491–502. [Google Scholar] [CrossRef]
- Scorletti, E.; Carr, R.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.-Y.; He, G.; Ali, S.R.; Holzer, R.G.; Österreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Giraldez, M.D.; Carneros, D.; Garbers, C.; Rose-John, S.; Bustos, M. New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 787–803. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Afkhami, H.; Javadi, A.; Kashfi, M. Understanding the complex function of gut microbiota: Its impact on the pathogenesis of obesity and beyond: A comprehensive review. Diabetol. Metab. Syndr. 2024, 16, 308. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, L.; Liao, Y.; Lin, Z.; Guo, C.; Luo, S.; Wang, F.; Zou, Z.; Zeng, Z.; Chen, C. Semaglutide alleviates gut microbiota dysbiosis induced by a high-fat diet. Eur. J. Pharmacol. 2024, 969, 176440. [Google Scholar] [CrossRef] [PubMed]
- Karačić, A.; Renko, I.; Krznarić, Ž.; Klobučar, S.; Liberati Pršo, A.-M. The Association between the Firmicutes/Bacteroidetes Ratio and Body Mass among European Population with the Highest Proportion of Adults with Obesity: An Observational Follow-Up Study from Croatia. Biomedicines 2024, 12, 2263. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Liu, S.; Niu, Y.; Fu, L. SESN2 ablation weakens exercise benefits on resilience of gut microbiota following high-fat diet consumption in mice. Food Sci. Hum. Wellness 2023, 12, 1961–1968. [Google Scholar] [CrossRef]
- Ke, W.; Bonilla-Rosso, G.; Engel, P.; Wang, P.; Chen, F.; Hu, X. Suppression of high-fat diet-induced obesity by platycodon grandiflorus in mice is linked to changes in the gut microbiota. J. Nutr. 2020, 150, 2364–2374. [Google Scholar] [CrossRef]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2020, 64, 103687. [Google Scholar] [CrossRef]
- Tong, L.-T.; Xiao, T.; Wang, L.; Lu, C.; Liu, L.; Zhou, X.; Wang, A.; Qin, W.; Wang, F. Plant protein reduces serum cholesterol levels in hypercholesterolemia hamsters by modulating the compositions of gut microbiota and metabolites. iScience 2021, 24, 103435. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. Iscience 2021, 24, 103342. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Fu, J.; Duan, Z.; Qiu, W.; Cai, Y.; Ma, W.; Zhou, H.; Chen, Y.; Zheng, J. Sex-and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort. Microorganisms 2023, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, J.; Luo, Y.; Wen, B.; Wu, W.; Zeng, H.; Zhonghua, L. Fuzhuan brick tea attenuates high-fat diet-induced obesity and associated metabolic disorders by shaping gut microbiota. J. Agric. Food Chem. 2019, 67, 13589–13604. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, J.; Zhu, L.; Guo, T.; Qin, D.; Hu, Z.; Han, S.; Wang, J.; Matias, F.B.; Wen, L. Oryzanol alleviates high fat and cholesterol diet-induced hypercholesterolemia associated with the modulation of the gut microbiota in hamsters. Food Funct. 2022, 13, 4486–4501. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-L.; Yang, J.-W.; Dou, H.-Y.; Li, G.-Q.; Li, X.-Y.; Shen, L.; Ji, H.-F. Anti-inflammatory effect of luteolin is related to the changes in the gut microbiota and contributes to preventing the progression from simple steatosis to nonalcoholic steatohepatitis. Bioorg. Chem. 2021, 112, 104966. [Google Scholar] [CrossRef]
- Tao, J.H.; Duan, J.A.; Qian, Y.Y.; Qian, D.W.; Guo, J.M. Investigation of the interactions between Chrysanthemum morifolium flowers extract and intestinal bacteria from human and rat. Biomed. Chromatogr. 2016, 30, 1807–1819. [Google Scholar] [CrossRef]
- Qiu, X.; Macchietto, M.G.; Liu, X.; Lu, Y.; Ma, Y.; Guo, H.; Saqui-Salces, M.; Bernlohr, D.A.; Chen, C.; Shen, S. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int. J. Obes. 2021, 45, 143–154. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef]
- Yang, C.; Yan, K.; Ma, C.; Xie, L.; Wang, W.; Chen, W.; Mao, B. Insight into the root growth, soil quality, and assembly of the root-associated microbiome in the virus-free Chrysanthemum morifolium. Ind. Crops Prod. 2022, 176, 114362. [Google Scholar] [CrossRef]
- Yan, K.; Du, X.; Mao, B. Production of virus-free chrysanthemum (Chrysanthemum morifolium Ramat) by tissue culture techniques. In Plant Virology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 171–186. [Google Scholar]
- Feng, X.; Chen, Y.; Luo, L.; Fang, Z.; Ma, S.; Li, Z.; Huang, J.; Pan, Y.; Lv, H.; Gong, S. Liubao insect tea polyphenols ameliorate DSS-induced experimental colitis by protecting intestinal barrier and regulating intestinal microbiota. Food Chem. 2025, 467, 142156. [Google Scholar] [CrossRef]
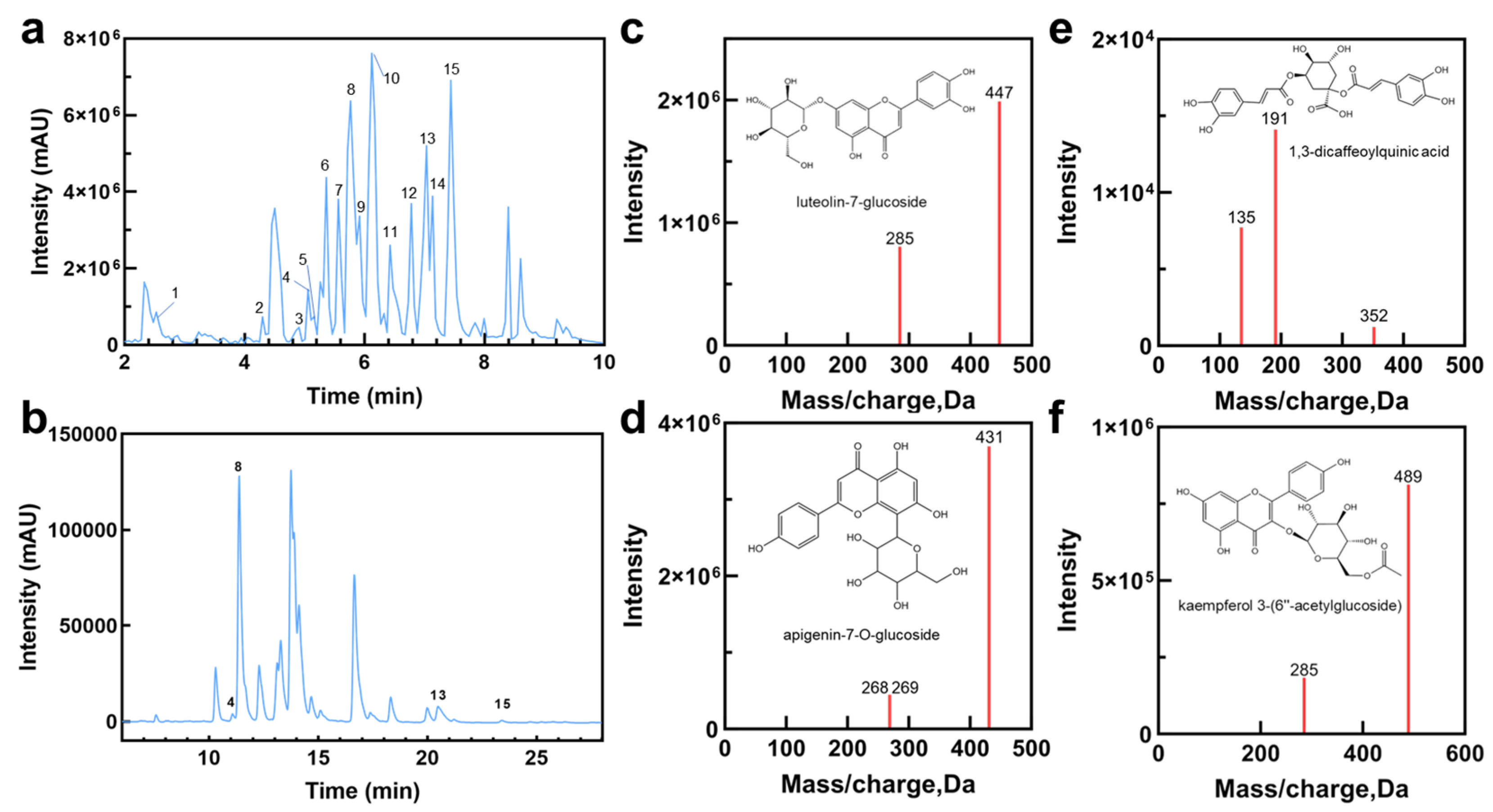
| No. | Rt (min) | Observed m/z | Formula | Mass Error (ppm) | MS/MS | Tentative Identification | Category | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.527 | 341.0886 | C15H18O9 | −2.04 | 89, 135, 161, 179, 207, 251 | Caffeoyl hexoside | Flavonoid glycoside | [31] |
| 2 | 4.299 | 401.1457 | C18H26O10 | −1.25 | 101, 161, 233, 269 | Benzyl-β-primeveroside | Flavonoid glycosides | [32] |
| 3 | 4.906 | 593.1518 | C27H30O15 | −0.64 | 353, 383, 473 | Safflor yellow A | Flavonoid | NIST 17 |
| 4 | 5.075 | 463.0892 | C21H17O12 | −2.15 | 113, 151, 175, 287 | 5,7,3′,5′-Tetrahydroxyflavanone 7-O-glucuronide | Flavonoid glycosides | [33] |
| 5 | 5.176 | 461.1668 | C20H30O12 | −0.84 | 59, 89, 149, 191, 287, 415 | Decaffeoyl verbascoside | Flavonoid glycosides | [34] |
| 6 | 5.362 | 449.1111 | C21H22O11 | −4.1 | 135, 151, 287 | Eriodicyol-7-O-glucoside | Flavonoid glycosides | NIST 17 |
| 7 | 5.564 | 515.1214 | C25H24O12 | 2.9 | 135, 191, 352 | 1,3-Dicaffeoylquinic acid (cynarin) | Caffeoyl quinic acid derivatives | NIST 17 [35] |
| 8 | 5.75 | 447.098 | C21H20O11 | −2 | 285, 447 | Luteolin-7-glucoside | Flavonoid glycosides | [33] |
| 9 | 5.936 | 515.1215 | C25H24O12 | −3.36 | 173, 353, 515 | 4,5-Dicaffeoylquinic acid | Caffeoyl quinic acid derivatives | [33] |
| 10 | 6.138 | 431.1049 | C21H20O10 | −0.9 | 268, 269, 311 | Apigenin-7-O-glucoside | Flavonoids and their glycosides | [33] |
| 11 | 6.425 | 287.0569 | C15H12O6 | −2.53 | 65, 107, 135, 151 | Okanin | Flavonoids derivatives | [33] |
| 12 | 6.78 | 489.1054 | C23H22O12 | −2.55 | 285, 489 | Kaempferol 3-O-β-D-6″-acetylglucoside | Flavonoid glycosides | [36] |
| 13 | 6.999 | 285.0427 | C15H10O6 | −6.7 | 65, 107, 133, 175 | Luteolin | Flavonoid glycosides | [33] |
| 14 | 7.134 | 473.1105 | C23H22O11 | −2.54 | 63, 151, 240, 268, 311 | Apigenin-7-O-6″-acetyl-glucoside | Flavonoid glycosides | [33] |
| 15 | 7.438 | 269.0492 | C15H10O5 | −3 | 107, 117, 151, 201, 225 | Apigenin | Flavonoid | NIST 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Huang, J.; Xiang, L.; Zhang, F.; Wang, X.; Yan, A.; Pan, Y.; Chen, P.; Mao, B.; Chu, Q. Polyphenol-Rich Extract of Chrysanthemum × morifolium (Ramat) Hemsl. (Hangbaiju) Prevents Obesity and Lipid Accumulation Through Restoring Intestinal Microecological Balance. Plants 2025, 14, 2393. https://doi.org/10.3390/plants14152393
Feng X, Huang J, Xiang L, Zhang F, Wang X, Yan A, Pan Y, Chen P, Mao B, Chu Q. Polyphenol-Rich Extract of Chrysanthemum × morifolium (Ramat) Hemsl. (Hangbaiju) Prevents Obesity and Lipid Accumulation Through Restoring Intestinal Microecological Balance. Plants. 2025; 14(15):2393. https://doi.org/10.3390/plants14152393
Chicago/Turabian StyleFeng, Xinyu, Jing Huang, Lin Xiang, Fuyuan Zhang, Xinxin Wang, Anran Yan, Yani Pan, Ping Chen, Bizeng Mao, and Qiang Chu. 2025. "Polyphenol-Rich Extract of Chrysanthemum × morifolium (Ramat) Hemsl. (Hangbaiju) Prevents Obesity and Lipid Accumulation Through Restoring Intestinal Microecological Balance" Plants 14, no. 15: 2393. https://doi.org/10.3390/plants14152393
APA StyleFeng, X., Huang, J., Xiang, L., Zhang, F., Wang, X., Yan, A., Pan, Y., Chen, P., Mao, B., & Chu, Q. (2025). Polyphenol-Rich Extract of Chrysanthemum × morifolium (Ramat) Hemsl. (Hangbaiju) Prevents Obesity and Lipid Accumulation Through Restoring Intestinal Microecological Balance. Plants, 14(15), 2393. https://doi.org/10.3390/plants14152393








