Unveiling the Impact of Climatic Factors on the Distribution Patterns of Caragana spp. in China’s Three Northern Regions
Abstract
1. Introduction
2. Materials and Methods
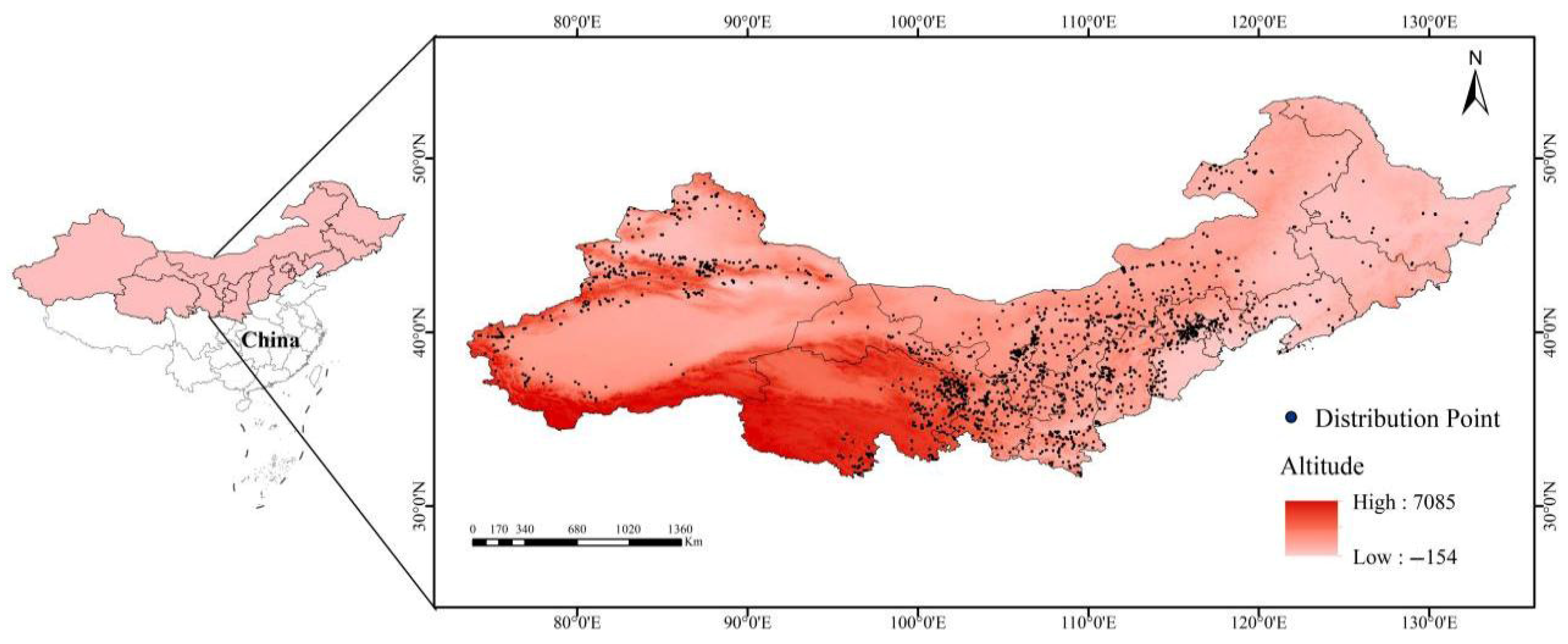
2.1. Acquisition and Screening of Species Distribution Data
2.2. Acquisition and Screening of Environmental Variables for Model Construction
2.3. Ensemble Model Construction and Accuracy Evaluation
2.4. Suitable Habitats Change and Centroid Migration
2.5. Acquisition and Calculation of Moisture–Temperature Indexes for Different Caragana Species
3. Results
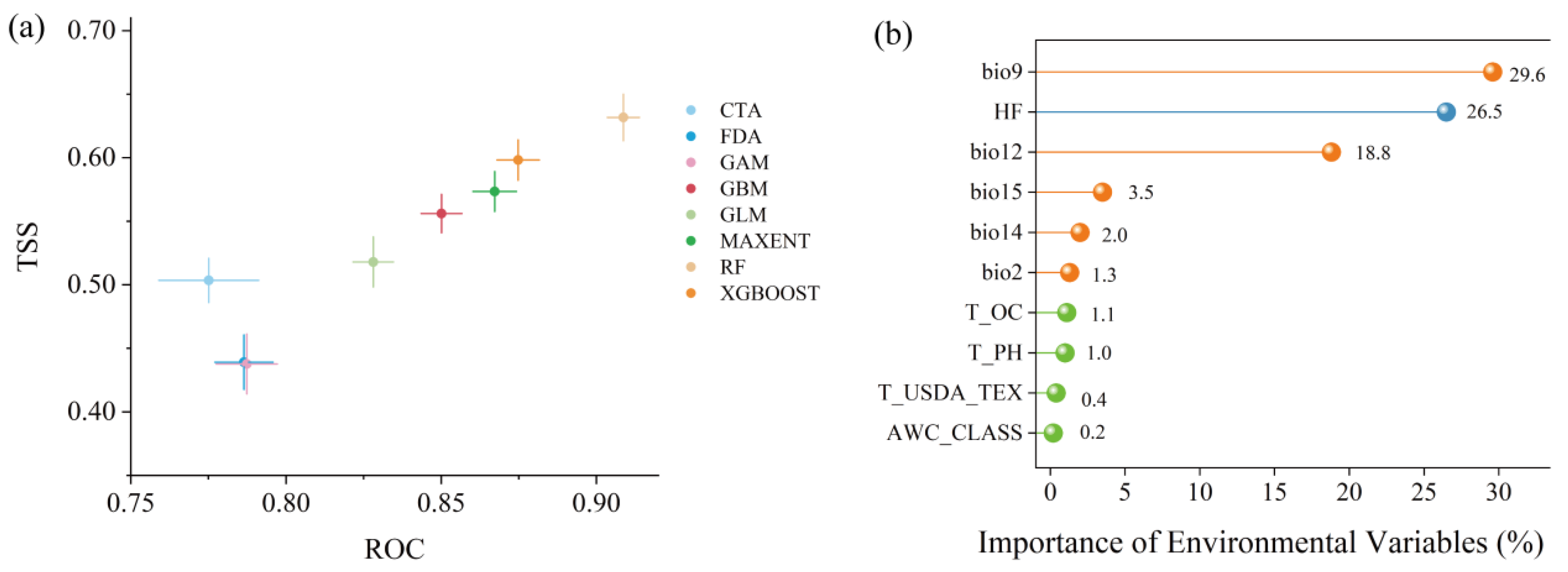
3.1. Evaluation of Model Accuracy and Variable Importance
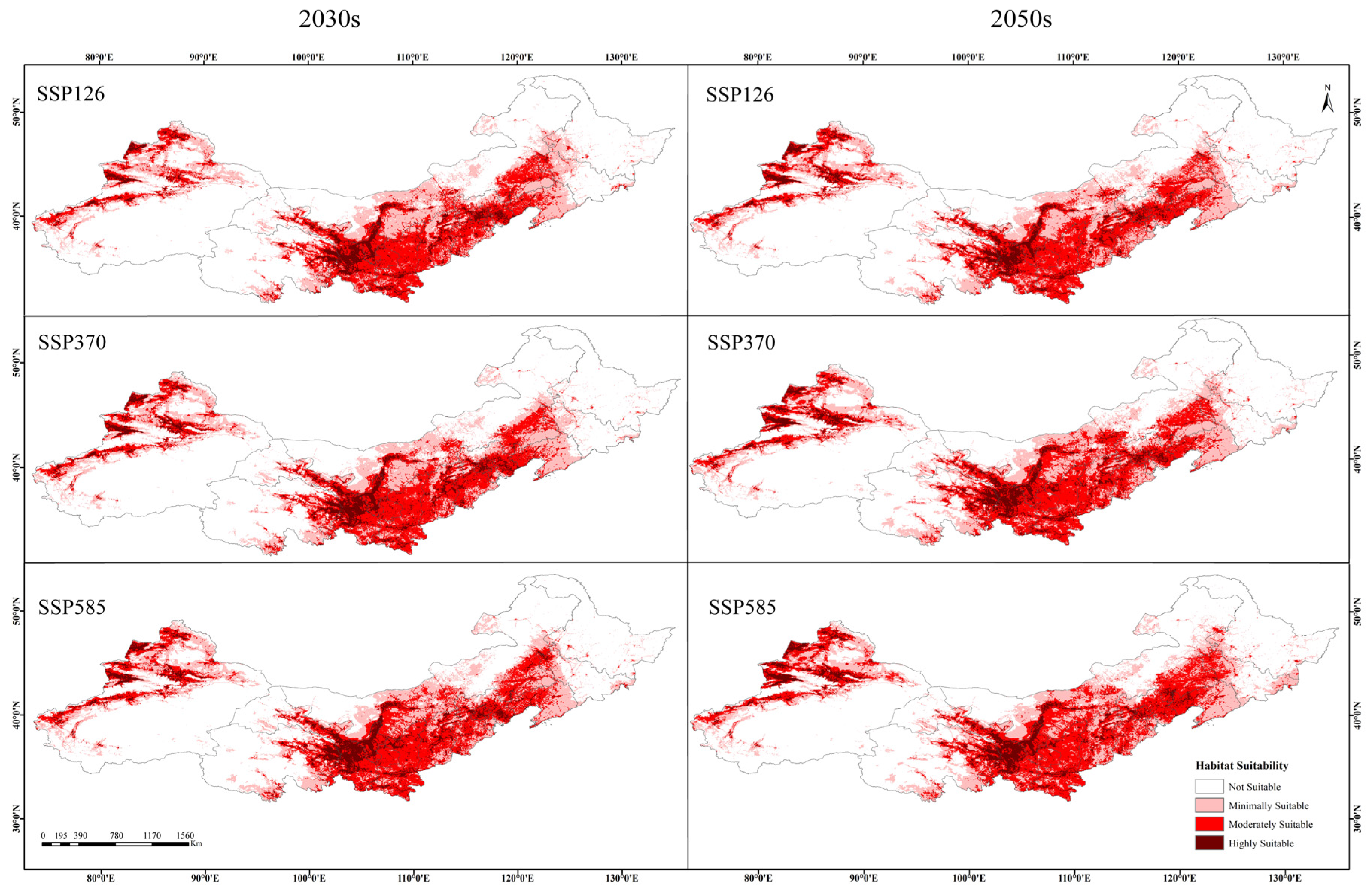
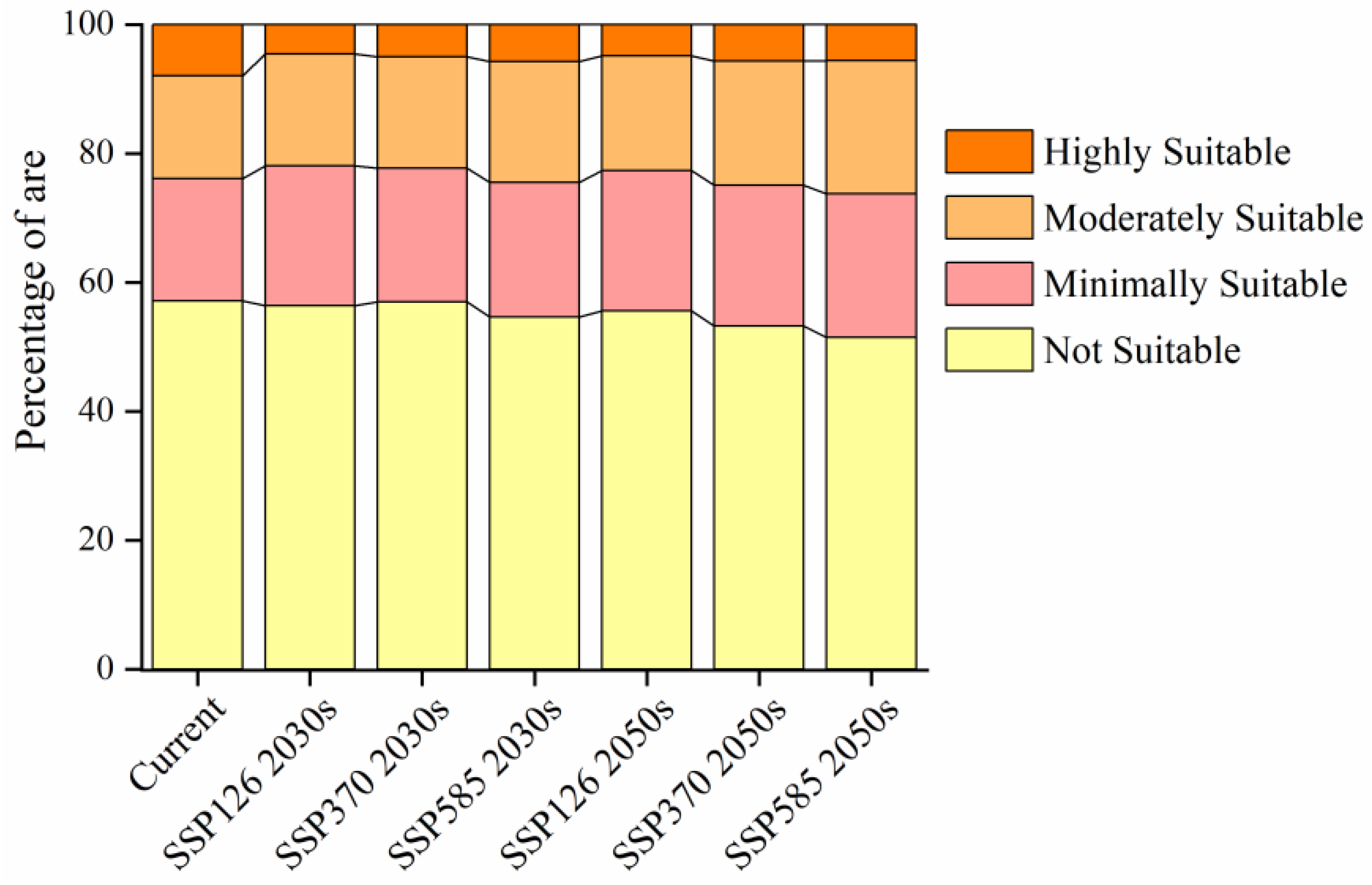
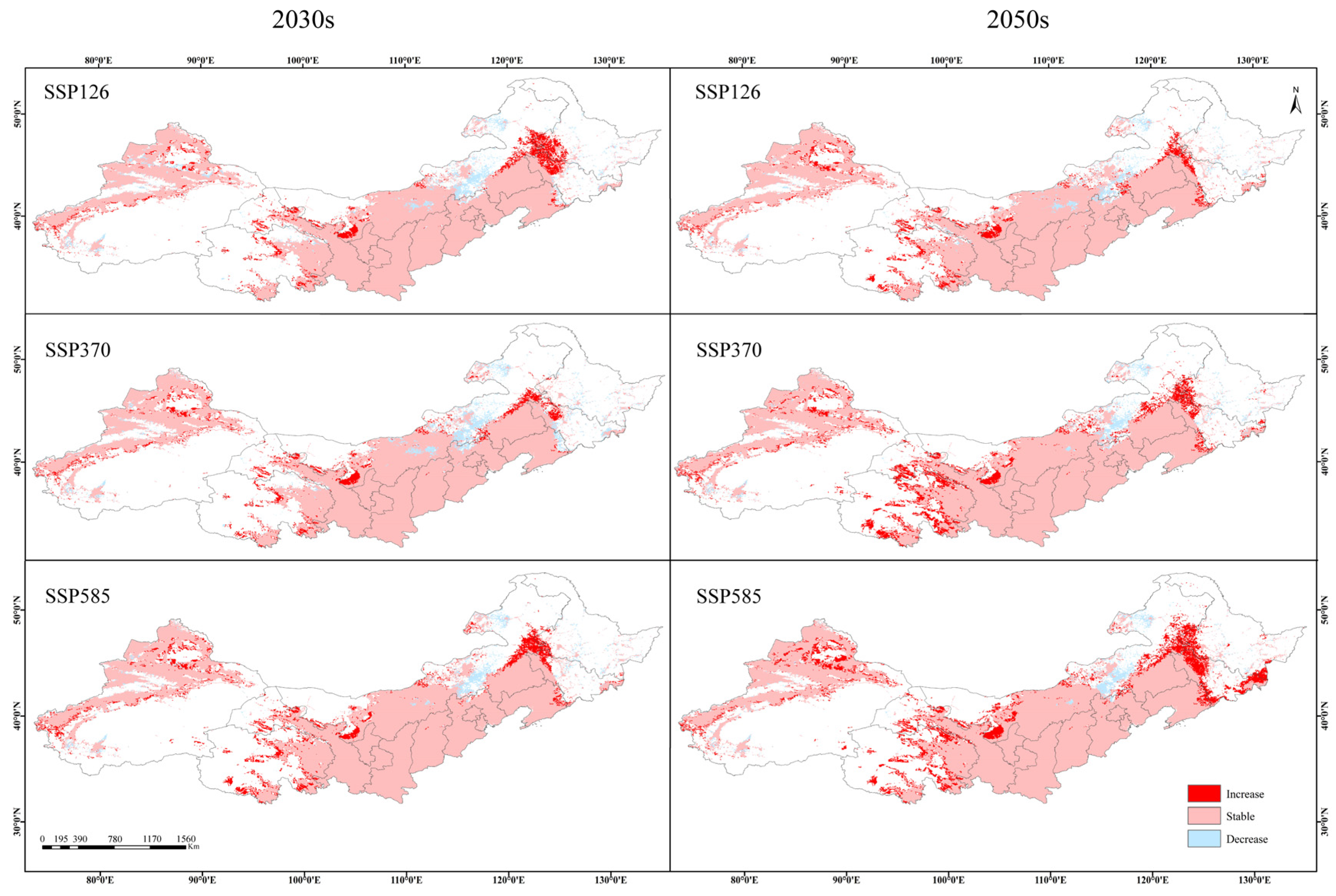
3.2. Current and Future Distribution of the Suitable Habitats of Caragana spp. in China’s Three Northern Regions
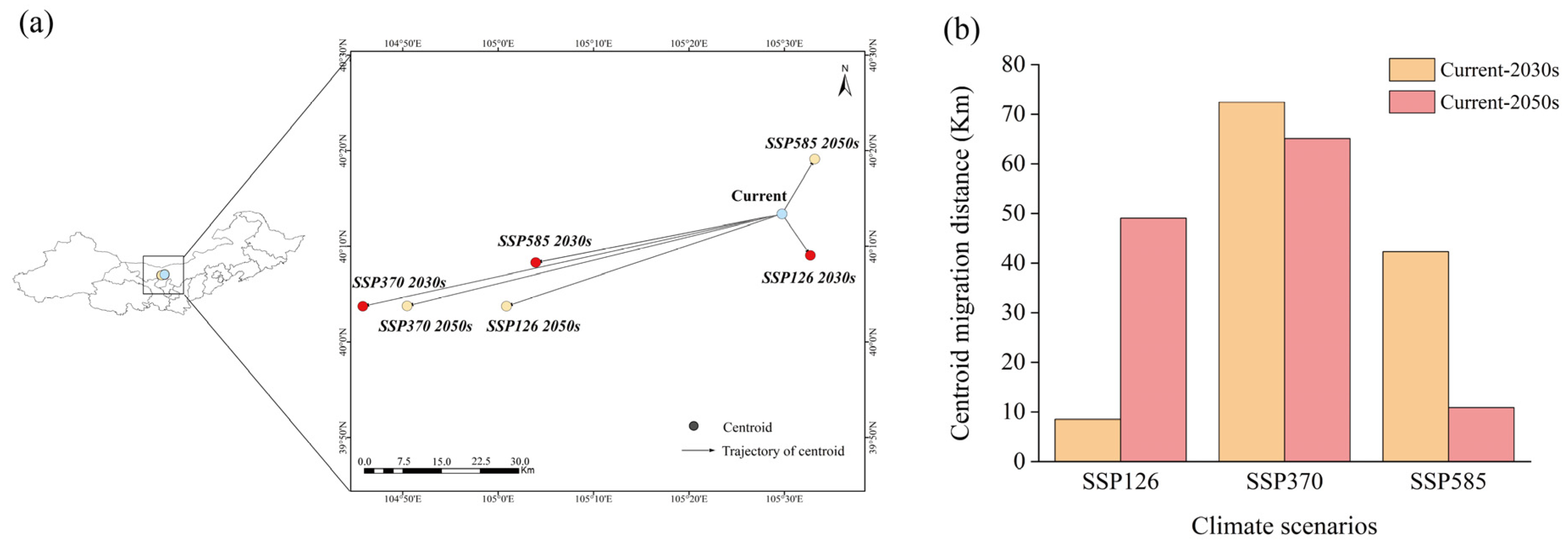
3.3. Suitable Habitat Overlap and Centroid Migration
3.4. Correlations Between the Geographic Distribution of Caragana Species in China’s Three Northern Regions and Climatic Factors
3.5. Moisture–Temperature Indexes Distribution Ranges of Caragana Species in China’s Three Northern Regions
3.6. Groups of Moisture–Temperature Distribution Caragana Species in China’s Three Northern Regions
- (1)
- Cold-Temperate Humid Type (20 ≤ WI < 60, H > 7.5). The climate is characterized by cold temperatures but relatively sufficient moisture availability. This type includes C. brevifolia, C. chinghaiensis, C. densa, C. erinacea, C. junatovii, C. tangutica, and C. versicolor. These species are characterized by high-altitude distributions, typical of alpine shrub communities. Except for a portion of C. densa distributed in the Tianshan Mountains of Xinjiang (which exhibits a disjunct distribution pattern), all other species within this type are confined to the mountain ranges of the northeastern marginal Qinghai–Tibet Plateau. Due to the cold and humid climate, the values of WI are very low, while the HI values are very high.
- (2)
- Mesothermal Xeric Type (60 ≤ WI < 75, 3.5 ≤ HI ≤ 7.5). The climate features moderate temperatures but suboptimal moisture availability. This type includes C. licentiana, C. opulens, C. pygmaea, C. stenophylla, and C. tibetica. These species are predominantly distributed in China’s semi-arid regions, concentrated in eastern Gansu, Ningxia, and Inner Mongoli, exhibiting relatively high values of WI and low HI.
- (3)
- Mesothermal Humid Type (60 ≤ WI < 75, HI > 7.5). The climate is characterized by moderate temperatures coupled with favorable moisture conditions. This type includes C.jubata. It is a typical discontinuous distribution species, which is distributed under the forests in the mountains of North China and Northwest China, separated by a vast arid grassland area in the middle. The humid habitat exhibits favorable thermal and moisture regimes, so the values of WI and HI are both higher.
- (4)
- Warm-Temperate Hyperxeric Type (75 ≤ WI < 90, HI < 3.5). The climate features warm temperatures coupled with extreme aridity. This type includes C. brachypoda, C. camilloi-schneideri, C. dasyphylla, C. kirghisorum, C. pleiophylla, C. polourensis, C. pruinosa, C. pumila, C. tragacanthoides, and C. turfanensis. Most are desert species, primarily distributed in arid regions of China, inhabiting desert areas such as western Inner Mongolia, Ningxia, and Xinjiang. These areas experience favorable thermal conditions but arid climates, resulting in high WI values and very low HI values.
- (5)
- Warm-Temperate Xeric Type (75 ≤ WI < 90, 3.5 ≤ HI ≤ 7.5). The climate is characterized by warm temperatures and limited moisture availability. This type contains the most species-rich group, accounting for 46% of all Caragana species. The genus Caragana exhibits a broad geographic distribution, spanning all provinces across China’s Three Northern Regions, yet its populations are most densely concentrated in the North China region. Compared to the Warm-Temperate Hyperxeric Type, this type exhibits a southeastern distributional shift, characterized by high WI values and low HI values. This type includes C. acanthophylla, C. arborescens, C. aurantiaca, C. boisii, C. bongardiana, C. davazamcii, C. kansuensis, C. korshinskii, C. leucophloea, C. leveillei, C. liouana, C. microphylla, C. pekinensis, C. potaninii, C. purdomii, C. rosea, C. sinica, C. soongorica, C. stipitata, C. turkestanica, and C. zahlbruckneri.
4. Discussion
4.1. Current Suitable Habitats and Key Environmental Variables for Caragana Species
4.2. Changes in the Suitable Habitats for Caragana spp. in the Future
4.3. Zonal Distribution of Caragana Species in the Three Northern Regions of China
4.4. Prospective Research Priorities and Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sales, L.; Ribeiro, B.R.; Chapman, C.A.; Loyola, R. Multiple dimensions of climate change on the distribution of Amazon primates. Perspect. Ecol. Conserv. 2020, 18, 83–90. [Google Scholar] [CrossRef]
- Forster, P.M.; Smith, C.J.; Walsh, T.; Lamb, W.F.; Lamboll, R.; Hauser, M.; Ribes, A.; Rosen, D.; Gillett, N.; Palmer, M.D.; et al. Indicators of Global Climate Change 2022: Annual update of large-scale indicators of the state of the climate system and human influence. Earth Syst. Sci. Data 2023, 15, 2295–2327. [Google Scholar] [CrossRef]
- Lynn, J.; Peeva, N. Communications in the IPCC’s Sixth Assessment Report cycle. Clim. Change 2021, 169, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, L.; Jia, J.; Gong, H.; Qi, X.; Xu, X. Effects of land use changes on the nonlinear trends of net primary productivity in arid and semiarid areas, China. Land Degrad. Dev. 2021, 32, 2183–2196. [Google Scholar] [CrossRef]
- Cao, X.; Cui, M.; Xi, L.; Feng, Y. Spatial-Temporal Process of Land Use/Land Cover and Desertification in the Circum-Tarim Basin during 1990–2020. Land 2024, 13, 735. [Google Scholar] [CrossRef]
- Gong, H.; Cao, L.; Duan, Y.; Jiao, F.; Xu, X.; Zhang, M.; Wang, K.; Liu, H. Multiple effects of climate changes and human activities on NPP increase in the Three-north Shelter Forest Program area. For. Ecol. Manag. 2023, 529, 120732. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L. Coordination of available soil water content and root distribution modifies water source apportionment of the shrub plant Caragana korshinskii. Sci. Total Environ. 2023, 900, 165893. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ma, L.; Jiang, W.; Wang, L.; Wei, L.; Zhang, H.; Yang, R. Anthropogenic Disturbance and Climate Change Impacts on the Suitable Habitat of Sphenomorphus incognitus in China. Ecol. Evol. 2025, 15, e70848. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Li, J.; Liao, L.; Jiang, X.; Xu, Y.; Yu, X.; Wu, L.; Zhao, L.; Fu, J.; et al. “Two zones and three centers” distribution and suitable areas shift of an evergreen oak in subtropical China under climate scenarios. Ecol. Evol. 2024, 14, e70300. [Google Scholar] [CrossRef]
- Subedi, S.C.; Drake, S.; Adhikari, B.; Coggeshall, M.V. Climate-change habitat shifts for the vulnerable endemic oak species (Quercus arkansana Sarg.). J. For. Res. 2023, 35, 23. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Lai, Y.; Shao, H. Comparative Study of Potential Habitats for Two Endemic Grassland Caterpillars on the Qinghai-Tibet Plateau Based on BIOMOD2 and Land Use Data. Insects 2024, 15, 781. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guan, B.-Q.; Chen, R.; Yi, R.; Jiang, X.-L.; Xie, K.-Q. Investigating the Distribution Dynamics of the Camellia Subgenus Camellia in China and Providing Insights into Camellia Resources Management Under Future Climate Change. Plants 2025, 14, 1137. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Burton, V.; Giannetti, F.; Moriondo, M.; Bernetti, I.; Bindi, M.; Chirici, G. Species distribution modelling to support forest management. A literature review. Ecol. Model. 2019, 411, 108817. [Google Scholar] [CrossRef]
- Biaou, S.; Gouwakinnou, G.N.; Noulèkoun, F.; Salako, K.V.; Houndjo Kpoviwanou, J.M.R.; Houehanou, T.D.; Biaou, H.S.S. Incorporating intraspecific variation into species distribution models improves climate change analyses of a widespread West African tree species (Pterocarpus erinaceus Poir, Fabaceae). Glob. Ecol. Conserv. 2023, 45, e02538. [Google Scholar] [CrossRef]
- Ma, F.; Na, X.; Xu, T. Drought responses of three closely related Caragana species: Implication for their vicarious distribution. Ecol. Evol. 2016, 6, 2763–2773. [Google Scholar] [CrossRef]
- Waseem, M.; Nie, Z.-F.; Yao, G.-Q.; Hasan, M.; Xiang, Y.; Fang, X.-W. Dew absorption by leaf trichomes in: An alternative water acquisition strategy for withstanding drought in arid environments. Physiol. Plant. 2021, 172, 528–539. [Google Scholar] [CrossRef]
- Du, Y.-X.; Qi, S.-H.; Tian, X.-Q.; Yao, G.-Q.; Zhang, L.; Li, F.-P.; Jiang, H.; Zhang, X.-Y.; Fang, X.-W. Higher Flower Hydraulic Safety, Drought Tolerance and Structural Resource Allocation Provide Drought Adaptation to Low Mean Annual Precipitation in Caragana Species. Plant Cell Environ. 2024. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Fan, J.; Shao, M.A.; Yang, Q.; Yang, X.; Zhang, S. Evaluating soil water dynamics and vegetation growth characteristics under different soil depths in semiarid loess areas. Geoderma 2024, 442, 116791. [Google Scholar] [CrossRef]
- Qu, W.-j.; Chen, L.; Wang, L.; Wang, X.; Song, N.-p.; Yang, X.-G. Local soil hydrological effects of nebkha creation in a degraded artificial Caragana shrubland. Ecol. Eng. 2019, 131, 27–33. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, W.; Li, S.-L.; Hua, B.; Sakai, T.; Singh, R.P.; Wu, C. Complex interactions of “water-light-heat” climatic conditions on spring phenology in the mid-high latitudes of the Northern Hemisphere. Agric. For. Meteorol. 2025, 367, 110520. [Google Scholar] [CrossRef]
- Petrenko, T.; Dzizyurova, V.; Altman, J.; Doležal, J.; Kislov, D.; Korznikov, K. Changes in Distribution Ranges of Abies Species Dominating in the Forests of Northeast Asia Since the Last Glacial Maximum. J. Biogeogr. 2025, 52, 599–620. [Google Scholar] [CrossRef]
- Kira, T. A climatological interpretation of Japanese vegetation zones. In Vegetation Science and Environmental Protection; Maruzen: Tokyo, Japan, 1977. [Google Scholar]
- Liu, H.; Bu, R.; Liu, J.; Leng, W.; Hu, Y.; Yang, L.; Liu, H. Predicting the wetland distributions under climate warming in the Great Xing’an Mountains, northeastern China. Ecol. Res. 2011, 26, 605–613. [Google Scholar] [CrossRef]
- Fang, J.; Lechowicz, M.J. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Tripathi, P.; Behera, M.D.; Roy, P.S. Predicting the patterns of plant species distribution under changing climate in major biogeographic zones of mainland India. Biodivers. Conserv. 2024, 33, 3495–3515. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Gao, B.; Zheng, K.; Wu, Y.; Wang, M. Construction of ecological security pattern of national ecological barriers for ecosystem health maintenance. Ecol. Indic. 2023, 146, 109801. [Google Scholar] [CrossRef]
- Luo, W.; Sun, C.; Yang, S.; Chen, W.; Sun, Y.; Li, Z.; Liu, J.; Tao, W.; Tao, J. Contrasting range changes and drivers of four forest foundation species under future climate change in China. Sci. Total Environ. 2024, 942, 173784. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Wang, L.; Li, Z.; Shi, Z.; Yang, X.; Yahdjian, L. Plant invasion risk assessment in Argentina’s arid and semi-arid rangelands. J. Environ. Manag. 2025, 377, 124648. [Google Scholar] [CrossRef]
- Yang, L.; Jia, H.; Hua, Q. Predicting suitable habitats of parasitic desert species based on Biomod2 ensemble model: Cynomorium songaricum rupr and its host plants as an example. BMC Plant Biol. 2025, 25, 351. [Google Scholar] [CrossRef]
- Mambo, W.W.; Zhu, G.-F.; Milne, R.I.; Wambulwa, M.C.; Oyebanji, O.O.; Ngarega, B.K.; Carver, D.; Liu, J. Shrinking horizons: Climate-induced range shifts and conservation status of hickory trees (Carya Nutt.). Ecol. Inform. 2024, 84, 102910. [Google Scholar] [CrossRef]
- Wang, R.; Wu, N.; Shi, Z.; Li, C.; Jiang, N.; Fu, C.; Wang, M. Biomod2 for evaluating the changes in the spatiotemporal distribution of Locusta migratoria tibetensis Chen in the Qinghai-Tibet Plateau under climate change. Glob. Ecol. Conserv. 2025, 58, e03508. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Wang, C.; Wan, Y.; Ji, M.; Ma, F.; Lu, Y. Estimating global geographical distribution and ecological niche dynamics of Ammannia coccinea under climate change based on Biomod2. Sci. Rep. 2024, 14, 30579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, G.; Sun, X.; Chen, S.; Huang, L. Assessing the quality ecology of endemic tree species in China based on machine learning models and UPLC methods: The example of Eucommia ulmoides Oliv. J. Clean. Prod. 2024, 452, 142021. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Wang, Q.; Huang, R.; Nie, W.; Yang, S.; Cheng, X.; Li, M. Occurrence Data Sources Matter for Species Distribution Modeling: A Case Study of Quercus variabilis Based on Biomod2. Ecol. Evol. 2025, 15, e71390. [Google Scholar] [CrossRef] [PubMed]
- Xu, W. Kira’s Temperature Indices and Their Application in the Study of Vegetation. J. Ecol. 1985, 3, 35–39. (In Chinese) [Google Scholar]
- Rahman, A.U.; Khan, S.M.; Ahmad, Z.; Alamri, S.; Hashem, M.; Ilyas, M.; Aksoy, A.; Dülgeroğlu, C.; Shahab Ali, G.K. -Impact of multiple environmental factors on species abundance in various forest layers using an integrative modeling approach. Glob. Ecol. Conserv. 2021, 29, e01712. [Google Scholar] [CrossRef]
- Coconis, A.C.; Nussear, K.E.; Rowe, R.J.; Hornsby, A.D.; Matocq, M.D. The role of climate and species interactions in determining the distribution of two elevationally segregated species of small mammals through time. Ecography 2025, 2025, e07556. [Google Scholar] [CrossRef]
- Tu, Y.; Yao, Z.; Guo, J.; Yang, L.; Zhu, Y.; Yang, X.; Shi, Z.; Indree, T. Predicting the potential risk of Caragana shrub encroachment in the Eurasian steppe under anthropogenic climate change. Sci. Total Environ. 2024, 944, 173925. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Knighton, J.; Evaristo, J.; Wassen, M. Contrasting adaptive strategies by Caragana korshinskii and Salix psammophila in a semiarid revegetated ecosystem. Agric. For. Meteorol. 2021, 300, 108323. [Google Scholar] [CrossRef]
- Mohamed Nisin, K.M.N.; Sreenath, K.R.; Paul Sreeram, M. Change in habitat suitability of the invasive Snowflake coral (Carijoa riisei) during climate change: An ensemble modelling approach. Ecol. Inform. 2023, 76, 102145. [Google Scholar]
- Sándor, R.; Iovino, M.; Lichner, L.; Alagna, V.; Forster, D.; Fraser, M.; Kollár, J.; Šurda, P.; Nagy, V.; Szabó, A.; et al. Impact of climate, soil properties and grassland cover on soil water repellency. Geoderma 2021, 383, 114780. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, L.; Yang, L.; Ren, Z.; Ale, R.; Ma, P.; Sun, J.; Liang, E.; Pugnaire, F.I. Caragana versicolor shrub expansion is enhanced by moderate warming but later constrained by climate extremes in the southwestern Tibetan Plateau. Glob. Ecol. Conserv. 2025, 59, e03566. [Google Scholar] [CrossRef]
- Seider, J.H.; Lantz, T.C.; Bone, C. Tundra shrub expansion in a warming climate and the influence of data type on models of habitat suitability. Arct. Antarct. Alp. Res. 2022, 54, 488–506. [Google Scholar] [CrossRef]
- Hofmann, M.; Volosciuk, C.; Dubrovský, M.; Maraun, D.; Schultz, H.R. Downscaling of climate change scenarios for a high-resolution, site-specific assessment of drought stress risk for two viticultural regions with heterogeneous landscapes. Earth Syst. Dyn. 2022, 13, 911–934. [Google Scholar] [CrossRef]
- Bommarito, C.; Noè, S.; Díaz-Morales, D.M.; Lukić, I.; Hiebenthal, C.; Rilov, G.; Guy-Haim, T.; Wahl, M. Co-occurrence of native and invasive macroalgae might be facilitated under global warming. Sci. Total Environ. 2024, 912, 169087. [Google Scholar] [CrossRef] [PubMed]
- Brito-Morales, I.; Schoeman, D.S.; Molinos, J.G.; Burrows, M.T.; Klein, C.J.; Arafeh-Dalmau, N.; Kaschner, K.; Garilao, C.; Kesner-Reyes, K.; Richardson, A.J. Climate velocity reveals increasing exposure of deep-ocean biodiversity to future warming. Nat. Clim. Change 2020, 10, 576–581. [Google Scholar] [CrossRef]
- Li, Y.; Peng, S.; Zhang, Z.; Duan, J. A new paleoclimate perspective shows that arid regions will be drier under global warming conditions. Quat. Res. 2025, 126, 1–13. [Google Scholar] [CrossRef]
- White, H.J.; Caplat, P.; Emmerson, M.C.; Yearsley, J.M. Predicting future stability of ecosystem functioning under climate change. Agric. Ecosyst. Environ. 2021, 320, 107600. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, J.; Zhang, Q.; Sanderson, S.C. Inferring ancestral distribution area and survival vegetation of Caragana (Fabaceae) in Tertiary. Plant Syst. Evol. 2015, 301, 1831–1842. [Google Scholar] [CrossRef]
- Xie, L.; Ma, C.; Guo, H.; Li, Q.; Gao, Y. Distribution pattern of Caragana species under the influence of climate gradient in the Inner Mongolia region, China. J. Arid Land 2014, 6, 311–323. [Google Scholar] [CrossRef]
- Cong, Y.; Gu, Y.; Wang, W.J.; Wang, L.; Xue, Z.; Chen, Y.; Jin, Y.; Xu, J.; Li, M.-H.; He, H.S.; et al. The interaction between temperature and precipitation on the potential distribution range of Betula ermanii in the alpine treeline ecotone on the Changbai Mountain. For. Ecosyst. 2024, 11, 100166. [Google Scholar] [CrossRef]
- Hickcox, R.P.; Mattern, T.; Young, M.J.; Rodríguez-Recio, M.; van Heezik, Y.; Seddon, P.J. Risks, resources, and refugia: Spatial overlap between yellow-eyed penguin foraging distribution and prey, commercial fisheries, and marine protected areas. Biol. Conserv. 2023, 284, 110197. [Google Scholar] [CrossRef]
- Ray, D.; Marchi, M.; Rattey, A.; Broome, A. A multi-data ensemble approach for predicting woodland type distribution: Oak woodland in Britain. Ecol. Evol. 2021, 11, 9423–9434. [Google Scholar] [CrossRef] [PubMed]
- Escoriza, D.; Hernandez, A. Using hierarchical spatial models to assess the occurrence of an island endemism: The case of Salamandra corsica. Ecol. Process. 2019, 8, 15. [Google Scholar] [CrossRef]


| Datasets | Variables | Description | Units |
|---|---|---|---|
| Bioclimatic variables | bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C |
| bio9 | Mean temperature of driest quarter | °C | |
| bio12 | Annual precipitation | mm | |
| bio14 | Precipitation of driest month | mm | |
| bio15 | Precipitation seasonality (coefficient of variation) | — | |
| Human activities | HF | The human footprint index | — |
| Topsoil variables | T_OC | Topsoil organic carbon | % weight |
| T_pH | Topsoil pH (H2O) | −log(H+) | |
| T_USDA_TEX | Topsoil USDA texture classification | name | |
| AWC_CLASS | Available water content class | code |
| No. | Type | Species | Frequency | WI | CI | HI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Range | Optimal Range | Mean | S.D. | Full Range | Mean | S.D. | Full Range | Mean | S.D. | ||||
| 1 | (5) | C. acanthophylla | 50 | 72.1~113.6 | 76.9~97.7 | 87.3 | 8.8 | −106.6~−40.0 | −58.7 | 9.1 | 0.9~12.2 | 6.3 | 3.1 |
| 2 | (5) | C. arborescens | 157 | 36.3~118.9 | 66.2~108.1 | 87.1 | 17.8 | −143.1~−2.7 | −48.8 | 30.2 | 2.0~12.0 | 6.2 | 2.0 |
| 3 | (5) | C. aurantiaca | 22 | 27.2~114.5 | 64.7~113.2 | 88.9 | 20.6 | −78.9~−32.3 | −47.8 | 10.9 | 0.6~17.4 | 3.8 | 4.4 |
| 4 | (5) | C. boisii | 7 | 76.3~117.8 | 78.4~119.8 | 99.1 | 17.6 | −40.3~−3.4 | −16.8 | 14.1 | 2.0~8.1 | 5.9 | 2.2 |
| 5 | (5) | C. bongardiana | 8 | 77.2~91.4 | 77.4~87.6 | 82.5 | 4.4 | −70.1~−57.9 | −63.2 | 4.2 | 2.1~11.3 | 5.4 | 3.6 |
| 6 | (4) | C. brachypoda | 48 | 60.3~92.4 | 67.8~92.6 | 80.2 | 10.5 | −92.2~−26.1 | −45.8 | 16.3 | 1.6~9.8 | 3.3 | 1.8 |
| 7 | (1) | C. brevifolia | 104 | 17.0~84.9 | 29.6~59.3 | 44.5 | 12.6 | −80.4~−12.8 | −45.5 | 12.5 | 3.9~44.5 | 14.1 | 9.0 |
| 8 | (4) | C. camilloi-schneideri | 10 | 75.9~97.0 | 77.4~97.4 | 87.4 | 8.5 | −77.0~−49.5 | −58.9 | 10.5 | 1.5~3.7 | 2.5 | 0.9 |
| 9 | (1) | C. chinghaiensis | 32 | 16.9~42.7 | 17.4~30.0 | 23.7 | 5.4 | −87.1~−37.4 | −67.1 | 12.1 | 9.5~52.0 | 29.1 | 12.0 |
| 10 | (4) | C. dasyphylla | 12 | 87.6~111.4 | 93.8~109.3 | 101.5 | 6.6 | −65.6~−23.5 | −33.4 | 10.8 | 0.4~6.5 | 1.9 | 1.6 |
| 11 | (5) | C. davazamcii | 5 | 72.9~89.9 | 74.1~90.9 | 82.5 | 7.1 | −40.6~−29.4 | −34.9 | 4.8 | 2.3~6.1 | 4.6 | 1.6 |
| 12 | (1) | C. densa | 35 | 18.9~101.5 | 28.8~81.1 | 55.0 | 22.2 | −109.9~−13.3 | −44.1 | 18.5 | 0.4~44.8 | 12.9 | 9.9 |
| 13 | (1) | C. erinacea | 46 | 15.7~83.6 | 13.2~53.8 | 33.5 | 17.3 | −81.0~−8.4 | −50.0 | 15.6 | 2.6~45.6 | 21.8 | 10.6 |
| 14 | (3) | C. jubata | 104 | 15.8~116.3 | 28.4~102.5 | 65.5 | 31.5 | −80.4~−11.2 | −43.8 | 14.0 | 0.7~44.5 | 10.7 | 9.8 |
| 15 | (1) | C. junatovii | 10 | 20.5~26.9 | 21.1~27.1 | 24.1 | 2.6 | −70.7~−39.5 | −55.5 | 11.7 | 17.6~29.2 | 22.6 | 4.5 |
| 16 | (5) | C. kansuensis | 56 | 64.5~110.1 | 70.2~93.0 | 81.6 | 9.7 | −48.3~−14.3 | −31.5 | 6.2 | 2.0~8.0 | 4.9 | 1.5 |
| 17 | (4) | C. kirghisorum | 6 | 90.4~96.9 | 91.6~97.4 | 94.5 | 2.5 | −59.5~−50.0 | −53.2 | 4.2 | 1.5~3.5 | 2.2 | 0.8 |
| 18 | (5) | C. korshinskii | 115 | 33.1~119.4 | 60.6~96.8 | 78.7 | 15.4 | −91.4~−3.3 | −44.1 | 17.9 | 0.8~14.9 | 4.8 | 2.5 |
| 19 | (5) | C. leucophloea | 112 | 48.5~126.8 | 69.0~100.2 | 84.6 | 13.3 | −94.2~−23.5 | −55.0 | 13.9 | 0.1~14.2 | 3.7 | 2.9 |
| 20 | (5) | C. leveillei | 40 | 83.5~122.0 | 87.6~114.6 | 101.1 | 11.5 | −43.4~−1.9 | −20.5 | 10.4 | 4.1~9.3 | 5.6 | 1.0 |
| 21 | (2) | C. licentiana | 44 | 32.0~91.6 | 51.4~83.2 | 67.3 | 13.5 | −57.1~−16.7 | −35.0 | 7.6 | 1.5~22.7 | 7.1 | 4.4 |
| 22 | (5) | C. liouana | 81 | 43.7~113.6 | 67.3~93.6 | 80.4 | 11.2 | −120.1~−5.0 | −44.1 | 16.9 | 1.6~14.2 | 5.0 | 1.8 |
| 23 | (5) | C. microphylla | 103 | 32.0~115.2 | 59.0~100.5 | 79.8 | 17.6 | −120.1~−6.2 | −52.2 | 29.6 | 2.1~22.7 | 5.6 | 2.3 |
| 24 | (2) | C. opulens | 169 | 22.9~112.7 | 46.1~92.9 | 69.5 | 19.9 | −73.2~−5.1 | −38.6 | 13.4 | 1.7~36.2 | 7.3 | 5.0 |
| 25 | (5) | C. pekinensis | 56 | 89.9~122.0 | 102.3~115.3 | 108.8 | 5.5 | −43.9~−12.0 | −24.5 | 5.3 | 4.1~6.0 | 5.1 | 0.4 |
| 26 | (4) | C. pleiophylla | 18 | 84.6~106.2 | 91.1~106.0 | 98.5 | 6.3 | −66.7~−26.5 | −38.3 | 11.5 | 0.4~7.6 | 1.9 | 1.8 |
| 27 | (4) | C. polourensis | 46 | 92.8~114.1 | 98.1~115.0 | 106.6 | 7.1 | −52.8~−20.9 | −28.2 | 6.4 | 0.2~3.1 | 1.1 | 0.9 |
| 28 | (5) | C. potaninii | 6 | 82.6~97.8 | 81.6~96.3 | 89.0 | 6.3 | −44.2~−19.6 | −34.7 | 9.9 | 4.6~6.6 | 5.3 | 0.8 |
| 29 | (4) | C. pruinosa | 18 | 73.8~113.6 | 83.4~110.4 | 96.9 | 11.5 | −79.2~−26.5 | −42.3 | 17.0 | 0.2~5.8 | 1.9 | 1.3 |
| 30 | (4) | C. pumila | 43 | 74.1~122.7 | 74.4~101.9 | 88.2 | 11.7 | −82.3~−33.2 | −57.6 | 11.5 | 0.6~10.1 | 3.3 | 2.4 |
| 31 | (5) | C. purdomii | 72 | 81.5~121.5 | 81.9~103.8 | 92.8 | 9.3 | −45.4~−2.0 | −26.5 | 9.2 | 1.7~9.0 | 5.7 | 1.2 |
| 32 | (2) | C. pygmaea | 50 | 58.8~104.2 | 58.5~84.3 | 71.4 | 11.0 | −92.3~−24.2 | −63.6 | 19.4 | 2.3~9.3 | 4.3 | 1.6 |
| 33 | (5) | C. rosea | 150 | 43.9~123.2 | 82.1~114.9 | 98.5 | 13.9 | −62.0~−0.9 | −28.2 | 11.9 | 3.3~15.2 | 5.8 | 1.4 |
| 34 | (5) | C. sinica | 94 | 49.7~122.5 | 86.5~121.5 | 104.0 | 14.9 | −50.1~−1.2 | −17.9 | 13.0 | 4.1~10.6 | 6.5 | 1.8 |
| 35 | (5) | C. soongorica | 23 | 76.9~113.6 | 79.6~98.1 | 88.8 | 7.9 | −94.6~−43.2 | −64.1 | 11.2 | 1.4~9.9 | 4.9 | 2.7 |
| 36 | (2) | C. stenophylla | 166 | 28.7~99.4 | 59.9~90.0 | 75.0 | 12.8 | −128.3~−23.9 | −55.9 | 25.1 | 1.4~15.2 | 4.0 | 2.0 |
| 37 | (5) | C. stipitata | 50 | 83.5~121.9 | 97.5~116.1 | 106.8 | 7.9 | −33.9~−3.4 | −13.6 | 7.5 | 4.1~9.3 | 7.1 | 1.3 |
| 38 | (1) | C. tangutica | 31 | 23.1~83.0 | 40.6~78.1 | 59.3 | 15.9 | −49.7~−15.5 | −32.4 | 10.8 | 2.9~27.7 | 8.6 | 6.0 |
| 39 | (2) | C. tibetica | 62 | 24.1~92.4 | 43.4~94.5 | 69.0 | 21.7 | −68.7~−26.5 | −41.2 | 10.7 | 1.7~22.9 | 6.4 | 5.8 |
| 40 | (4) | C. tragacanthoides | 11 | 73.1~84.8 | 73.3~84.0 | 78.7 | 4.5 | −76.7~−61.8 | −66.5 | 4.2 | 1.9~2.8 | 2.4 | 0.3 |
| 41 | (4) | C. turfanensis | 17 | 87.6~111.4 | 94.1~107.8 | 100.9 | 5.8 | −65.6~−23.5 | −35.2 | 10.0 | 0.7~6.5 | 1.8 | 1.4 |
| 42 | (5) | C. turkestanica | 8 | 73.8~106.2 | 71.4~98.7 | 85.0 | 11.6 | −100.7~−26.5 | −63.3 | 21.0 | 1.3~11.1 | 5.0 | 3.8 |
| 43 | (1) | C. versicolor | 9 | 16.3~58.8 | 12.5~53.7 | 33.1 | 17.5 | −79.8~−35.1 | −58.8 | 18.4 | 7.6~43.2 | 24.7 | 15.6 |
| 44 | (5) | C. zahlbruckneri | 49 | 65.1~115.2 | 80.7~111.2 | 95.9 | 13.0 | −66.4~−15.2 | −34.7 | 11.7 | 4.3~7.5 | 5.5 | 0.8 |
| (Kira’s WI-Xu’s HI) | |||||
|---|---|---|---|---|---|
| Class of HI (mm/°C·Month) | Total | ||||
| <3.5 | 3.5~7.5 | >7.5 | |||
| 20~60 | 7 * 9 12 13 15 38 43 | 7 | |||
| Class of WI (°C·month) | 60~75 | 21 24 32 36 39 | 14 | 6 | |
| 75~90 | 6 8 10 17 26 27 29 30 40 41 | 1 2 3 4 5 11 16 18 19 20 22 23 25 28 31 33 34 35 37 42 44 | 31 | ||
| Total | 10 | 26 | 8 | 44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, Y.; Li, Y.; Zou, C.; Shimizu, H. Unveiling the Impact of Climatic Factors on the Distribution Patterns of Caragana spp. in China’s Three Northern Regions. Plants 2025, 14, 2368. https://doi.org/10.3390/plants14152368
Zhao W, Liu Y, Li Y, Zou C, Shimizu H. Unveiling the Impact of Climatic Factors on the Distribution Patterns of Caragana spp. in China’s Three Northern Regions. Plants. 2025; 14(15):2368. https://doi.org/10.3390/plants14152368
Chicago/Turabian StyleZhao, Weiwei, Yujia Liu, Yanxia Li, Chunjing Zou, and Hideyuki Shimizu. 2025. "Unveiling the Impact of Climatic Factors on the Distribution Patterns of Caragana spp. in China’s Three Northern Regions" Plants 14, no. 15: 2368. https://doi.org/10.3390/plants14152368
APA StyleZhao, W., Liu, Y., Li, Y., Zou, C., & Shimizu, H. (2025). Unveiling the Impact of Climatic Factors on the Distribution Patterns of Caragana spp. in China’s Three Northern Regions. Plants, 14(15), 2368. https://doi.org/10.3390/plants14152368





