A Water Solution from the Seeds, Seedlings and Young Plants of the Corn Cockle (Agrostemma githago) Showed Plant-Growth Regulator Efficiency
Abstract
1. Introduction
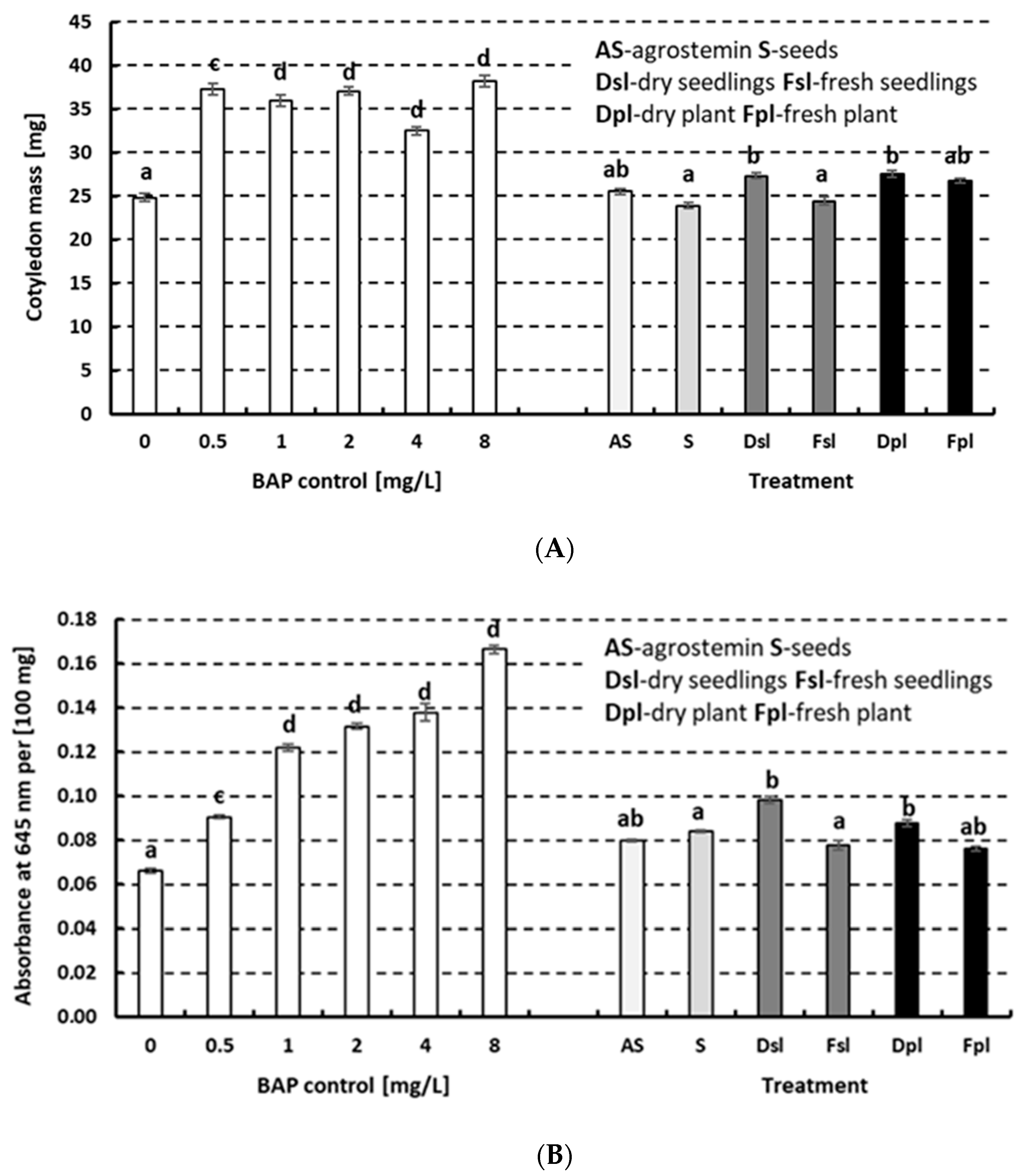
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Biological Activity
4.3. Plant Hormone Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A global perspective. Front. Plant Sci. 2016, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- European Biostimulants Industry Council [EBIC]. Available online: https://biostimulants.eu/plant-biostimulants/ (accessed on 26 June 2025).
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Ancín, M.; Soba, D.; Picazo, P.J.; Gámez, A.L.; Le Page, J.F.; Houdusse, D.; Aranjuelo, I. Optimizing oilseed rape growth: Exploring the effect of foliar biostimulants on the interplay among metabolism, phenology, and yield. Physiol. Plant. 2024, 176, e14561. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Hasan, M.M.; Rahman, M.A. Hormones and biostimulants in plants: Physiological and molecular insights on plant stress responses. Front. Plant Sci. 2024, 15, 1413659. [Google Scholar] [CrossRef] [PubMed]
- Smakosz, A.; Matkowski, A.; Nawrot-Hadzik, I. Phytochemistry and Biological Activities of Agrostemma Genus—A Review. Plants 2024, 13, 1673. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, B.; Doll, H. A positive allelopathic effect of corn cockle, Agrostemma githago, on wheat, Triticum aestivum. Can. J. Bot. 1992, 70, 1916–1918. [Google Scholar] [CrossRef]
- Mian, S.; Leitch, I.J.; Royal Botanic Gardens Kew Genome Acquisition Lab; Plant Genome Sizing collective; Darwin Tree of Life Barcoding Collective; Wellcome Sanger Institute Tree of Life Management; Samples and Laboratory Team; Wellcome Sanger Institute Scientific Operations: Sequencing Operations; Wellcome Sanger Institute Tree of Life Core Informatics team; Tree of Life Core Informatics Collective; et al. The genome sequence of the corn cockle, Agrostemma githago L., 1753 (Caryophyllaceae). Wellcome Open Res. 2024, 9, 590. [Google Scholar] [CrossRef]
- Agrostemin®. Available online: https://www.agrostemin.co.rs/opstipodacieng.php (accessed on 24 June 2025).
- Ambrožič-Dolinšek, J.; Ornik, D.; Branda, R.; Molnár, Z.; Ciringer, T. Does biostimulant Agrostemin® exhibit plant growth regulator activities? Phyton 2021, 61, 109–116. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Skripnikov, A. Bioassays for Identifying and Characterizing Plant Regulatory Peptides. Biomolecules 2023, 13, 1795. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, V.; Zandoli, R.; Di Nardo, A.; Biondi, S.; Antognoni, F.; Calandriello, F. Biological activity of different botanical extracts as evaluated by means of an array of in vitro and in vivo bioassays. In Proceedings of the I World Congress on the Use of Biostimulants in Agriculture, Strasbourg, France, 26–29 November 2012; Volume 1009, pp. 61–66. [Google Scholar]
- Vrbaški, M.M.; Grujić-Injac, B.; Gajić, D. Preparation, identification and biological activity of substance “A” from seeds of Agrostemma githago. Biochem. Physiol. Pflanz. 1973, 171, 69–74. [Google Scholar] [CrossRef]
- Gajic, D.; Nikocevic, G. Chemical allelopathic effect of Agrostemma githago upon wheat. Fragm. Herbol. Jugosl. 1973, 18, 1–5. [Google Scholar]
- Krajnc, U.A.; Ivanuš, A.; Kristl, J.; Susek, A. Seaweed extract elicits the metabolic responses in leaves and enhances growth of Pelargonium cuttings. Eur. J. Hortic. Sci. 2012, 77, 170–181. [Google Scholar] [CrossRef]
- Gad, M.M.; Ibrahim, M.M. Effect of IBA and some natural extracts on rooting and vegetative growth of Picual olive sucker and shoot cuttings. Curr. Sci. Int. 2018, 7, 191–203. [Google Scholar]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Avice, J.C.; Vasconcelos, M.W.; Du Jardin, P.; Brown, P.H. Biostimulants in Agriculture. Physiol. Plant. 2025, 177, e70046. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Z.; Huang, G.; Li, G. An improved disk bioassay for determining activities of plant growth regulators. J. Plant Growth Regul. 1992, 11, 209–213. [Google Scholar] [CrossRef]
- Yopp, J.H. Bioassays for plant hormones and other naturally occurring plant growth regulators. In Handbook of Natural Pesticides: Methods; CRC Press: Boca Raton, FL, USA, 1985; pp. 329–477. [Google Scholar]
- Semenarna Ljubljana. Ljubljana, Slovenia. Available online: https://www.semenarna.si/files/files/Veliki vrtni katalog_2025.pdf (accessed on 26 June 2025).
- Kühnle, J.A.; Fuller, G.; Corse, J.; Mackey, B.E. Antisenescent activity of natural cytokinins. Physiol. Plant. 1977, 41, 14–21. [Google Scholar] [CrossRef]
- Creative Proteomics. New York, USA. Available online: https://www.creative-proteomics.com/plantmet/plant-hormones-analysis-service.html (accessed on 26 June 2025).

| Sample | IAA | Methyl IAA | IAA-Ala | IAA-Asp | IAA-Trp |
|---|---|---|---|---|---|
| ng g−1 of dry weight | |||||
| Agrostemin® | 1.2 | ND | ND | ND | ND |
| Seeds | 3.3 | ND | ND | 28.0 | 0.4 |
| Seedlings | 53.4 | ND | ND | 7.9 | ND |
| Young plants | 15.9 | ND | ND | ND | ND |
| LOD, nM 10 uL injection | 0.16 | 8 | 0.4 | 0.4 | |
| Sample | cZ | cZR | tZ | tZR |
|---|---|---|---|---|
| ng g−1 of dry weight | ||||
| Agrostemin® | 0.01 | 0.00 | ND | ND |
| Seeds | 0.15 | 0.11 | 0.21 | 0.06 |
| Seedlings | 0.09 | 1.88 | 0.52 | 2.09 |
| Young plants | 0.30 | 1.73 | 0.81 | 2.13 |
| LOD, nM 10 uL injection | 0.032 | 0.0128 | ||
| Sample | ABA | SA | JA | JA-ILE | OPDA |
|---|---|---|---|---|---|
| ng g−1 of dry weight | |||||
| Agrostemin® | 0.5 | 129.4 | ND | ND | ND |
| Seeds | 5.3 | 157.0 | ND | ND | 5.9 |
| Seedlings | 33.4 | 193.4 | 65.6 | 22.0 | 22.3 |
| Young plants | 22.1 | 121.1 | 36.6 | 5.3 | ND |
| LOD, nM 10 uL injection | 0.3 | 0.1 | 8.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrožič-Dolinšek, J.; Golič, V.; Saco, V.R.; Peranić, P.; Grujić, V.J.; Ciringer, T. A Water Solution from the Seeds, Seedlings and Young Plants of the Corn Cockle (Agrostemma githago) Showed Plant-Growth Regulator Efficiency. Plants 2025, 14, 2349. https://doi.org/10.3390/plants14152349
Ambrožič-Dolinšek J, Golič V, Saco VR, Peranić P, Grujić VJ, Ciringer T. A Water Solution from the Seeds, Seedlings and Young Plants of the Corn Cockle (Agrostemma githago) Showed Plant-Growth Regulator Efficiency. Plants. 2025; 14(15):2349. https://doi.org/10.3390/plants14152349
Chicago/Turabian StyleAmbrožič-Dolinšek, Jana, Vid Golič, Víctor Rouco Saco, Petra Peranić, Veno Jaša Grujić, and Terezija Ciringer. 2025. "A Water Solution from the Seeds, Seedlings and Young Plants of the Corn Cockle (Agrostemma githago) Showed Plant-Growth Regulator Efficiency" Plants 14, no. 15: 2349. https://doi.org/10.3390/plants14152349
APA StyleAmbrožič-Dolinšek, J., Golič, V., Saco, V. R., Peranić, P., Grujić, V. J., & Ciringer, T. (2025). A Water Solution from the Seeds, Seedlings and Young Plants of the Corn Cockle (Agrostemma githago) Showed Plant-Growth Regulator Efficiency. Plants, 14(15), 2349. https://doi.org/10.3390/plants14152349






