Bark Extracts of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Attenuate LPS-Induced Inflammatory Responses in RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
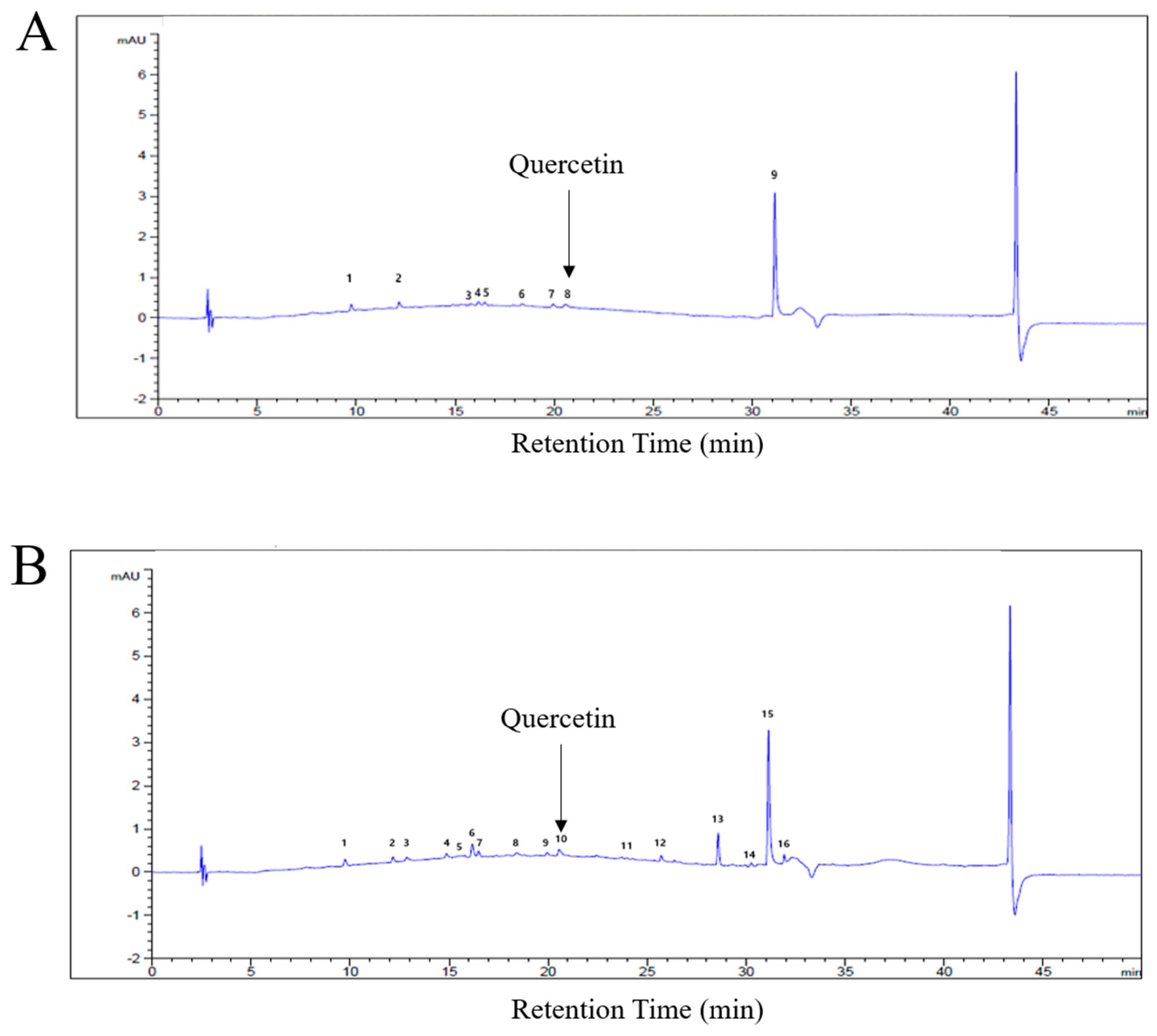
2.1. Fingerprint Analysis of C. obtusa Bark Extracts
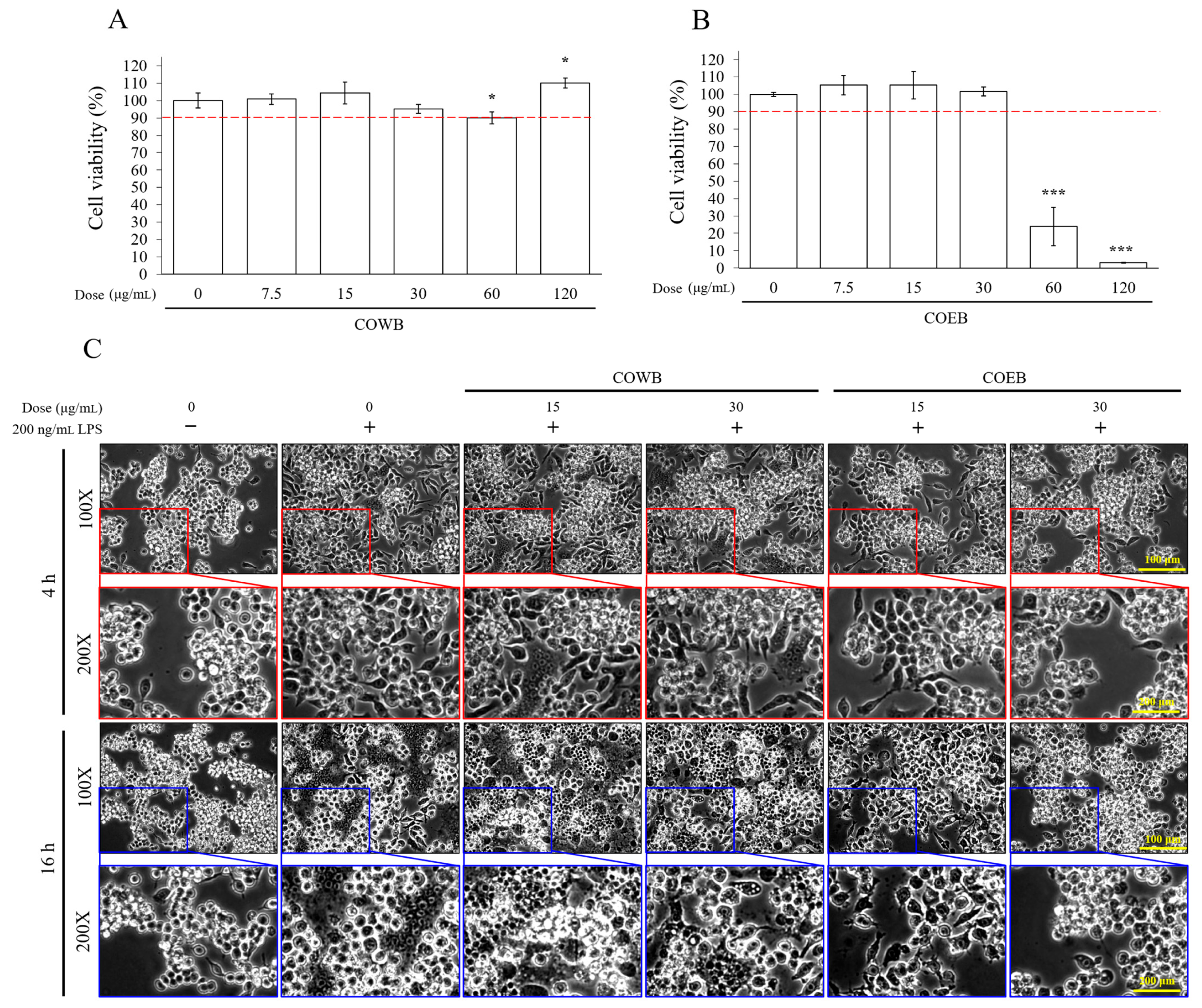
2.2. Cytotoxicity and Anti-Inflammatory Effects of COWB and COEB on LPS-Stimulated RAW264.7 Cells
2.3. Comparison of Anti-Inflammatory Effects of COWB and COEB in LPS-Stimulated RAW264.7 Cells
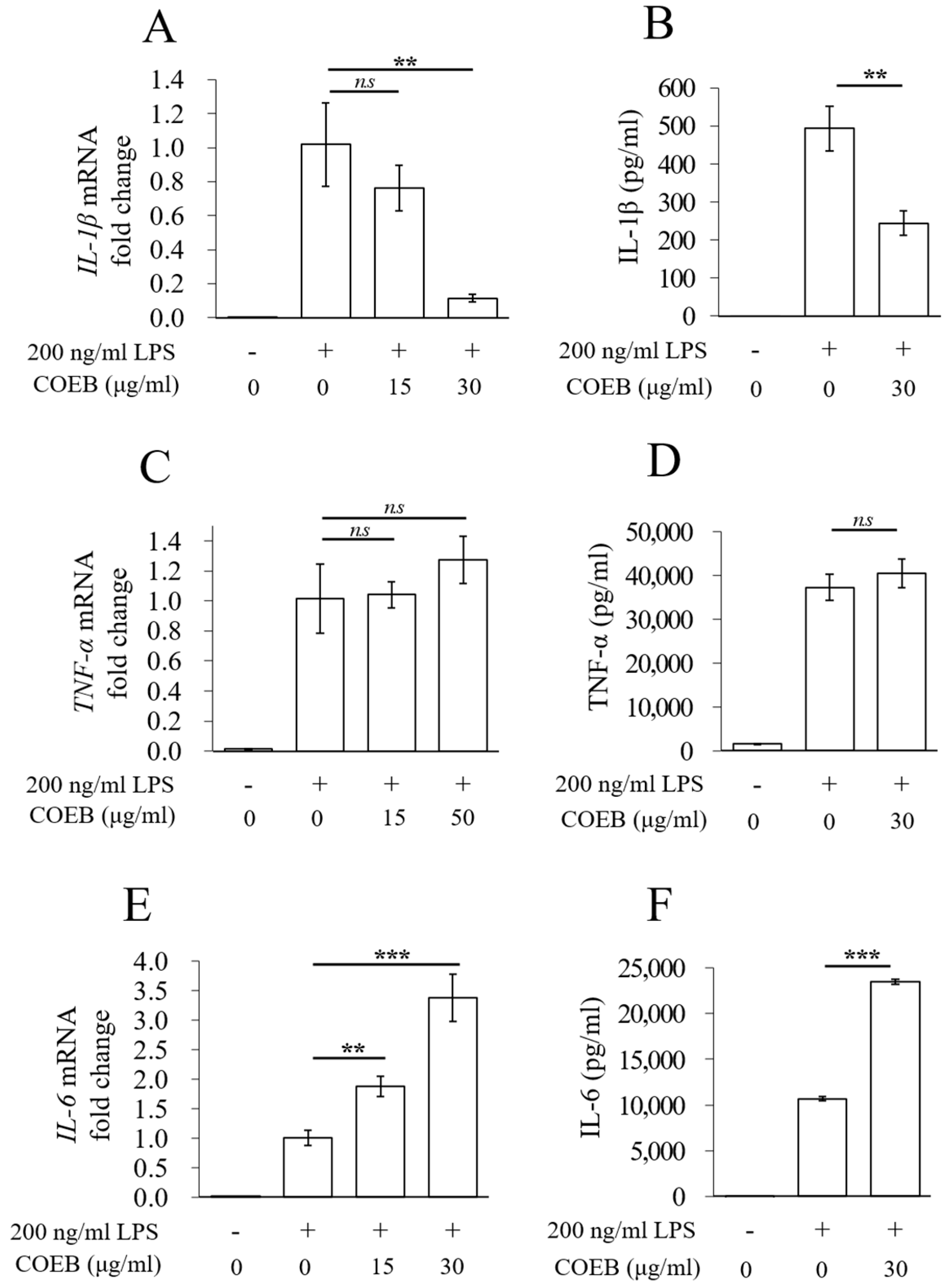
2.4. COEB Regulates LPS-Induced Pro-Inflammatory Cytokines
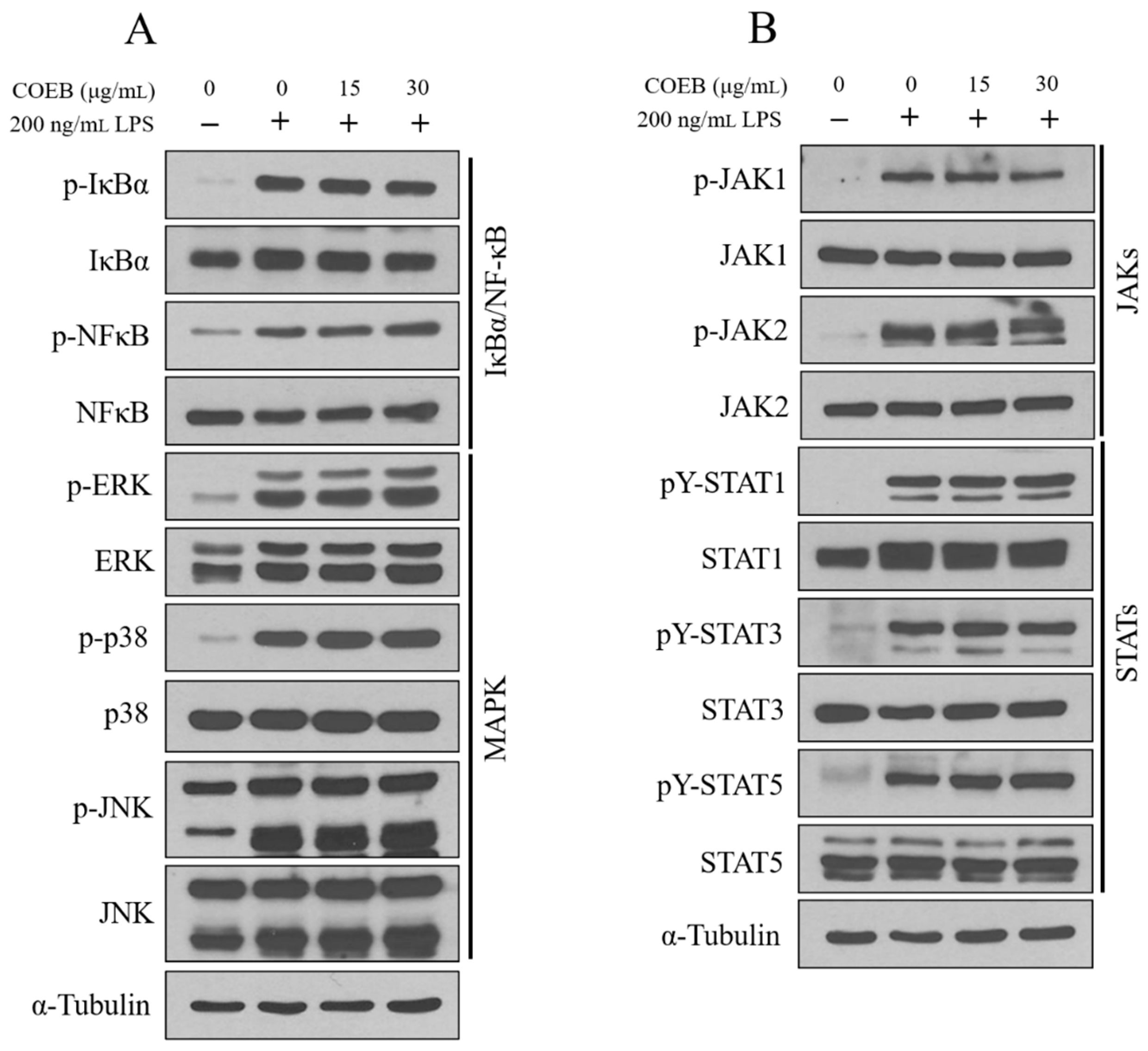
2.5. The Anti-Inflammatory Effects of COEB Are Independent of NF-κB, MAPK, and JAK/STAT Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Antibodies
4.3. Extraction Process of C. obtusa Bark Extracts
4.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.5. Cell Culture Condition
4.6. Cell Viability Analysis
4.7. Nitric Oxide (NO) Production
4.8. Prostaglandin E2 (PGE2) Production
4.9. Immunoblotting
4.10. Quantitative Real-Time PCR (qPCR)
4.11. ELISA Analysis of Cytokines
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azuma, M. Fundamental mechanisms of host immune responses to infection. J. Periodontal Res. 2006, 41, 361. [Google Scholar] [CrossRef]
- Pezzolo, E.; Naldi, L. Epidemiology of major chronic inflammatory immune-related skin diseases in 2019. Expert Rev. Clin. Immunol. 2020, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Nair, P. Autoimmune responses in severe asthma. Allergy Asthma Immunol. Res. 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Beyaert, R. Role of Toll-like receptors in pathogen recognition. Clin. Microbiol. Rev. 2003, 16, 637. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Sautebin, L. Prostaglandins and nitric oxide as molecular targets for anti-inflammatory therapy. Fitoterapia 2000, 71, S48. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Nailwal, N.P.; Doshi, G.M. Role of intracellular signaling pathways and their inhibitors in the treatment of inflammation. Inflammopharmacology 2021, 29, 617. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Bird, H.; Giannoudis, P.V. Nonsteroidal anti-inflammatory drugs: Prostaglandins, indications, and side effects. Int. J. Interferon Cytokine Mediat. Res. 2011, 2011, 19–27. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140. [Google Scholar] [CrossRef]
- Nunes, C.d.R.; Arantes, M.B.; de Faria Pereira, S.M.; Da Cruz, L.L.; de Souza Passos, M.; De Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Recio, M.C.; Andujar, I.; Rios, J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767. [Google Scholar] [CrossRef] [PubMed]
- Górski, K.M.; Kowalczyk, T.; Picot, L.; Rijo, P.; Ghorbanpour, M.; Sitarek, P. The precious potential of the sacred tree Chamaecyparis obtusa (Siebold & Zucc.) Endl. as a source of secondary metabolites with broad biological applications. Int. J. Mol. Sci. 2024, 25, 2723. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Seo, E.-B.; Kim, S.-K.; Noh, K.H.; Lee, H.; Joung, Y.-W.; Shin, H.M.; Jang, Y.-A.; Kim, Y.M.; Lee, J.-T.; et al. Chamaecyparis obtusa (Siebold & Zucc.) Endl. leaf extracts prevent inflammatory responses via inhibition of the JAK/STAT axis in RAW264. 7 cells. J. Ethnopharmacol. 2022, 282, 114493. [Google Scholar] [CrossRef]
- Kim, B.E.; Goleva, E.; Hall, C.F.; Park, S.H.; Lee, U.H.; Brauweiler, A.M.; Streib, J.E.; Richers, B.N.; Kim, G.; Leung, D.Y. Skin wound healing is accelerated by a lipid mixture representing major lipid components of Chamaecyparis obtusa plant extract. J. Investig. Dermatol. 2018, 138, 1176. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Seo, E.-B.; Kim, S.-K.; Lee, H.-S.; Lee, H.; Jang, Y.-A.; Kim, Y.M.; Kim, Y.-N.; Lee, J.-T.; Ye, S.-K. Pharmacological anti-tumor effects of natural Chamaecyparis obtusa (siebold & zucc.) endl. Leaf extracts on breast cancer. J. Ethnopharmacol. 2023, 313, 116598. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, S.-G.; Oh, T.-J.; Lim, S.R.; Kim, S.-H.; Lee, H.J.; Kim, Y.-S.; Choi, H.-K. Antiproliferative and apoptotic activity of chamaecyparis obtusa leaf extract against the HCT116 human colorectal cancer cell line and investigation of the bioactive compound by gas chromatography-mass spectrometry-based metabolomics. Molecules 2015, 20, 18066. [Google Scholar] [CrossRef]
- Park, S.-H.; Kwon, Y.-J. Evaluation of Whitening and Antibacterial Efficacy using Chamaecyparis Obtusa (Siebold & Zucc.) Endl Root: Focusing on Functional Cosmetic Materials. J. Altern. Anim. Exp. 2024, 18, 27. [Google Scholar]
- Joung, Y.-W.; Kim, Y.-M.; Jang, Y.-A. Studies on the antioxidant and whitening effects of Chamaecyparis obtusa extract. J. Korean Appl. Sci. Technol. 2020, 37, 1496. [Google Scholar] [CrossRef]
- Marimuthu, P.; Wu, C.; Chang, H.; Chang, S. Antioxidant activity of the ethanolic extract from the bark of Chamaecyparis obtusa var. formosana. J. Sci. Food Agric. 2008, 88, 1400. [Google Scholar] [CrossRef]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, L.M.H.; Yagi, S.; Mohamed, H.M.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential oils composition and biological activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia grown wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef]
- Khalil, A.S.E.; Lukasiewicz, M. The optimization of the hot water extraction of the polysaccharide-rich fraction from Agaricus bisporus. Molecules 2024, 29, 4783. [Google Scholar] [CrossRef]
- Liu, J.; Wen, X.-Y.; Zhang, X.-Q.; Pu, H.-M.; Kan, J.; Jin, C.-H. Extraction, characterization and in vitro antioxidant activity of polysaccharides from black soybean. Int. J. Biol. Macromol. 2015, 72, 1182–1190. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Cuevas-Valenzuela, J.; Pérez-Correa, J.R. Pressurized hot water extraction of polyphenols from plant material. In Biotechnology of Bioactive Compounds: Sources and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 63–101. [Google Scholar] [CrossRef]
- Kaundal, R.; Kumar, R.; Kumar, D. Optimized extraction technique and solvent to enhance the recovery of bioactive polyphenols from Viola canescens Wall. ex Roxb.: A case study using OFAT and chemical characterization using UPLC-PDA, UHPLC-Q-TOF-IMS and GC–MS. Microchem. J. 2025, 212, 113170. [Google Scholar] [CrossRef]
- Pi, J.; Li, T.; Liu, J.; Su, X.; Wang, R.; Yang, F.; Bai, H.; Jin, H.; Cai, J. Detection of lipopolysaccharide induced inflammatory responses in RAW264. 7 macrophages using atomic force microscope. Micron 2014, 65, 1. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-Borojeni, A.A. Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res. 2022, 36, 1216. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 1, 448. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Śliwiński, T.; Zajdel, R. Anti-inflammatory activity of extracts and pure compounds derived from plants via modulation of signaling pathways, especially PI3K/AKT in macrophages. Int. J. Mol. Sci. 2020, 21, 9605. [Google Scholar] [CrossRef]
- Tang, P.; Li, Q.; Liao, S.; Wei, S.; Cui, L.; Xu, W.; Zhu, D.; Luo, J.; Kong, L. Shizukaol A exerts anti-inflammatory effect by regulating HMGB1/Nrf2/HO-1 pathway. Phytomedicine 2021, 82, 153472. [Google Scholar] [CrossRef]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; A Flavell, R. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447. [Google Scholar] [CrossRef]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Zhang, T.; Niu, J.; Li, X. Dihydroquercetin inhibits LPS-induced inflammation by activating AMPK/Nrf2/HO-1 pathway in RAW264.7 macrophages. Front. Pharmacol. 2020, 11, 662. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J.; Dai, Y.; Xiong, H. Nardochinoid C inhibits inflammation via activating Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Kurashima, Y.; Kiyono, H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu. Rev. Immunol. 2017, 35, 119. [Google Scholar] [CrossRef]
- Lacina, L.; Kolář, M.; Pfeiferová, L.; Gál, P.; Smetana, K. Wound healing: Insights into autoimmunity, ageing, and cancer ecosystems through inflammation and IL-6 modulation. Front. Immunol. 2024, 15, 1403570. [Google Scholar] [CrossRef]
- Sanghavi, N.; Bhosale, S.D.; Malode, Y. RP-HPLC method development and validation of Quercetin isolated from the plant Tridax procumbens L. J. Sci. Innov. Res. 2014, 3, 594–597. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Herrmann, M.G.; Moss, A.A.; Rasmussen, R.P. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 1997, 22, 130–138. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-A.; Byeon, J.-A.; Jang, Y.-A.; Kwon, Y.-J. Bark Extracts of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Attenuate LPS-Induced Inflammatory Responses in RAW264.7 Macrophages. Plants 2025, 14, 2346. https://doi.org/10.3390/plants14152346
Kim B-A, Byeon J-A, Jang Y-A, Kwon Y-J. Bark Extracts of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Attenuate LPS-Induced Inflammatory Responses in RAW264.7 Macrophages. Plants. 2025; 14(15):2346. https://doi.org/10.3390/plants14152346
Chicago/Turabian StyleKim, Bo-Ae, Ji-A Byeon, Young-Ah Jang, and Yong-Jin Kwon. 2025. "Bark Extracts of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Attenuate LPS-Induced Inflammatory Responses in RAW264.7 Macrophages" Plants 14, no. 15: 2346. https://doi.org/10.3390/plants14152346
APA StyleKim, B.-A., Byeon, J.-A., Jang, Y.-A., & Kwon, Y.-J. (2025). Bark Extracts of Chamaecyparis obtusa (Siebold & Zucc.) Endl. Attenuate LPS-Induced Inflammatory Responses in RAW264.7 Macrophages. Plants, 14(15), 2346. https://doi.org/10.3390/plants14152346




