Vernalization of Winter Crops Increases Photosynthetic Energy Conversion Efficiency and Seed Yield
Abstract
1. Introduction
2. Photosynthetic Energy Conversion Efficiency vs. Photoprotection
3. Vernalization and Photosynthetic Performance
4. Vernalization and Photostasis
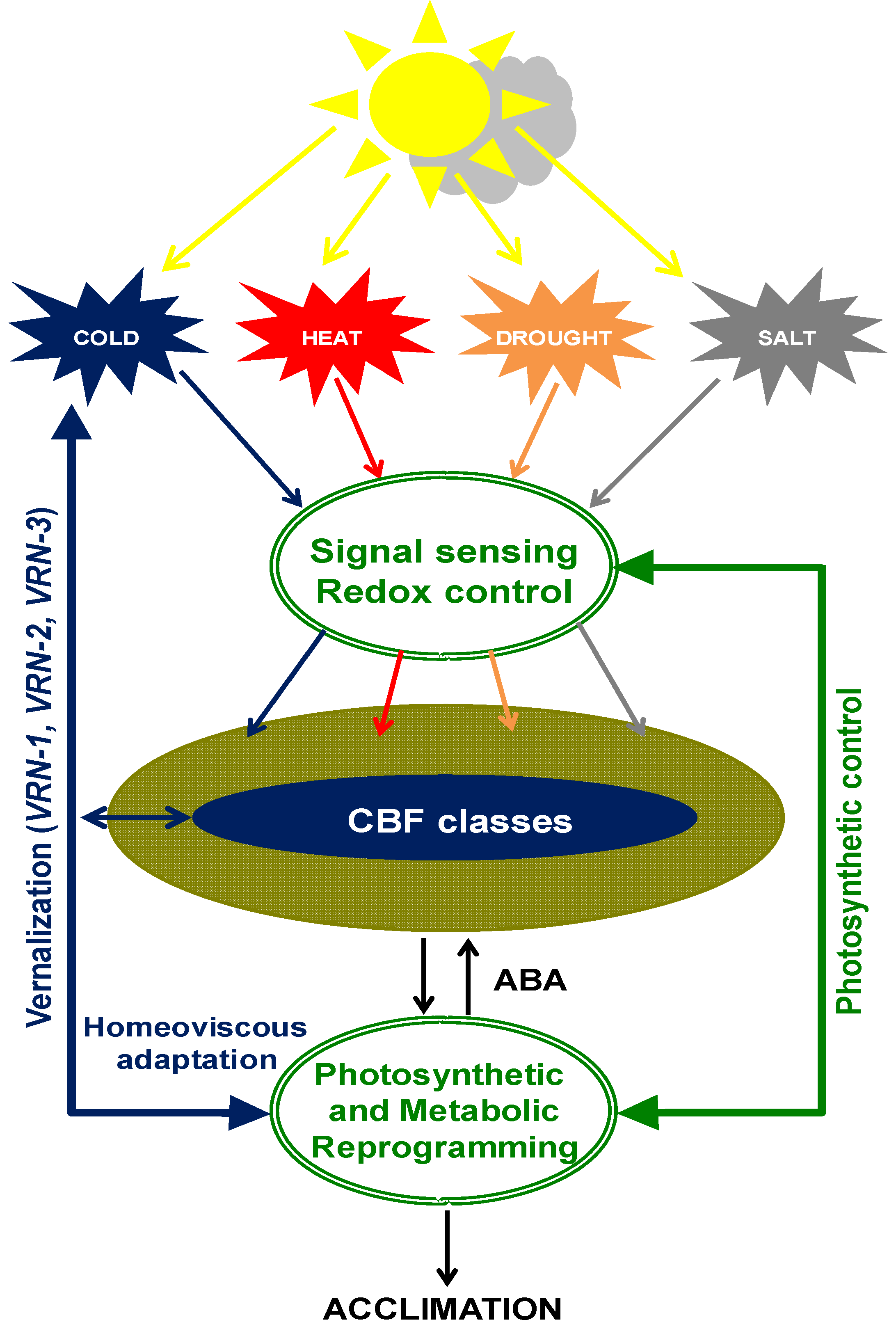
5. Cold Acclimation, Photostasis and CBFs
6. Vernalization, Photostasis, and CBFs Converge on Gibberellin Biosynthesis
7. Conclusions
8. Dedication
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Murchie, E.H.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009, 81, 532–552. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2023/2050: The 2012 Revision; ESA Working Paper No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar][Green Version]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Caccialupi, G.; Milc, J.; Cardonia, F.; Francia, E. The Triticeae CBF gene cluster- to frost resistance and beyond. Cells 2023, 12, 2606. [Google Scholar] [CrossRef]
- Stirbet, A.; Guo, Y.; Lazár, D.; Govindjee, G. From leaf to multiscale models of photosynthesis: Applications and challenges for crop improvement. Photosynth. Res. 2024, 161, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on improving photosynthesis to increase crop yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team], Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Horton, P. Prospects for crop improvement through the genetic manipulation of photosynthesis: Morphological and biochemical aspects of light capture. J. Exp. Bot. 2000, 51, 475–485. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Soto-Gomez, D.; Perez-Rodriguez, P. Sustainable agriculture through perennial grains: Wheat, rice, maize and other species. A review. Agric. Ecosyst. Environ. 2022, 325, 107747. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Dahal, K.; Bode, R.; Kurepin, L.V.; Ivanov, A.G. Photosynthetic acclimation, vernalization, crop productivity and ‘the grand design of photosynthesis’. J. Plant Physiol. 2016, 203, 29–43. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R. When there is too much light. Plant Physiol. 2001, 125, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Strand, Å.; Hurry, V.; Henkes, S.; Hüner, N.; Gustafsson, P.; Gardeström, P.; Stitt, M. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin Cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 1999, 119, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Őquist, G. Effects of low temperature on photosynthesis: Review. Plant Cell Environ. 1983, 6, 281–301. [Google Scholar] [CrossRef]
- Hayden, D.B.; Baker, N.R.; Percival, M.P.; Beckwith, P.B. Modification of the Photosystem II light-harvesting chlorophyll a/b protein complex in maize during chill-induced photoinhibition. Biochim. Biophys. Acta 1986, 851, 86–92. [Google Scholar] [CrossRef]
- Baker, N.R. A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol. Plant. 1991, 81, 563–570. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Somersalo, S.; Krause, G.H. Reversible photoinhibition of unhardened and cold-acclimated spinach leaves at chilling temperatures. Planta 1990, 180, 181–187. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef]
- Osmond, C.B. What is photoinhibition? Some insights from comparison of shade and sun plants. In Photoinhibition of Photosynthesis-from Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; Bios Scientific Publishers: Oxford, UK, 1994; pp. 1–24. [Google Scholar]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Krol, M.; Sveshnikov, D.; Malmberg, G.; Gardestrom, P.; Hurry, V.; Őquist, G.; Hüner, N.P.A. Characterization of the photosynthetic apparatus in cortical bark chlorenchyma of Scots pine. Planta 2006, 223, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.G.; Sane, P.; Hurry, V.; Krol, M.; Sveshnikov, D.; Huner, N.P.A.; Oquist, G. Low-temperature modulation of the redox properties of the acceptor side of photosystem II: Photoprotection through reaction centre quenching of excess energy. Physiol. Plant. 2003, 199, 376–383. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Sane, P.V.; Hurry, V.; Öquist, G.; Hüner, N.P.A. Photosystem II reaction centre quenching: Mechanisms and physiological role. Photosynth. Res. 2008, 98, 565–574. [Google Scholar] [CrossRef]
- Sveshnikov, D.; Ensminger, I.; Ivanov, A.G.; Campbell, D.; Lloyd, J.; Funk, C.; Hüner, N.P.A.; Öquist, G. Excitation energy partitioning and quenching during cold acclimation in Scots pine. Tree Physiol. 2006, 26, 325–336. [Google Scholar] [CrossRef]
- Timm, S.; Sun, H.; Huang, W. Photorespiration–emerging insights into photoprotection mechanisms. Trends Plant 2024, 29, 1052–1055. [Google Scholar] [CrossRef]
- Melis, A. Photostasis in plants. In Photostasis and Related Phenomena; Williams, T.P., Thistle, A.B., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 207–220. [Google Scholar]
- McDonald, A.E.; Ivanov, A.G.; Bode, R.; Maxwell, D.P.; Rodermel, S.R.; Hüner, N.P.A. Flexibility in photosynthetic electron transport: The physiological role of plastoquinol terminal oxidase (PTOX). Biochim. Biophys. Acta 2011, 1807, 954–967. [Google Scholar] [CrossRef]
- Ramírez, C.F.; Cavieres, L.A.; Sanhueza, C.; Vallejos, V.; Gómez-Espinoza, O.; Bravo, L.A.; Sáez, P.L. Ecophysiology of Antarctic vascular plants: An update on the extreme environment resistance mechanisms and their importance in facing climate change. Plants 2024, 13, 449. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Cohu, C.; Muller, O.; Adams, W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef]
- Kulheim, C.; Agren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef]
- Bag, P.; Ivanov, A.G.; Hüner, N.P.A.; Jansson, J. Photosynthetic advantages of conifers in boreal forests. Trends Plant Sci. 2025, 30, 409–423. [Google Scholar] [CrossRef]
- Teh, J.T.; Leitz, V.; Holzer, V.J.C.; Neusius, D.; Marino, G.; Meitzel, T.; García-Cerdán, J.G.; Dent, R.M.; Niyogi, K.K.; Geigenberger, P.; et al. NTRC regulates CP12 to activate Calvin–Benson cycle during cold acclimation. Proc. Natl. Acad. Sci. USA 2023, 120, e2306338120. [Google Scholar] [CrossRef]
- Bascunan-Godoy, L.; Uribe, E.; Zuniga-Feest, A.; Corcuera, L.J.; Bravo, L.A. Low temperature regulates sucrose-phosphate synthase activity in Colobanthus quitensis (Kunth) Bartl. by decreasing its sensitivity to Pi and increased activation by glucose-6-phosphate. Polar Biol. 2006, 29, 1011–1017. [Google Scholar] [CrossRef]
- Perez-Torres, E.; Bascuñán, L.; Sierra, A.; Bravo, L.A.; Corcuera, L.J. Robustness of activity of Calvin cycle enzymes after high light and low temperature conditions in Antarctic vascular plants. Polar Biol. 2006, 29, 909–916. [Google Scholar] [CrossRef]
- Savitch, L.V.; Gray, G.R.; Hüner, N.P.A. Feedback-limited photosynthesis and regulation of sucrose-starch accumulation during cold acclimation and low-temperature stress in a spring and winter wheat. Planta 1997, 201, 18–26. [Google Scholar] [CrossRef]
- Savitch, L.V.; Harney, T.; Hüner, N.P.A. Sucrose metabolism in spring and winter wheat in response to high irradiance, cold stress and cold acclimation. Physiol. Plant. 2000, 108, 270–278. [Google Scholar] [CrossRef]
- Savitch, L.V.; Leonardos, E.D.; Krol, M.; Jansson, S.; Grodzinski, B.; Hüner, N.P.A.; Őquist, G. Two different strategies for light utilization in photosynthesis in relation to growth and cold acclimation. Plant Cell Environ. 2002, 25, 761–771. [Google Scholar] [CrossRef]
- Leonardos, E.D.; Savitch, L.V.; Hüner, N.P.A.; Őquist, G.; Grodzinski, B. Daily photosynthetic and C-export patterns in winter wheat leaves during cold stress and acclimation. Physiol. Plant. 2003, 117, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.R.; Heath, D. A global reorganization of the metabolome in Arabidopsis during cold acclimation is revealed by metabolic fingerprinting. Physiol. Plant. 2005, 124, 236–248. [Google Scholar] [CrossRef]
- Gray, G.R.; Boese, S.R.; Hüner, N.P.A. A comparison of low temperature growth vs. low temperature shifts to induce resistance to photoinhibition in spinach (Spinacia oleracea). Physiol. Plant. 1994, 90, 560–566. [Google Scholar] [CrossRef]
- Gray, G.R.; Chauvin, L.-P.; Sarhan, F.; Hüner, N.P.A. Cold acclimation and freezing tolerance. A complex interaction of light and temperature. Plant Physiol. 1997, 114, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Kane, K.; Gadapati, W.; Webb, E.; Savitch, L.V.; Singh, J.; Sharma, P.; Sarhan, F.; Longstaffe, F.J.; Grodzinski, B.; et al. The effects of phenotypic plasticity on photosynthetic performance in winter rye, winter wheat and Brassica napus. Physiol. Plant. 2012, 144, 169–188. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Stitt, M.; Heinke, D.; Gerhardt, R.; Raschke, K.; Heldt, H.W. Limitations of photosynthesis by carbon metabolism. II. O2-insensitive CO2 uptake results from limitation of triose phosphate utilization. Plant Physiol. 1986, 81, 1123–1129. [Google Scholar] [CrossRef]
- Sharkey, T.D. Feedback limitation of photosynthesis and the physiological role of ribulose bisphosphate carboxylase carbamylation. Bot. Mag. 1990, 2, 87–105. [Google Scholar]
- Pammenter, N.W.; Loreto, F.; Sharkey, T.D. End product feedback effects on photosynthetic electron transport. Photosynth. Res. 1993, 35, 5–14. [Google Scholar] [CrossRef]
- Dahal, K.; Knowles, V.L.; Plaxton, W.C.; Hüner, N.P.A. Enhancement of photosynthetic performance, water use efficiency and grain yield during long-term growth under elevated CO2 in wheat and rye is growth temperature and cultivar dependent. Environ. Exp. Bot. 2014, 106, 207–220. [Google Scholar] [CrossRef]
- Dahal, K.; Weraduwage, S.M.; Kane, K.; Rauf, S.A.; Leonardos, E.D.; Gadapati, W.; Savitch, L.; Singh, J.; Marillia, E.-F.; Taylor, D.C.; et al. Enhancing biomass production and yield by maintaining enhanced capacity for CO2 uptake in response to elevated CO2. Can. J. Plant Sci. 2014, 94, 1075–1083. [Google Scholar] [CrossRef]
- Amasino, R. Competence, and the Vernalization, Epigenetic Memory of Winter. Plant Cell 2004, 16, 2553–2559. [Google Scholar] [CrossRef]
- Sung, S.; Amasino, R.M. Remembering winter: Toward a Molecular Understanding of Vernalization. Annu. Rev. Plant Biol. 2005, 56, 491–508. [Google Scholar] [CrossRef]
- Trevaskis, B. The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct. Plant Biol. 2010, 37, 479–487. [Google Scholar] [CrossRef]
- Trevaskis, B.; Hemming, M.N.; Dennis, E.S.; Peacock, W.J. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 2007, 12, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Casao, M.C.; Wang, P.; Sato, K.; Hayes, P.M.; Finnegan, E.J.; Trevaskis, B. Direct links between the vernalization response and other key traits of cereal crops. Nat. Commun. 2015, 6, 5882. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Parida, S.; Guru, A. Vernalization: An approach to increase plant productivity. Agrobios Newsl. 2020, 18, 46–48. [Google Scholar]
- Krol, M.; Griffith, M.; Hüner, N.P.A. An appropriate physiological control for environmental temperature studies: Comparative growth kinetics for winter rye. Can. J. Bot. 1984, 62, 1062–1068. [Google Scholar] [CrossRef]
- Boese, S.R.; Hüner, N.P.A. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990, 94, 1830–1836. [Google Scholar] [CrossRef]
- Boese, S.R.; Hüner, N.P.A. Developmental history affects the susceptibility of spinach leaves to in vivo low temperature photoinhibition. Plant Physiol. 1992, 99, 1141–1145. [Google Scholar] [CrossRef]
- Crosatti, C.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Cattivelli, L. Harden the chloroplast to protect the plant. Physiol. Plant. 2013, 147, 55–63. [Google Scholar] [CrossRef]
- Giorni, E.; Crosatti, C.; Baldi, P.; Grossi, M.; Mare, C.; Stanca, A.M.; Cattivelli, L. Cold-regulated gene expression during winter in frost tolerant and frost susceptible barley cultivars grown under field conditions. Euphytica 1999, 106, 149–157. [Google Scholar] [CrossRef]
- Garen, J.C.; Michaletz, S.T. Acclimation unifies the scaling of carbon assimilation across climate gradients and levels of organization. Ecol. Lett. 2024, 27, e70004. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Bode, R.; Dahal, K.; Busch, F.A.; Possmayer, M.; Szyszka, B.; Rosso, D.; Ensminger, I.; Krol, M.; Ivanov, A.G.; et al. Shedding some light on cold acclimation, cold adaptation, and phenotypic plasticity. Botany 2013, 91, 127–136. [Google Scholar] [CrossRef]
- Hon, W.C.; Griffith, M.; Chong, P.; Yang, D.S.C. Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol. 1994, 104, 971–980. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.-Q.; Tai, H.; Creelman, A.; Bizimungu, B. Improving potato stress tolerance and tuber yield under a climate change scenario—A current overview. Front. Plant Sci. 2019, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Rosso, D.; Ivanov, A.G.; Fu, A.; Geisler-Lee, J.; Hendrickson, L.; Geisler, M.; Stewart, G.; Krol, M.; Hurry, V.; Rodermel, S.R.; et al. IMMUTANS does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady-state photosynthesis. Plant Physiol. 2006, 142, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Rosso, D.; Bode, R.; Li, W.; Krol, M.; Saccon, D.; Wang, S.; Schillaci, L.; Rodermel, S.R.; Maxwell, D.P.; Hüner, N.P.A. Photosynthetic redox imbalance governs leaf sectoring in the Arabidopsis thaliana variegation mutants immutans, spotty, var1, and var2. Plant Cell 2009, 21, 3473–3492. [Google Scholar] [CrossRef] [PubMed]
- Bode, R.; Ivanov, A.G.; Hüner, N.P.A. Global transcriptome analyses provide evidence that chloroplast redox state contributes to intracellular as well as long-distance signalling in response to stress and acclimation in Arabidopsis. Photosynth. Res. 2016, 128, 287–312. [Google Scholar] [CrossRef]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherchia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Krol, M.; Hüner, N.P.A.; Williams, J.P.; Maissan, E. Chloroplast biogenesis at cold hardening temperatures. Kinetics of trans- Δ3-hexadecenoic acid accumulation and the assembly of LHCII. Photosynth. Res. 1988, 15, 115–132. [Google Scholar] [CrossRef]
- Krol, M.; Hüner, N.P.A.; Williams, J.P.; Maissan, E. Prior accumulation of phosphatidylglycerol high in trans-Δ3-hexadecenoic acid enhances the in vitro stability of oligomeric light harvesting complex II. J. Plant Physiol. 1989, 135, 75–90. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Krol, M.; Williams, J.P.; Maissan, E.; Low, P.; Roberts, D.; Thompson, J.E. Low temperature development induces a specific decrease in trans-Δ3-hexadecenoic acid content which influences LHCII organization. Plant Physiol. 1987, 84, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; Hüner, N.P.A.; McIntosh, A. Chloroplast biogenesis at cold hardening temperatures. Development of photosystem I and Photosystem II activities in relation to pigment accumulation. Photosynth. Res. 1987, 14, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Elfman, B.; Hüner, N.P.A.; Griffith, M.; Krol, M.; Hayden, D.B.; Hopkins, W.G. Growth and development at cold hardening temperatures. Chlorophyll-protein complexes and thylakoid membrane polypeptides. Can. J. Bot. 1984, 62, 61–67. [Google Scholar] [CrossRef]
- Krupa, Z.; Hüner, N.P.A.; Williams, J.P.; Maissan, E.; James, D.R. Development at cold-hardening temperatures. The structure and composition of purified rye light harvesting complex II. Plant Physiol. 1987, 84, 19–24. [Google Scholar] [CrossRef]
- Krupa, Z.; Williams, J.P.; Hüner, N.P.A. The role of acyl lipids in reconstitution of lipid-depleted light harvesting complex II from cold hardened and nonhardened rye. Plant Physiol. 1992, 100, 931–938. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Elfman, B.; Krol, M.; MacIntosh, A. Growth and development at cold hardening temperatures. Chloroplast ultrastructure, pigment content and composition. Can. J. Bot. 1984, 62, 53–60. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Jeknic, Z.; Pillman, K.A.; Dhillon, T.; Skinner, J.S.; Veisz, O.; Cuesta-Marcos, A.; Hayes, P.M.; Jacobs, A.K.; Chen, T.H.H.; Stockinger, E.J. Hv-CBF2A overexpression in barley accelerates COR gene transcript accumulation and acquisition of freezing tolerance during cold acclimation. Plant Mol. Biol. 2014, 84, 67–82. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signalling in cold acclimation. Trends Plant Sci. 2018, 23, 623–636. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Sarwar, R.; Zhang, W.; Geng, R.; Zhu, K.-M.; Tan, X.-L. Research progress on the physiological response and molecular mechanism of cold response in plants. Front. Plant Sci. 2024, 15, 1334913. [Google Scholar] [CrossRef] [PubMed]
- Öquist, G.; Hurry, V.M.; Hüner, N.P.A. Low temperature effects on photosynthesis and correlation with freezing tolerance in spring and winter cultivars of wheat and rye. Plant Physiol. 1993, 101, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.R.; Savitch, L.V.; Ivanov, A.G.; Hüner, N.P.A. Photosystem II excitation pressure and development of resistance to photoinhibition. II. Adjustment of photosynthetic capacity in winter wheat and winter rye. Plant Physiol. 1996, 110, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the CBF cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Kerbler, S.M.; Wigge, P.A. Temperature sensing in plants. Annu. Rev. Plant Biol. 2023, 74, 341–366. [Google Scholar] [CrossRef]
- Hurry, V.; Hüner, N.P.A. Low growth temperature affects a differential inhibition of photosynthesis in spring and winter wheat. Plant Physiol. 1991, 96, 491–497. [Google Scholar] [CrossRef]
- Hurry, V.; Hüner, N.P.A. Effects of cold hardening on sensitivity of winter and spring wheat leaves to short-term photoinhibition and recovery of photosynthesis. Plant Physiol. 1992, 100, 1283–1290. [Google Scholar] [CrossRef]
- Őquist, G.; Hüner, N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Sebolt, A.M.; Salazar, M.P.; Everard, J.D.; Thomashow, M.F. Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 2000, 124, 1854–1865. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Fowler, S.G.; Thomashow, M.F. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Mol. Biol. 2004, 54, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, I.; Busch, F.; Hüner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Mock, T.; Valentin, K. Photosynthesis and cold acclimation: Molecular evidence from a polar diatom. J. Phycol. 2004, 40, 732–741. [Google Scholar] [CrossRef]
- Matula, C.V.; Quartino, M.L.; Nunez, J.D.; Zacher, K. Effects of seawater temperature and seasonal irradiance on growth, reproduction and survival of the endemic Antarctic brown alga Desmarestia menziezii (Phaephyceae). Polar Biol. 2022, 45, 559–572. [Google Scholar] [CrossRef]
- Bravo, L.A.; Ulloa, N.; Zuniga, G.E.; Casanova, A.; Corcuera, L.J.; Alberdi, M. Cold resistance in Antarctic angiosperms. Physiol. Plant. 2001, 111, 55–65. [Google Scholar] [CrossRef]
- Bravo, L.; Saavedra-Mella, F.A.; Vera, F.; Guerra, A.; Cavieres, L.A.; Ivanov, A.G.; Hüner, N.P.A.; Corcuera, L.J. Effect of cold acclimation on the photosynthetic performance of two ecotypes of Colobanthus quitensis (Kunth) Bartl. J. Exp. Bot. 2007, 58, 3581–3590. [Google Scholar] [CrossRef]
- Byun, M.Y.; Lee, J.; Cui, L.H.; Kang, Y.; Oh, T.K.; Park, H.; Lee, H.; Kim, W.T. Constitutive expression of DaCBF7, an Antarctic vascular plant Deschampsia antarctica resulted in improved cold tolerance in transgenic rice plants. Plant Sci. 2015, 236, 61–74. [Google Scholar] [CrossRef]
- Wong, M.L.; Cleland, C.E.; Arend, D.; Barlett, S.; Cleaves, H.J.; Demarest, H.; Prabhu, A.; Luine, J.I.; Hazen, R.M. On the roles of function and selection in evolving systems. Proc. Natl. Acad. Sci. USA 2023, 120, e2310223120. [Google Scholar] [CrossRef]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Hüner, N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Hüner, N.P.A.; Smith, D.R. Chilling out: The evolution and diversification of psychrophilic algae with a focus on Chlamydomonadales. Polar Biol. 2017, 40, 1169–1184. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Szyszka-Mroz, B.; Possmayer, M.; Pittock, P.; Lajoie, G.; Smith, D.R.; Hüner, N.P.A. Characterization of photosynthetic ferredoxin from the Antarctic alga Chlamydomonas sp. UWO241 reveals novel features of cold adaptation. New Phytol. 2018, 219, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovska, M.; Vakulenko, G.; Smith, D.R.; Zhang, X.; Hüner, N.P.A. Temperature stress in psychrophilic green microalgae: Mini review. Physiol. Plant. 2022, 174, e13811. [Google Scholar] [CrossRef] [PubMed]
- Hüner, N.P.A.; Szyszka-Mroz, B.; Ivanov, A.G.; Kata, V.; Lye, H.; Smith, D.R. Photosynthetic adaptation and multicellularity in the Antarctic psychrophile, Chlamydomonas priscuii. Algal Res. 2023, 74, 103220. [Google Scholar] [CrossRef]
- Poirier, M.; Osmers, P.; Wilkins, K.; Morgan-Kiss, R.M.; Cvetkovska, M. Aberrant light sensing and motility in the green alga Chlamydomonas priscuii from the ice-covered Antarctic Lake Bonney. Plant Signal. Behav. 2023, 18, 2184588. [Google Scholar] [CrossRef]
- Gajardo, H.A.; Morales, M.; Larama, G.; Luengo-Escobar, A.; López, D.; Machado, M.; Nunes-Nesi, A.; Reyes-Díaz, M.; Planchais, S.; Savouré, A.; et al. Physiological, transcriptomic and metabolomic insights of three extremophyte woody species living in the multi-stress environment of the Atacama Desert. Planta 2024, 260, 55. [Google Scholar] [CrossRef]
- Chang, C.Y.-Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of winter survival: Cold acclimation and molecular regulation of cold hardiness in evergreen conifers. New Phytol. 2021, 229, 675–691. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Stangl, Z.R.; Ivanov, A.G.; Bui, V.; Mema, M.; Hüner, N.P.A.; Öquist, G.; Way, D.; Hurry, V. Contrasting acclimation abilities of two dominant boreal conifers to elevated CO2 and temperature. Plant Cell Environ. 2018, 41, 1331–1345. [Google Scholar] [CrossRef]
- Kurepin, L.; Dahal, K.; Savitch, L.; Singh, J.; Bode, R.; Ivanov, A.G.; Hurry, V.; Hüner, N.P.A. Role of CBFs as integrators of chloroplast redox, phytochrome and plant hormone signaling during cold acclimation. Int. J. Mol. Sci. 2013, 14, 12729–12763. [Google Scholar] [CrossRef]
- Karpinski, S.; Reynolds, H.; Karpinska, B.; Wingsle, G.; Creissen, G.; Mullineaux, P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A Probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Stirbet, A.; Govindjee. Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef]
- Horton, P. Optimization of light harvesting and photoprotection: Molecular mechanisms and physiological consequences. Philos. Trans. R. Soc. B Biol Sci. 2012, 367, 3455–3465. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Őquist, G.; Melis, A. Photostasis in plants, green algae and cyanobacteria: The role of light harvesting antenna complexes. In Light Harvesting Antennas in Photosynthesis; Green, B.R., Parson, W.W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 13, pp. 401–421. [Google Scholar]
- Arnon, D.I. Sunlight, Earth Life: The grand design of photosynthesis. N. Y. Acad. Sci. 1982, 22, 22. [Google Scholar]
- Fujita, Y.; Murakami, A.; Aizawa, K.; Ohki, K. Short-term and long-term adaptation of the photosynthetic apparatus: Homeostatic properties of thylakoids. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1994; Volume 1, pp. 677–692. [Google Scholar]
- Anderson, J.M.; Chow, W.S.; Park, Y.-I. The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 1995, 46, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, J.-M.; Lomas, M.; LaRoche, J.; Falkowski, P.G. Light intensity regulates cab gene transcription via the redox state of the plastoquinone pool in the green alga, Dunaliella tertiolecta. Proc. Nat. Acad. Sci. USA 1995, 92, 10237–10241. [Google Scholar] [CrossRef] [PubMed]
- Pfannschmidt, T. Chloroplast redox signals: How photosynthesis controls its own genes. Trends Plant Sci. 2003, 8, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Redox signal integration: From stimulus to networks and genes. Physiol. Plant. 2008, 133, 459–468. [Google Scholar] [CrossRef]
- Winfield, M.O.; Lu, C.; Wilson, I.D.; Coghill, J.A.; Edwards, K.J. Plant responses to cold: Transcriptome analysis of wheat. Plant Biotechnol. J. 2010, 8, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Barajas-López, J.D.; Blanco, N.E.; Strand, Å. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta 2013, 1833, 425–437. [Google Scholar] [CrossRef]
- Sakamoto, W.; Miyagishima, S.; Jarvis, P. Chloroplast biogenesis: Control of plastid development, protein Import, division and inheritance. Arab. Book 2008, 6, e0110. [Google Scholar] [CrossRef]
- Jarvis, P.; Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Chen, Y.-B. Photoacclimation of light harvesting systems in eukaryotic algae. In Light Harvesting Systems in Photosynthesis; Green, B.R., Parson, W.W., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 13, pp. 423–447. [Google Scholar]
- Woodson, J.D.; Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Zhang, G.-C.; Zhu, F.; Zhang, D.-W.; Yuan, S. The roles of tetrapyrroles in plastid retrograde signaling and tolerance to environmental stresses. Planta 2015, 242, 1263–1276. [Google Scholar] [CrossRef]
- Eberhard, S.; Finazzi, G.; Wollman, F.-A. The dynamics of photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Quail, P.H.; Boylan, M.T.; Parks, B.M.; Short, T.W.; Xu, Y.; Wagner, D. Phytochromes: Photosensory perception and signal transduction. Science 1995, 268, 675–680. [Google Scholar] [CrossRef]
- Casal, J.J.; Fankhauser, C.; Coupland, G.; Blazquez, M.A. Signalling for developmental plasticity. Trends Plant Sci. 2004, 9, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kianianmomeni, A.; Hallmann, A. Algal photoreceptors: In vivo functions and potential applications. Planta 2014, 239, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Gerken, U.; Tang, K.; Philipp, M.; Zurbriggen, M.D.; Köhler, J.; Möglich, A. Plant phytochrome interactions decode light and temperature signals. Plant Cell 2024, 36, 4819–4839. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Toledo-Ortiz, G.; Pyott, D.E.; Halliday, K.J. Interaction of light and temperature signalling. J. Exp. Bot. 2014, 65, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Pogson, B.J.; Woo, N.S.; Förster, B.; Small, I.D. Plastid signalling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 1984, 35, 583–584. [Google Scholar] [CrossRef]
- Guy, C.L. Cold acclimation and freezing tolerance: Role of protein metabolism. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, C.W.; Choi, S.I.; Kim, J.K. Expression of barley HvCBF4 enhances tolerance to abiotic stress in transgenic rice. Plant Biotechnol. J. 2007, 5, 646–656. [Google Scholar] [CrossRef]
- Lourenco, T.; Saibo, N.; Batista, R.; Pinto Ricardo, C.; Oliviera, M.M. Inducible and constitutive expression of HvCBF4 in rice leads to differential gene expression and drought tolerance. Biol. Plant 2011, 55, 653–663. [Google Scholar] [CrossRef]
- Soltesz, A.; Smedley, M.; Vashegyi, I.; Galiba, G.; Harwood, W.; Vagujfalvi, A. Transgenic barley lines prove the involvement of TaCBF14 and TaCBF15 in the cold acclimation process and in frost tolerance. J. Exp. Bot. 2013, 64, 1849–1862. [Google Scholar] [CrossRef]
- Galiba, G.; Vágújfalvi, A.; Li, C.; Soltész, A.; Dubcovsky, J. Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci. 2009, 176, 12–19. [Google Scholar] [CrossRef]
- Savitch, L.V.; Allard, G.; Seki, M.; Robert, L.S.; Tinker, N.A.; Hüner, N.P.A.; Shinozaki, K.; Singh, J. The effect of over-expression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and freezing tolerance in Brassica napus. Plant Cell Physiol. 2005, 46, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.-L.; Mathesius, U. Root-to-shoot signalling: Integration of diverse molecules, pathways and functions. Funct. Plant Biol. 2016, 43, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.B.; Byrns, B.M.; Greer, K.J. Overwinter low-temperature responses of cereals: Analyses and simulation. Crop Sci. 2014, 54, 2395–2405. [Google Scholar] [CrossRef]
- Fowler, D.B.; Dvorak, J.; Gusta, L.V. Comparative cold hardiness of several Triticum species and Secale cereale L. Crop Sci. 1977, 17, 941–943. [Google Scholar] [CrossRef]
- Fowler, D.B. Cold acclimation threshold induction temperatures in cereals. Crop Sci. 2008, 48, 1147–1154. [Google Scholar] [CrossRef]
- Dietz, K.J. Plant peroxiredoxins. Annu. Rev. Plant Biol. 2003, 54, 93–107. [Google Scholar] [CrossRef]
- Dietz, K.J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Kim, C.; Apel, K. Singlet oxygen-mediated signaling in plants: Moving from flu to wild type reveals an increasing complexity. Photosynth. Res. 2013, 116, 455–464. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer: Sunderland, MA, USA, 2002. [Google Scholar]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; Wiley and Sons: Hoboken, NJ, USA, 2009; 503. [Google Scholar]
- Reinecke, D.M.; Wickramarathna, A.D.; Ozga, J.A.; Kurepin, L.V.; Jin, A.L.; Good, A.G.; Pharis, R.P. Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis, growth, and development in pea. Plant Physiol. 2013, 163, 929–945. [Google Scholar] [CrossRef]
- Hedden, P. The genes of the green revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Carol, P.; Richards, D.E.; King, K.E.; Cowling, R.J.; Murphy, G.P.; Harberd, N.P. The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Genes Dev. 1997, 11, 3194–3205. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Harberd, N.P. Giberellin deficiency and response mutations suppress stem elongation phenotype of phytochrome-deficient mutants of Arabidopsis. Plant Physiol. 1997, 113, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Hussain, A.; Peng, J. Della proteins and GA signalling in Arabidopsis. J. Plant Growth Regul. 2003, 22, 134–140. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef]
- Siddiqua, M.; Nassuth, A. Vitis CBF1 and Vitis CBF4 differ in their effect on Arabidopsis abiotic stress tolerance, development and gene expression. Plant Cell Environ. 2011, 34, 1345–1359. [Google Scholar] [CrossRef]
- Limin, A.E.; Fowler, D.B. Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): Response to photoperiod, vernalization, and plant development. Planta 2006, 224, 360–366. [Google Scholar] [CrossRef]
- Zhu, J.; Pearce, S.; Burke, A.; See, D.; Skinner, D.; Dubcovsky, J.; Garland-Campbell, K. Copy number and haplotype variation at the VRN-A1 and central FR-A2 loci are associated with frost tolerance in hexaploid wheat. Theor. Appl. Genet. 2014, 127, 1183–1197. [Google Scholar] [CrossRef]
- Mahfoozi, S.; Limin, A.E.; Fowler, D.B. Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Sci. 2001, 41, 1006–1011. [Google Scholar] [CrossRef]
- Kidokoro, S.; Watnabe, K.; Ohori, T.; Moriwaki, T.; Mzoi, J.; Myint, N.; Htwe, P.S.; Fujita, Y.; Sekita, S.; Shinozaki, K.; et al. Soybean DREB1/CBF-type transcroption factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Senthil-Kumar, M. Unmasking complexities of combined stresses for creating climate-smart crops. Trends Plant Sci. 2024, 29, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Ceasar, S.A.; Baker, A. Millets for food security and agricultural sustainability. Planta 2025, 621, 39. [Google Scholar] [CrossRef]
- Lai, X.; Yan, J.; Chen, Z.; Zhang, Y.; Luo, F.; Cai, G.; Yan, L. Dynamic changes in the transcriptome of tropical region-originated king grasses in response to cold stress. Front. Plant Sci. 2025, 16, 1511466. [Google Scholar] [CrossRef]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts—Beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Zhou, L.; Ullah, F.; Zou, J.; Zeng, X. Molecular and physiological responses of plants that enhance cold tolerance. Int. J. Mol. Sci. 2025, 26, 1157. [Google Scholar] [CrossRef]

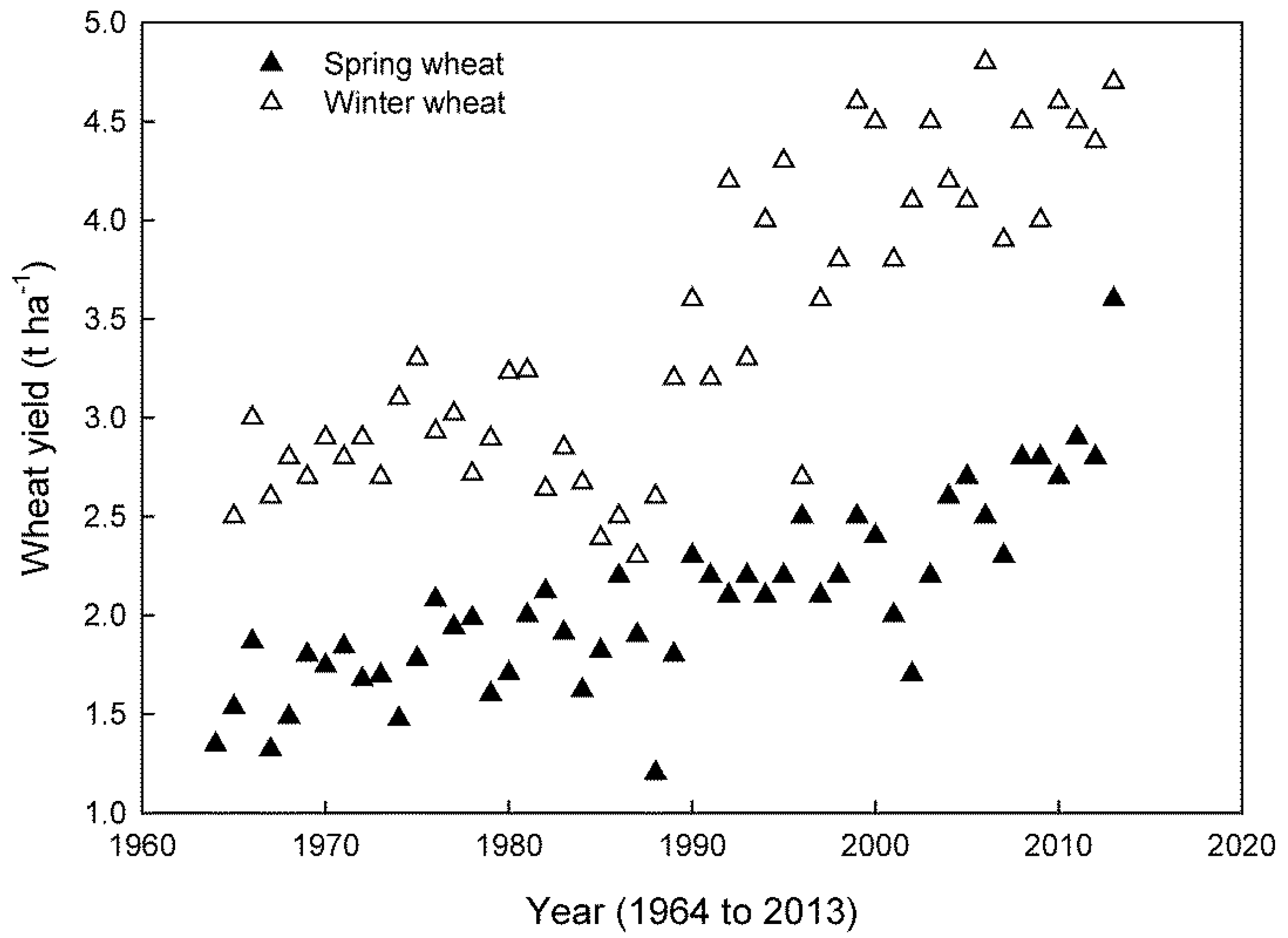
| Winter Varieties | Cold Acclimated (CA) | Non-Acclimated (NA) | CA/NA |
|---|---|---|---|
| Wheat cv Norstar | 0.288 | 0.120 | 2.40 |
| Rye cv Musketeer | 0.360 | 0.148 | 2.43 |
| Spinach | 0.400 | 0.176 | 2.27 |
| Brassica WT | 0.252 | 0.108 | 2.33 |
| Brassica CBF-OE | --- | 0.200 | 1.85 |
| Spring Wheat Varieties | |||
| SR4A | 0.160 | 0.132 | 1.21 |
| Katepwa | 0.168 | 0.144 | 1.17 |
| Country | Total (Mt) | Winter (%) | Spring (%) |
|---|---|---|---|
| China | 140 | 95 | 5 |
| European Union | 121.3 | 96 | 4 |
| India | 113.3 | ||
| Russia | 81.5 | 72 | 28 |
| United States | 53.7 | 70 | 30 |
| Canada | 35 | 4 | 96 |
| Australia | 32 | ||
| Pakistan | 31.4 | ||
| Ukraine | 22.9 | ||
| Turkey | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hüner, N.P.A.; Ivanov, A.G.; Szyszka-Mroz, B.; Bravo, L.A.; Savitch, L.V.; Krol, M. Vernalization of Winter Crops Increases Photosynthetic Energy Conversion Efficiency and Seed Yield. Plants 2025, 14, 2357. https://doi.org/10.3390/plants14152357
Hüner NPA, Ivanov AG, Szyszka-Mroz B, Bravo LA, Savitch LV, Krol M. Vernalization of Winter Crops Increases Photosynthetic Energy Conversion Efficiency and Seed Yield. Plants. 2025; 14(15):2357. https://doi.org/10.3390/plants14152357
Chicago/Turabian StyleHüner, Norman P. A., Alexander G. Ivanov, Beth Szyszka-Mroz, Leon A. Bravo, Leonid V. Savitch, and Marianna Krol. 2025. "Vernalization of Winter Crops Increases Photosynthetic Energy Conversion Efficiency and Seed Yield" Plants 14, no. 15: 2357. https://doi.org/10.3390/plants14152357
APA StyleHüner, N. P. A., Ivanov, A. G., Szyszka-Mroz, B., Bravo, L. A., Savitch, L. V., & Krol, M. (2025). Vernalization of Winter Crops Increases Photosynthetic Energy Conversion Efficiency and Seed Yield. Plants, 14(15), 2357. https://doi.org/10.3390/plants14152357







