Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth
Abstract
1. Introduction
2. Results
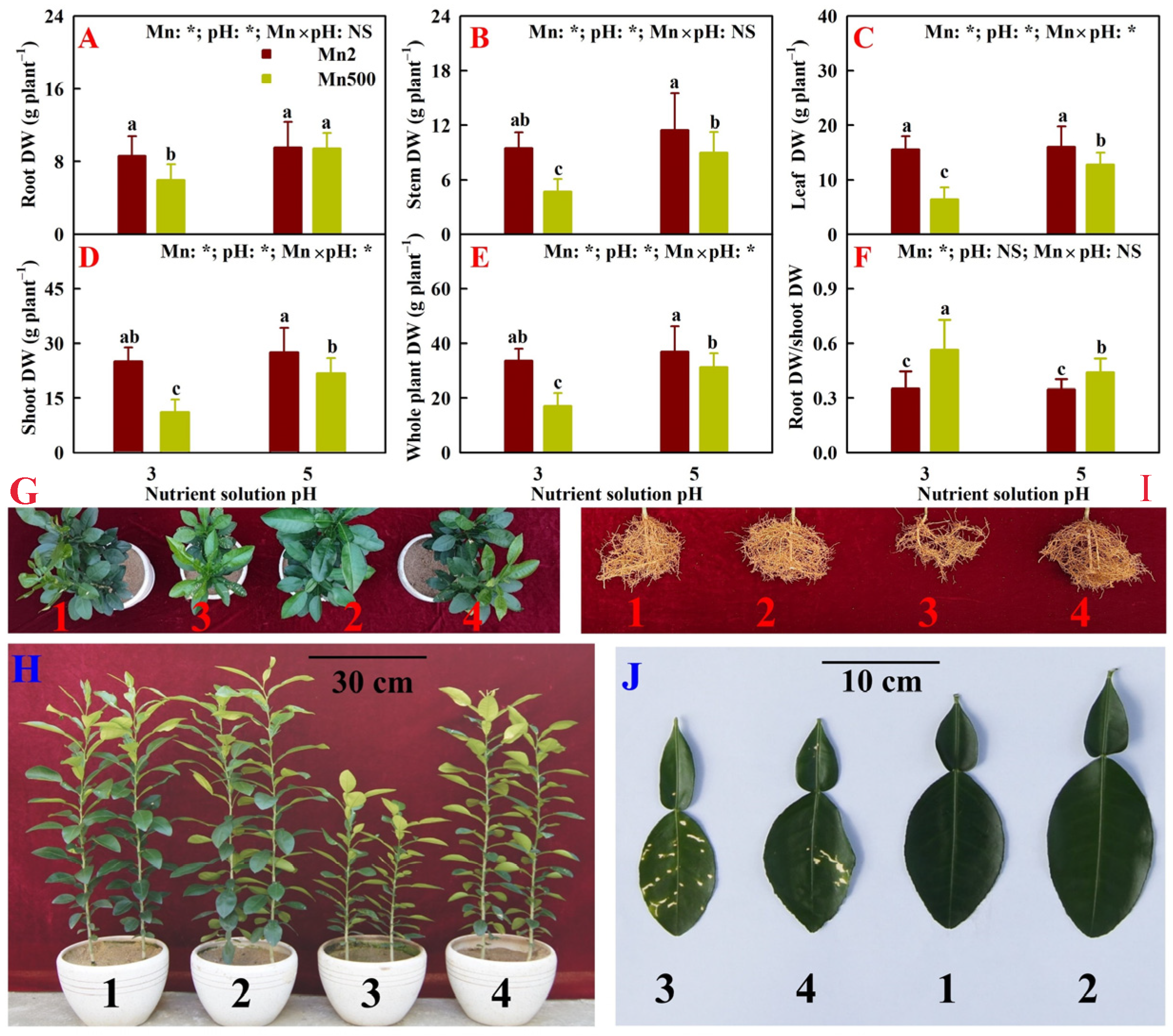
2.1. Effects of Mn-pH Treatments on Seedling Growth
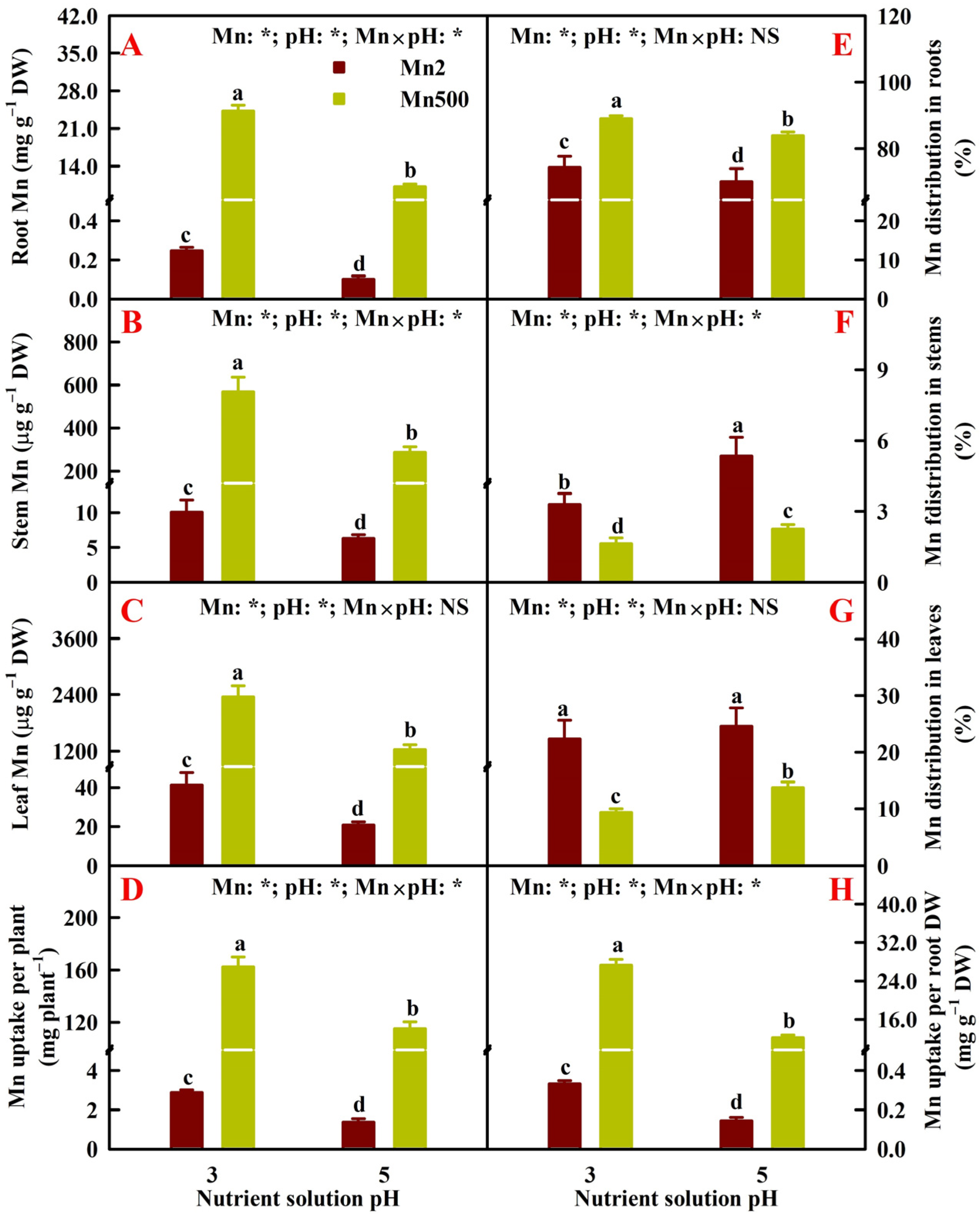
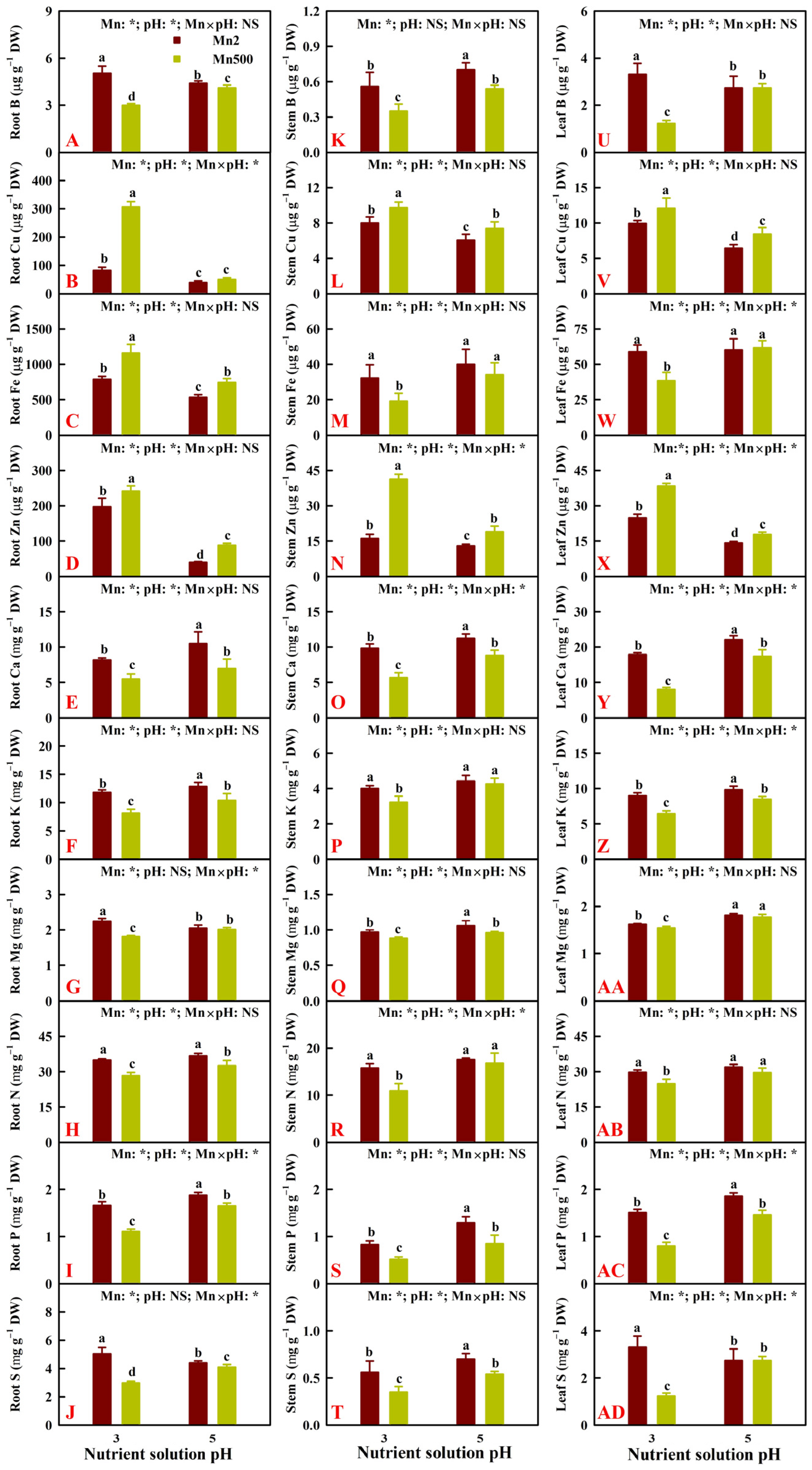
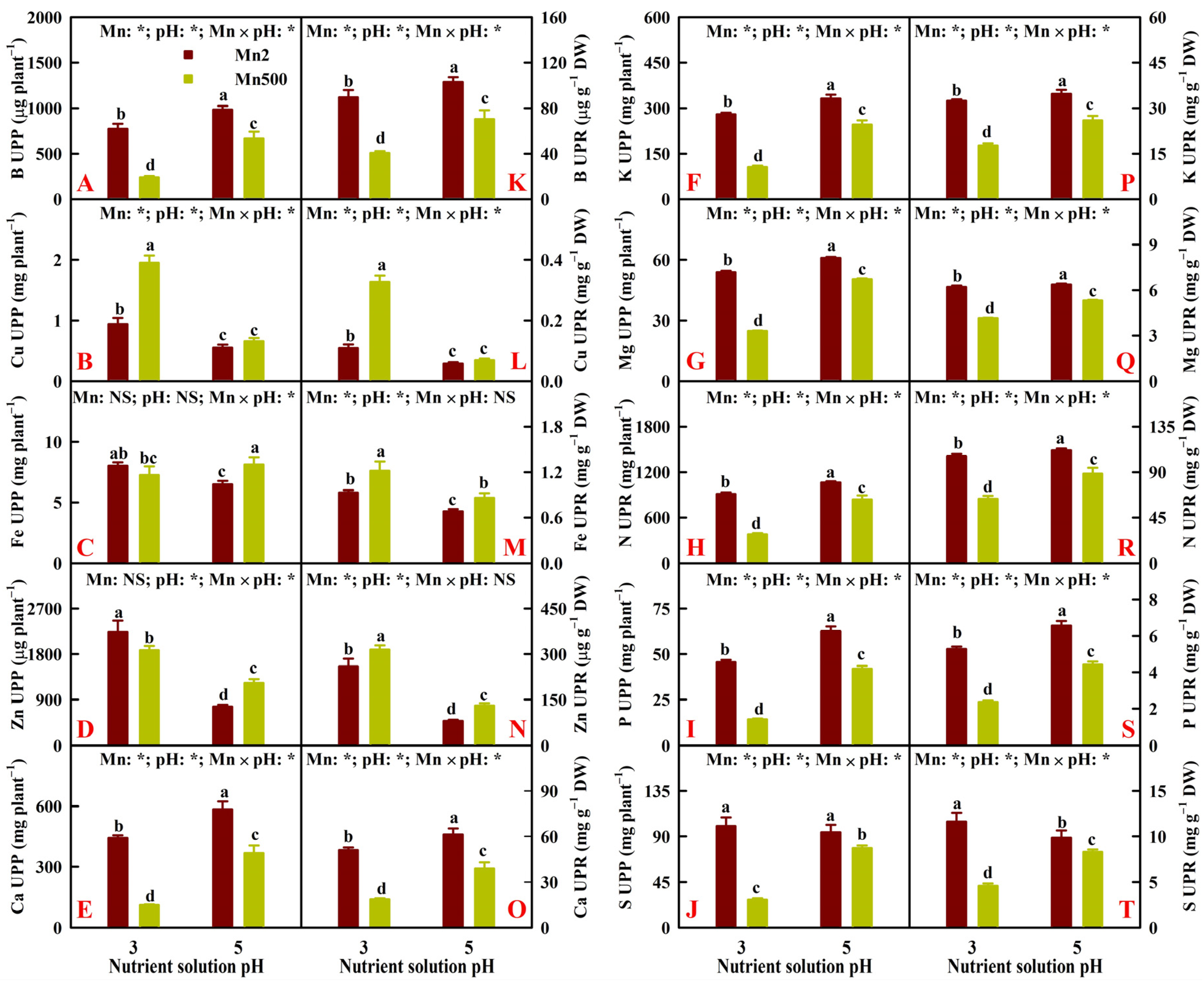
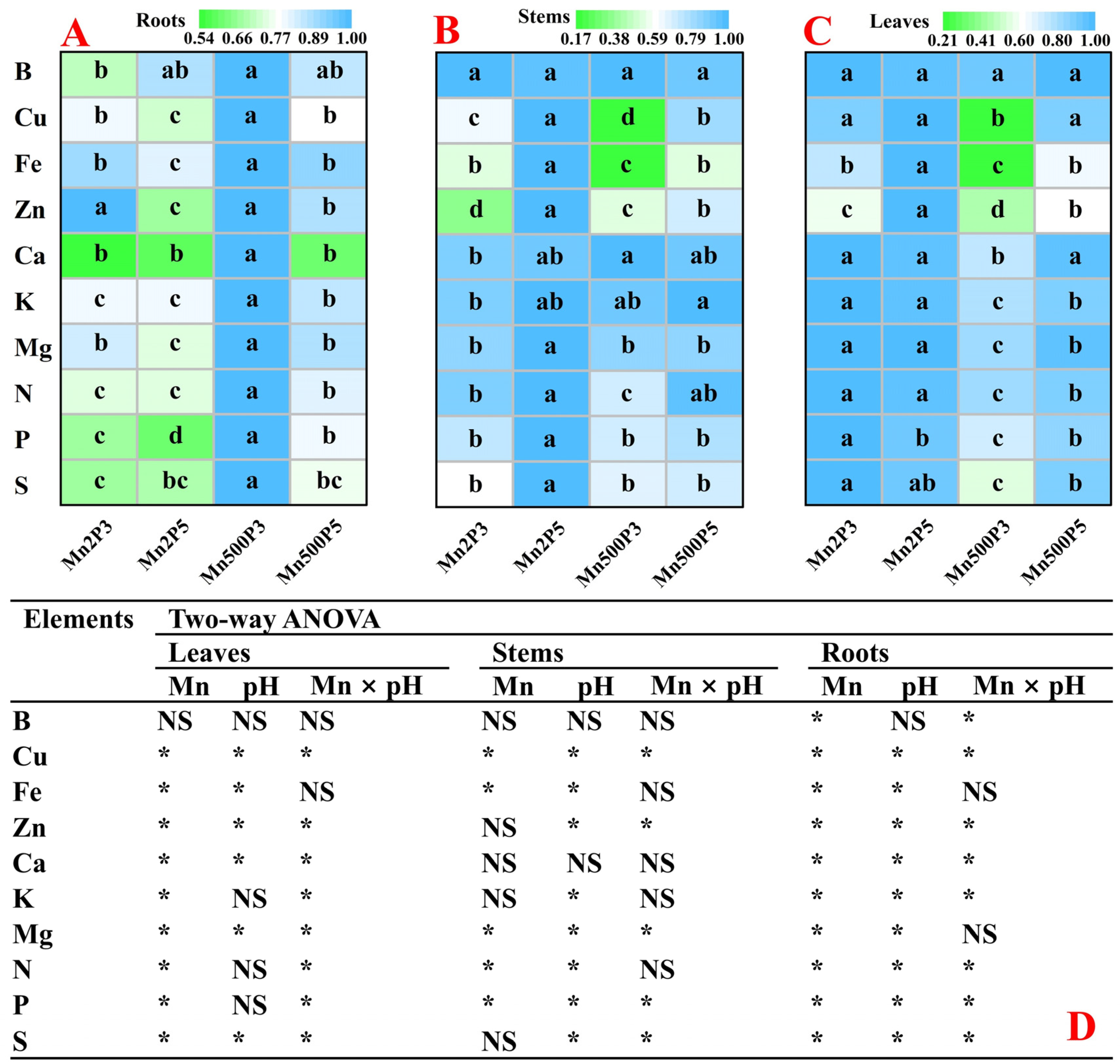
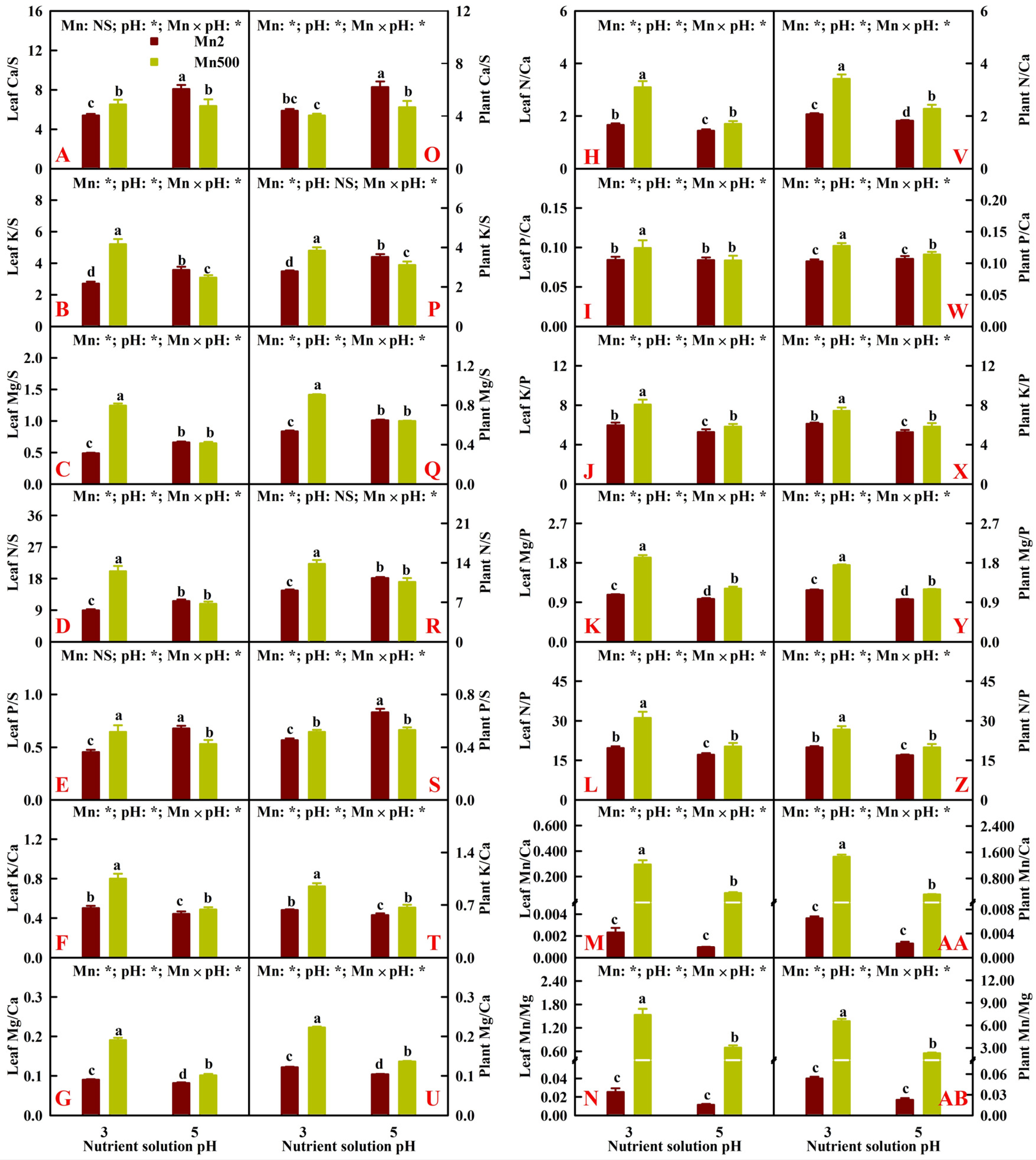
2.2. Effects of Mn-pH Treatments on Nutrient Uptake, Distributions, and Balance in Roots, Stems, and Leaves
2.3. Effects of Mn-pH Treatments on Leaf Pigments and Gas Exchange
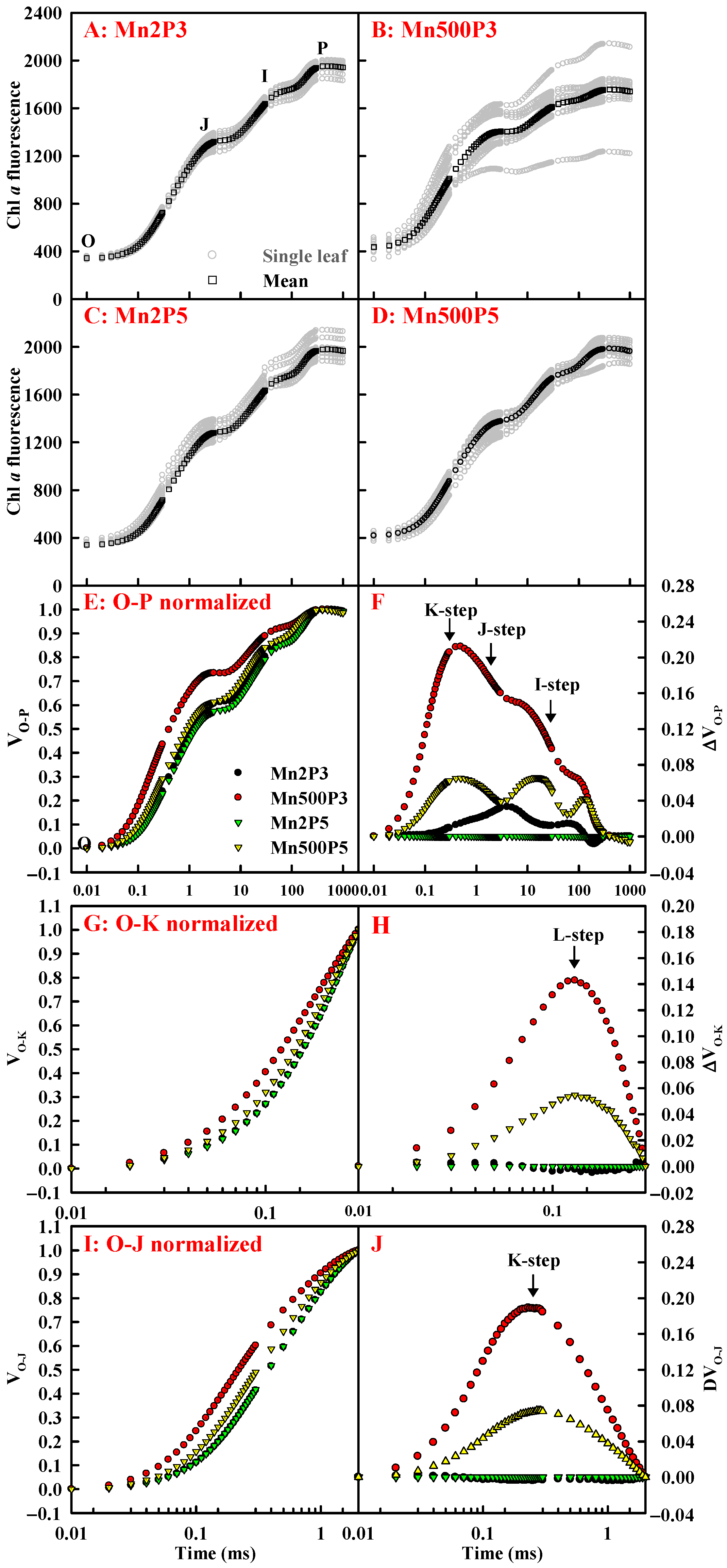
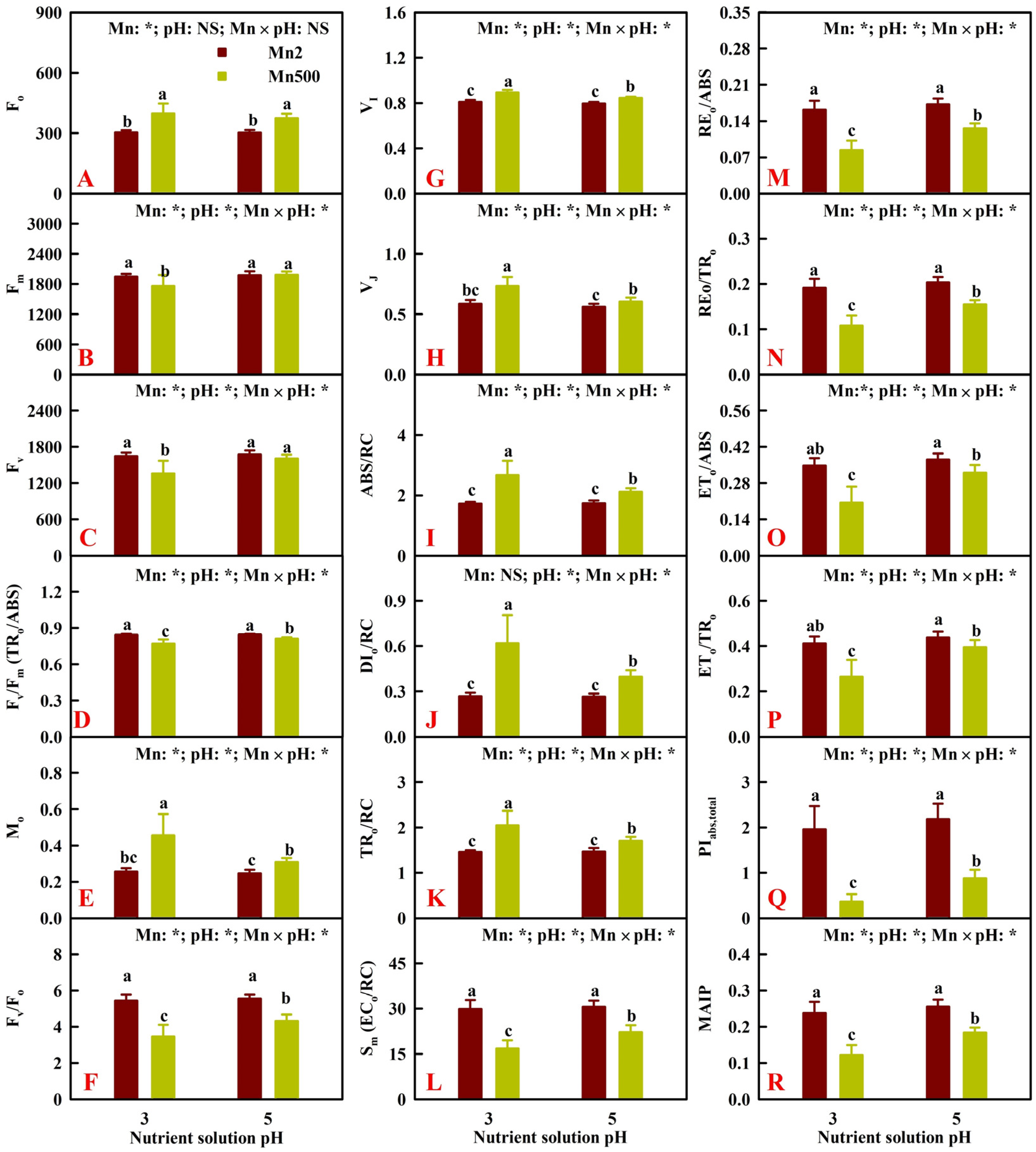
2.4. Effects of Mn-pH Treatments on Leaf OJIP Transients and Fluorescence Parameters
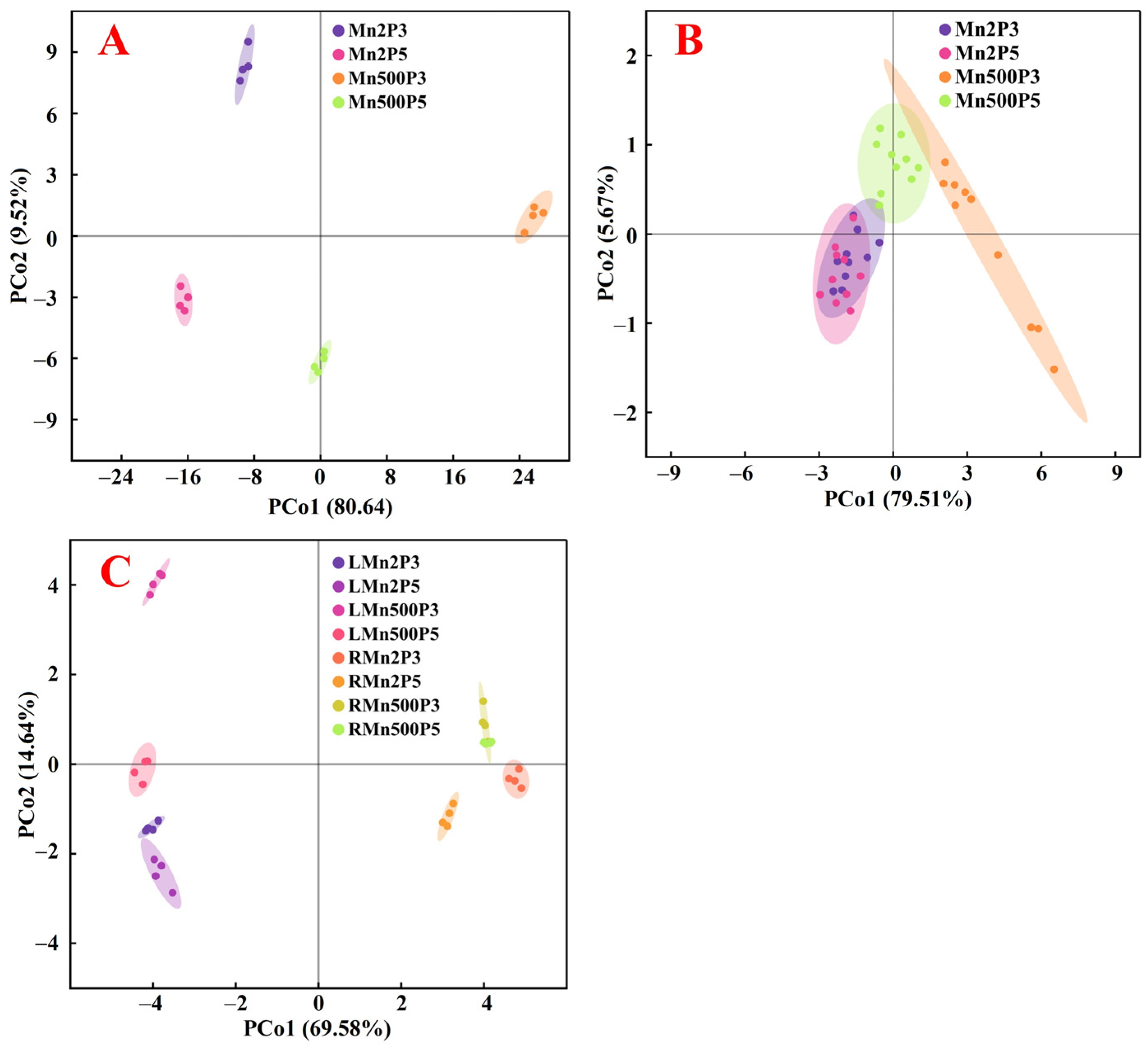
2.5. Principal Coordinate Analysis, Regression Analysis, and the Different Alterations in Physiological Parameters Between Roots and Leaves Caused by Mn-pH Treatments
3. Discussion
3.1. Increased pH-Mediated Mitigation of Mn500 in ‘Sour Pummelo’ Involved Lessened Uptake of Mn Rather than Elevated Mn Distribution in Roots
3.2. Increased pH-Mediated Mitigation of Mn500 in ‘Sour Pummelo’ Involved Efficient Maintenance of Nutrient Homeostasis
3.3. Increasing pH Mitigated Mn500-Induced Decline in Leaf ACO2 and Subsequent Decrease in Seedling Growth
4. Materials and Methods
4.1. Plant Materials
4.2. Measurements of Leaf Gas Exchange and OJIP Transient
4.3. Measurements of Biomass, Pigments, and Nutrients
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Zheng, Z.-C.; Chen, H.-H.; Yang, H.; Shen, Q.; Chen, X.-F.; Huang, W.-L.; Yang, L.-T.; Guo, J.; Chen, L.-S. Citrus sinensis manganese tolerance: Insight from manganese-stimulated secretion of root exudates and rhizosphere alkalization. Plant Physiol. Biochem. 2024, 206, 108318. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Liu, L.; Dong, R.; Liu, G.; Rao, I.M.; Chen, Z. Phenylalanine ammonia-lyase 2 regulates secondary metabolism and confers manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2025, 197, kiaf005. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Wang, Z.; Liu, G.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Hasanuzzaman, M.; Hussain, S.; Li, G.; Liu, J. Phosphorus confers tolerance against manganese toxicity in Prunus persica by reducing oxidative stress and improving chloroplast ultrastructure. Chemosphere 2022, 291, 132999. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.Y.; Yuan, M.; Zhu, P.P.; Ling, L.L.; Cao, L.; Fu, X.Z.; Peng, L.Z. Tolerance and physiological response of citrus rootstock cultivars to manganese toxicity. J. Plant Nutr. Fert. 2021, 27, 109–121. [Google Scholar]
- Papadakis, I.E.; Giannakoula, A.; Therios, I.N.; Bosabalidis, A.M.; Moustakas, M.; Nastou, A. Mn-induced changes in leaf structure and chloroplast ultrastructure of Citrus volkameriana (L.) plants. J. Plant Physiol. 2007, 164, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xu, W.; Sharma, A.; Singh, V.P.; Tripathi, D.K.; Yan, D.; Yuan, H.; Chen, H.; Wu, R.; Zheng, B.; et al. Physiological mechanisms of Carya illinoensis tolerance to manganese stress. Plant Physiol. Biochem. 2025, 219, 109461. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.-S.; Jiang, H.-X.; Tang, N.; Yang, L.-T.; Lin, Z.-H.; Li, Y.; Yang, G.-H. Effects of manganese-excess on CO2 assimilation, ribulose-1, 5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol. 2010, 10, 42. [Google Scholar] [CrossRef]
- Xu, F.Q.; Meng, L.L.; Lei, A.Q.; Hashem, A.A.A.; Elsayed, F.; Wu, Q.S. Arbuscular mycorrhizal fungi mitigate manganese toxicity on trifoliate orange. Sci. Horti. 2024, 338, 113722. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T. Comparison of photosynthetic responses to manganese toxicity of deciduous broad-leaved trees in northern Japan. Environ. Pollut. 1997, 97, 113–118. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, A.K.; Biswal, U.C. Manganese induced peroxidation of thylakoid lipids and changes in chlorophyll a fluorescence during aging of cell free chloroplasts in light. Phytochemistry 1987, 26, 3217–3219. [Google Scholar] [CrossRef]
- St Clair, S.B.; Lynch, J.P. Photosynthetic and antioxidant enzyme responses of sugar maple and red maple seedlings to excess manganese in contrasting light environments. Funct. Plant Biol. 2004, 31, 1005–1014. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T. Effects of manganese toxicity on photosynthesis of white birch (Betula platyphylla var. japonica) seedlings. Physiol. Plant. 1997, 101, 249–256. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Pan, Y.; Chen, J.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef]
- Xu, F.Q.; Meng, L.L.; Kuča, K.; Wu, Q.S. The mechanism of arbuscular mycorrhizal fungi-alleviated manganese toxicity in plants: A review. Plant Physiol. Biochem. 2024, 213, 108808. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Berríos, G.; Rodrigues-Salvador, A.; Nunes-Nesi, A.; Deppe, M.; Demanet, R.; Rengel, Z.; Alberdi, M. Physiological and biochemical responses to manganese toxicity in ryegrass (Lolium perenne L.) genotypes. Plant Physiol. Biochem. 2017, 113, 89–97. [Google Scholar] [CrossRef]
- Hafeez, A.; Rasheed, R.; Ashraf, M.A.; Rizwan, M.; Ali, S. Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 2022, 308, 136523. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Wentao, C.; Zhu, K.; Hasanuzzaman, M.; Li, G.; Liu, J. Phosphorus-induced restructuring of the ascorbate-glutathione cycle and lignin biosynthesis alleviates manganese toxicity in peach roots. Tree Physiol. 2024, 44, tpae098. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.G. Soil pH and magnesium effects on manganese toxicity in peanuts. J. Plant Nutr. 1996, 19, 535–550. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.J.; Carvalho, M.L.; Van Beusichem, M.L.; Kirkby, E.A. The significance of the magnesium to manganese ratio in plant tissues for growth and alleviation of manganese toxicity in tomato (Lyconersicon esculentum) and wheat (Triticum aestivum) plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.J.G.P.R. Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant Soil 1992, 139, 91–98. [Google Scholar] [CrossRef]
- Liu, B.; Wang, B.; Chen, T.; Zhang, M. Hydrogen sulfide mitigates manganese-induced toxicity in Malus hupehensis plants by regulating osmoregulation, antioxidant defense, mineral homeostasis, and glutathione ascorbate circulation. Horticulturae 2025, 11, 133. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; Li, C.; Howard, D.L.; Cheng, M.; Tang, C.; Scheckel, K.G.; Noerpel, M.R.; Wang, P.; Menzies, N.W.; Kopittke, P.M. Evaluating effects of iron on manganese toxicity in soybean and sunflower using synchrotron-based X-ray fluorescence microscopy and X-ray absorption spectroscopy. Metallomics 2019, 11, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Vose, P.B.; Randall, P.J. Resistance to aluminum and manganese toxicities in plants related to variety and cation exchange capacity. Nature 1962, 196, 85–86. [Google Scholar] [CrossRef]
- You, X.; Yang, L.-T.; Lu, Y.-B.; Li, H.; Zhang, S.-Q.; Chen, L.-S. Proteomic changes of citrus roots in response to long-term manganese toxicity. Trees Struct. Funct. 2014, 28, 1383–1399. [Google Scholar] [CrossRef]
- Zhou, C.-P.; Qi, Y.-P.; You, X.; Yang, L.-T.; Guo, P.; Ye, X.; Zhou, X.-X.; Ke, F.-J.; Chen, L.-S. Leaf cDNA-AFLP analysis of two citrus species differing in manganese tolerance in response to long-term manganese-toxicity. BMC Genom. 2013, 14, 621. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Zhang, D.; Zhu, K.; Shen, W.; Pan, J.; Hasanuzzaman, M.; Li, G.; Liu, J. Silencing of PpNRAMP5 improves manganese toxicity tolerance in peach (Prunus persica) seedlings. J. Hazard. Mater. 2023, 454, 131442. [Google Scholar] [CrossRef] [PubMed]
- Hue, N.V.; Vega, S.; Silva, J.A. Manganese toxicity in a Hawaiian oxisol affected by soil pH and organic amendments. Soil Sci. Soc. Am. J. 2001, 65, 153–160. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The effect of pH on morphological and physiological root traits of Lupinus angustifolius treated with struvite as a recycled phosphorus source. Plant Soil 2018, 434, 65–78. [Google Scholar] [CrossRef]
- Tóth, B.; Juhász, C.; Labuschagne, M.; Moloi, M.J. The Influence of soil acidity on the physiological responses of two bread wheat cultivars. Plants 2020, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Rosas, A.; Rengel, Z.; Mora, M.L. Manganese supply and pH influence growth, carboxylate exudation and peroxidase activity of ryegrass and white clover. J. Plant Nutr. 2007, 30, 253–270. [Google Scholar] [CrossRef]
- Cai, L.-Y.; Zhang, J.; Ren, Q.-Q.; Lai, Y.-H.; Peng, M.-Y.; Deng, C.-L.; Ye, X.; Yang, L.-T.; Huang, Z.-R.; Chen, L.-S. Increased pH-mediated alleviation of copper-toxicity and growth response function in Citrus sinensis seedlings. Sci. Horti. 2021, 288, 110310. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Cai, L.-Y.; Qi, Y.-P.; Yang, L.-T.; Lai, N.-W.; Chen, L.-S. Increasing nutrient solution pH alleviated aluminum-induced inhibition of growth and impairment of photosynthetic electron transport chain in Citrus sinensis seedlings. BioMed Res. Int. 2019, 2019, 9058715. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.-Q.; Lin, F.; Ten, Y.; Lin, J.; Zhu, D.-H.; Guo, P.; Weng, Y.-B.; Chen, L.-S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015, 15, 615–628. [Google Scholar] [CrossRef]
- Wu, S.; Liang, S.; Hu, C.; Tan, Q.; Zhang, J.; Dong, Z. Ecological region division of soil based supplementary fertilization and decrement fertilization in China citrus orchards. J. Huazhong Agri. Univ. 2022, 41, 9–19. [Google Scholar]
- Long, A.; Zhang, J.; Yang, L.-T.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Effects of low pH on photosynthesis, related physiological parameters and nutrient profile of citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef]
- Long, A.; Huang, W.-L.; Qi, Y.P.; Yang, L.-T.; Lai, N.-W.; Guo, J.-X.; Chen, L.-S. Low pH effects on reactive oxygen species and methylglyoxal metabolisms in citrus roots and leaves. BMC Plant Biol. 2019, 19, 477. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Qi, Y.P.; Huang, W.-L.; Yang, L.-T.; Lai, N.-W.; Ye, X.; Chen, L.-S. Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in citrus leaves. BMC Plant Biol. 2018, 18, 188. [Google Scholar] [CrossRef]
- Lai, N.-W.; Zheng, Z.-C.; Hua, D.; Zhang, J.; Chen, H.-H.; Ye, X.; Huang, Z.-R.; Guo, J.; Yang, L.-T.; Chen, L.-S. Molecular and physiological responses of Citrus sinensis leaves to long-term low pH revealed by RNA-Seq integrated with targeted metabolomics. Int. J. Mol. Sci. 2022, 23, 5844. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Chatzistathis, T.; Alifragis, D.; Papaioannou, A. The influence of liming on soil chemical properties and on the alleviation of manganese and copper toxicity in Juglans regia, Robinia pseudoacacia, Eucalyptus sp. and Populus sp. plantations. J. Environ. Manage. 2015, 150, 149–156. [Google Scholar] [CrossRef]
- Shad, M.I.; Ashraf, M.A.; Rasheed, R.; Hussain, I.; Ali, S. Exogenous coumarin decreases phytotoxic effects of manganese by regulating ascorbate–glutathione cycle and glyoxalase system to improve photosynthesis and nutrient acquisition in sesame (Sesamum indicum L.). J. Soil Sci. Plant. Nutr. 2023, 23, 251–274. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Marastoni, L.; Sandri, M.; Pii, Y.; Valentinuzzi, F.; Brunetto, G.; Cesco, S.; Mimmo, T. Synergism and antagonisms between nutrients induced by copper toxicity in grapevine rootstocks: Monocropping vs. intercropping. Chemosphere 2019, 214, 563–578. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to sub-cellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Force, L.; Critchley, C.; van Rensen, J.J.S. New fluorescence parameters for monitoring photosynthesis in plants. 1. The effect of illumination on the fluorescence parameters of the JIP-test. Photosynth. Res. 2003, 78, 17–33. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrovam, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Šetlík, I.; Allakhverdiev, S.I.; Nedbal, L.; Šetlíková, E.; Klimov, V.V. Three types of photosystem II photoinactivation. 1. Damaging processes on the acceptor side. Photosynth. Res. 1990, 23, 39–48. [Google Scholar] [CrossRef]
- Chen, X.-F.; Hua, D.; Zheng, Z.-C.; Zhang, J.; Huang, W.-T.; Chen, H.-H.; Huang, Z.-R.; Yang, L.-T.; Ye, X.; Chen, L.S. Boron-mediated amelioration of copper-toxicity in sweet orange [Citrus sinensis (L.) Osbeck cv. Xuegan] seedlings involved reduced damage to roots and improved nutrition and water status. Ecotoxicol. Environ. Saf. 2022, 234, 113423. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-T.; Xie, Y.-Z.; Chen, X.-F.; Zhang, J.; Chen, H.-H.; Ye, X.; Guo, J.-X.; Yang, L.-T.; Chen, L.-S. Growth, mineral nutrients, photosynthesis and related physiological parameters of citrus in response to nitrogen deficiency. Agronomy 2021, 11, 1859. [Google Scholar] [CrossRef]
- Huang, W.-T.; Shen, Q.; Yang, H.; Chen, X.-F.; Huang, W.-L.; Wu, H.-X.; Lai, N.-W.; Yang, L.-T.; Huang, Z.-R.; Chen, L.-S. Effects of humic acid-copper interactions on growth, nutrient absorption, and photosynthetic performance of Citrus sinensis seedlings in sand culture. J. Plant Growth Regul. 2024, 43, 3920–3938. [Google Scholar] [CrossRef]
- Santos, E.F.; Kondo Santini, J.M.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.-P.; Cai, Y.-T.; Yang, T.-Y.; Yang, L.-T.; Huang, Z.-R.; Chen, L.-S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef]
- Jiang, H.-X.; Chen, L.-S.; Zheng, J.-G.; Han, S.; Tang, N.; Smith, B.R. Aluminum-induced effects on photosystem II photochemistry in citrus leaves assessed by the chlorophyll a fluorescence transient. Tree Physiol. 2008, 28, 1863–1871. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Kowalenko, C.G.; Lavkulich, L.M. A modified curcumin method for boron analysis of soil extracts. Can. J. Soil Sci. 1976, 56, 537–539. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, R.-Y.; Huang, W.-L.; Yang, H.; Shen, Q.; Huang, W.-T.; Lu, F.; Ye, X.; Yang, L.-T.; Huang, Z.-R.; Chen, L.-S. Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth. Plants 2025, 14, 2390. https://doi.org/10.3390/plants14152390
Rao R-Y, Huang W-L, Yang H, Shen Q, Huang W-T, Lu F, Ye X, Yang L-T, Huang Z-R, Chen L-S. Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth. Plants. 2025; 14(15):2390. https://doi.org/10.3390/plants14152390
Chicago/Turabian StyleRao, Rong-Yu, Wei-Lin Huang, Hui Yang, Qian Shen, Wei-Tao Huang, Fei Lu, Xin Ye, Lin-Tong Yang, Zeng-Rong Huang, and Li-Song Chen. 2025. "Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth" Plants 14, no. 15: 2390. https://doi.org/10.3390/plants14152390
APA StyleRao, R.-Y., Huang, W.-L., Yang, H., Shen, Q., Huang, W.-T., Lu, F., Ye, X., Yang, L.-T., Huang, Z.-R., & Chen, L.-S. (2025). Raising pH Reduces Manganese Toxicity in Citrus grandis (L.) Osbeck by Efficient Maintenance of Nutrient Homeostasis to Enhance Photosynthesis and Growth. Plants, 14(15), 2390. https://doi.org/10.3390/plants14152390






