Changes in the Quality of Idesia polycarpa Maxim Fruits from Different Ecotypes During the Growth Process
Abstract
1. Introduction
2. Results
2.1. Changes in Phenotypic Traits During Fruit Growth of I. polycarpa from Different Ecotypes
2.2. Changes in Oil and Water Content of Fruits from Different Ecotypes of I. polycarpa
2.3. Changes of Antioxidant Enzymes in Fruit I. polycarpa from Different Ecotypes
2.4. Changes in Fruit Content of I. polycarpa from Different Ecotypes
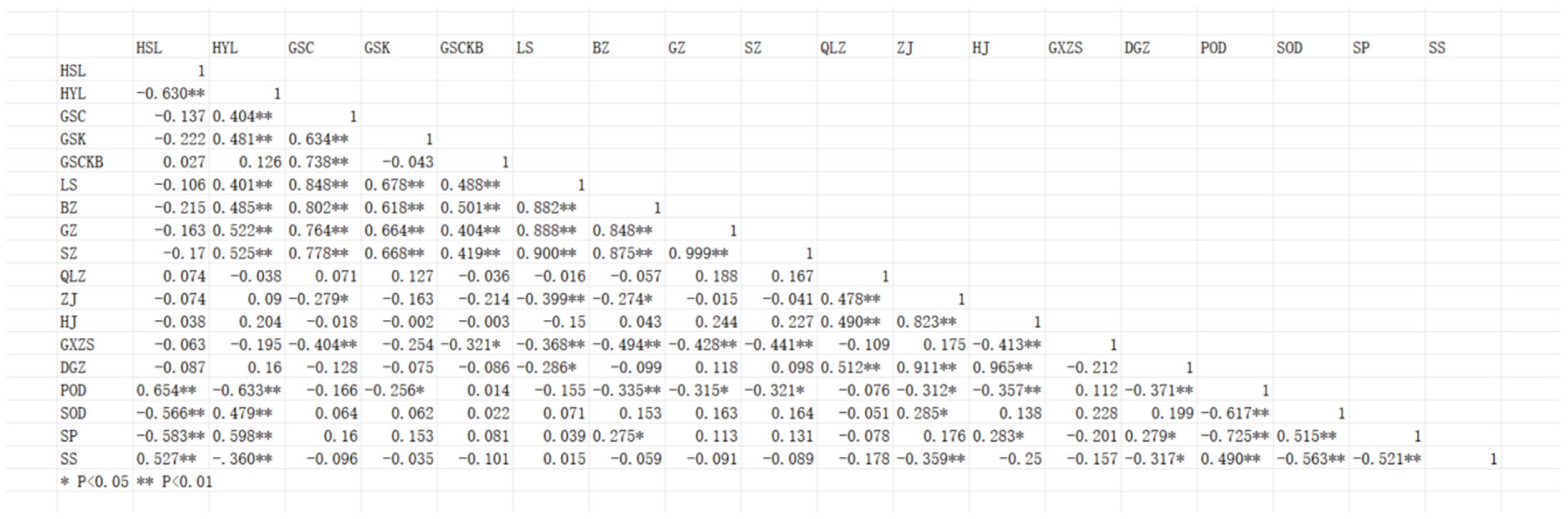
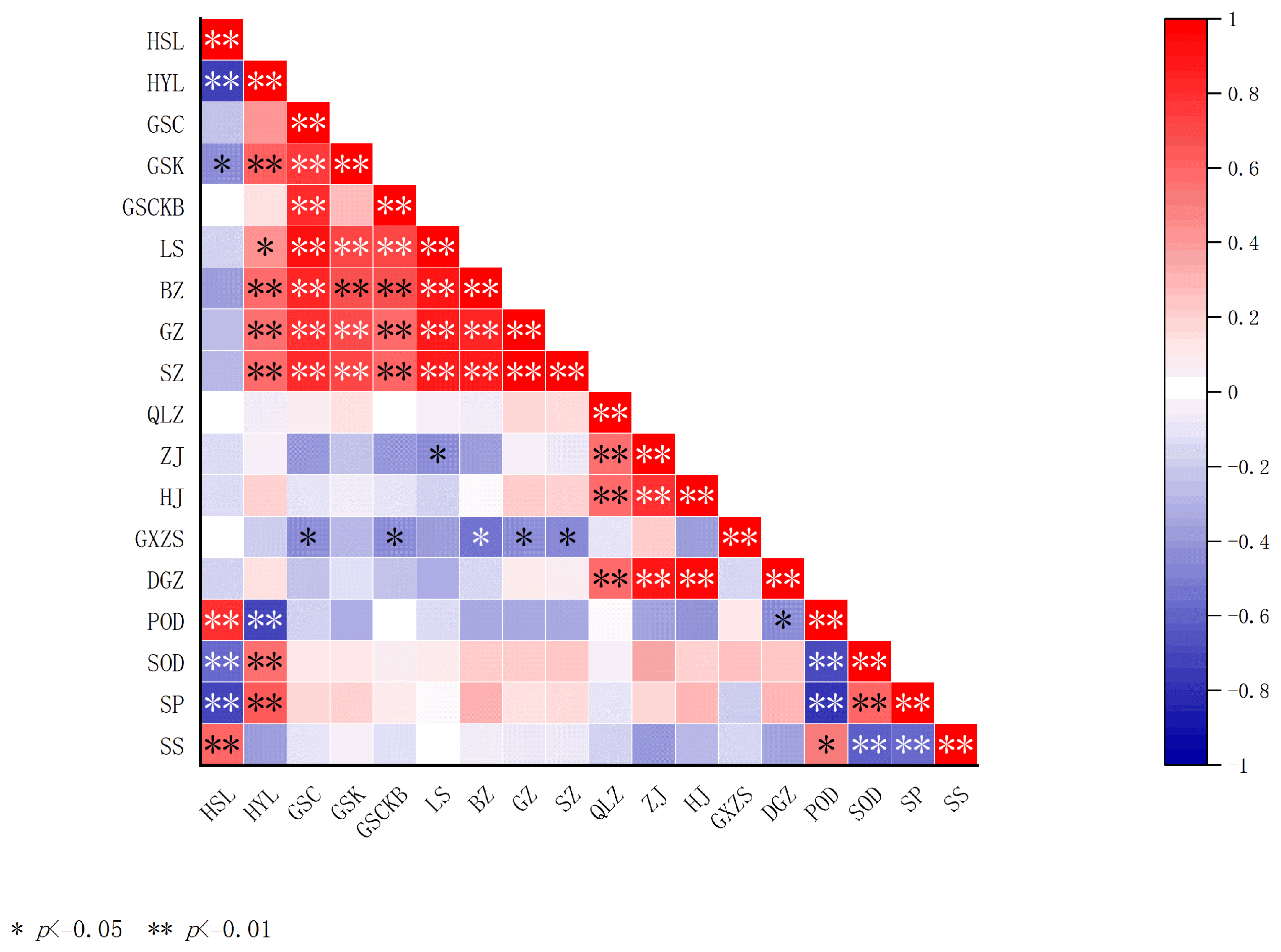
2.5. Correlation Analysis of Fruit Quality Indexes of I. polycarpa
2.6. Principal Component Analysis of I. polycarpa Fruit
3. Discussion
4. Materials and Methods
4.1. Overview of the Test Site
4.2. Test Material
4.3. Test Methods
4.3.1. Determination of Fruit Phenotypic Traits
4.3.2. Determination of Water and Oil Content of Fruit
4.3.3. Determination of Fruit Contents and Antioxidant Enzymes
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Zhu, L.; Guo, X.H. Cultivation technology of Idesia polycarpa, an excellent woody oilseed tree species. Shaanxi For. 2008, 5, 34. [Google Scholar]
- Li, X. Current situation and prospect of oil grape (Idesia polycarpa) industry in China. China For. Ind. 2019, 2, 68–73. [Google Scholar]
- Cai, Q.F.; Wang, S.; Xu, Z.H.; Song, Y.; Liu, Z. Analysis of the differences in chlorophyll fluorescence characteristics between different seed sources of Idesia polycarpa. Henan Sci. 2021, 39, 715–719. [Google Scholar]
- Tian, X.X.; Fang, X.Z.; Du, M.H. Nutritional quality and antioxidant capacity of oil from different parts of Idesia polycarpa fruit. Chin. J. Cereals Oils 2020, 35, 91–95. [Google Scholar]
- Jiang, H.Y. Preparation of Conjugated Linoleic Acid and Edible Oil Processes from Idesia polycarpa Oil. Master’s Thesis, Chengdu University, Chengdu, China, 2023. [Google Scholar]
- Jiang, H.Y.; Pang, G.Y.; Guo, X.Q.; Chen, L.M.; Kang, T.R. Response surface optimisation for the preparation of conjugated linoleic acid from Idesia polycarpa oil. China Flavour. 2022, 47, 92–98. [Google Scholar]
- Wang, Q.Y.; Du, K.F.; Jia, C.M.; Li, X.; Yao, S.; Song, H. Optimization of Linoleic Acid from Idesia polycarpa Maxim. var. vestita Diels by Response Surface Methodology. Adv. Mater. Res. 2011, 396–398, 1222–1227. [Google Scholar] [CrossRef]
- Liu, D.J.; Yan, P. Analysis of the industrialisation system and management strategy of China’s bio-industrial tree species Idesia polycarpa. For. Resour. Manag. 2015, 2, 9–12. [Google Scholar]
- Yang, F.X.; Su, Y.Q.; Li, X.H.; Zhang, Q.; Sun, R.C. Preparation of biodiesel from Idesia polycarpa var. vestita fruit oil. Int. News Fats Oils Relat. Mater. INFORM 2009, 20, 320. [Google Scholar]
- Zhu, Z.Y.; Wang, Q.; Ruan, X.; Li, Z.H.; Xue, J.; Jiang, H.; Lu, X. Oil content and fatty acid content of fruits from different geographic populations of Idesia polycarpa. For. Sci. 2010, 46, 176–180. [Google Scholar]
- Wu, L.L.; Deng, W.F.; Lu, X.Y.; Niu, C.T.; Tian, H.; Li, Z. Progress of research on the development and utilisation of Idesia polycarpa. Res. Econ. For. 2023, 41, 242–252. [Google Scholar]
- Wang, Y.M.; Wang, D.H.; Liu, Z.; Wang, L.; Jing, Z.J.; Dai, L. GC/MS analyses of oil content and fatty acids in seeds of Idesia polycarpa from different seed sources. Econ. For. Res. 2011, 29, 14–21. [Google Scholar]
- Jiang, X.B.; Li, D.W.; Gong, B.C.; Wu, K.Y. A preliminary study on seedling growth pattern of Idesia polycarpa from different origins. Henan Agric. Sci. 2012, 41, 133–136. [Google Scholar]
- Gong, B.C.; Li, D.W.; Jiang, X.B.; Wu, K.Y.; Peng, J.L.; Bai, J.J. Analysis of variations in fatty acid composition of fruits from different seed sources of Idesia polycarpa. J. Plant Physiol. 2012, 48, 505–510. [Google Scholar]
- Hernández, M.L.; Sicardo, M.D.; Belaj, A.; Martínez-Rivas, J.M. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Extraplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 653997. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhao, C.W.; Karrar, E.; Du, M.; Jin, Q.Z.; Wang, X.G. Analysis of Chemical Composition and Antioxidant Activity of Idesia polycarpa Pulp Oil from Five Regions in China. Foods 2023, 12, 1251. [Google Scholar] [CrossRef]
- Ye, Y.; Tang, X.S.; Chen, F.; Tang, L. Optimization of Phenolics Extracted from Idesia polycarpa Defatted Fruit Residue and Its Antioxidant and Depigmenting Activity In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. eCAM 2014, 2014, 931269. [Google Scholar] [CrossRef]
- Xu, Z.H.; Fang, L.S.; Liu, Z.; Li, Z.; Cai, Q.F.; Geng, X.D.; Zhai, W.J.; Wang, B.Y.; Zhan, Z.W.; Wang, Y.M. Analysis of photosynthetic characteristics of different seed sources of Idesia polycarpa. J. Henan Agric. Univ. 2021, 55, 44–51. [Google Scholar]
- Zhou, K.; Chang, Y.H.; Song, M.F.; Liu, X.Y.; He, J.; Ma, L.Z. Quality analysis and comprehensive evaluation of Idesia polycarpa from different origins. China Oil Fats 2025, 50, 129–134+152. [Google Scholar]
- Xu, S. Growth performance and stress tolerance of Sanguisorba officinalis under multi-environmental conditions. For. Sci. Technol. Intell. 2025, 57, 53–55. [Google Scholar]
- Geng, X.D.; Yue, J.F.; Liu, Y.; Tao, Y.H.; Zhou, Y.F.; Zhang, Z.Z.; Xie, H.D.; Cai, Q.F.; Liu, Z. Response of “Yuji” shantung seedling growth to drought stress. Econ. For. Res. 2025, 43, 29–39. [Google Scholar]
- Geng, X.D.; Zhou, Y.F.; Liu, Y.; Tao, Y.H.; Xie, H.D.; Yue, J.F.; Zhang, Z.Z.; Cai, Q.F.; Dai, L.; Liu, Z. Effects of flooding stress on growth and physiology of “Yuji” shantung seed. J. Henan Agric. Univ. 2025, 59, 389–399. [Google Scholar]
- Tian, K.X.; Xu, J.Y.; Dai, L.; Li, Z.; Geng, X.D.; Liu, Z.; Wang, Y.M. Physiological response of ldesia polycarpa reedlings to extreme high temperature and high temperature plus drought stress. For. Sci. 2024, 60, 84–92. [Google Scholar]
- Feng, J.; Yuan, Q.P.; Chen, X.Z.; Fang, L.S.; Zhang, T.; Liu, Z.; Wang, Y.M.; Geng, X.D.; Cai, Q.F.; Li, Z. Identification and Chemical Control of Stem Canker Pathogen of Idesia polycarpa. Plants 2025, 14, 1393. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Feng, J.; Rana, S.; Wang, S.S.; Wang, H.M.; Zhang, T.; Wang, Y.M.; Guo, G.P.; Cai, Q.F.; et al. Evaluation of Fine Root Morphology and Rhizosphere Environmental Characteristics of the Dioecious Idesia polycarpa Maxim. Forests 2024, 15, 234. [Google Scholar] [CrossRef]
- Zhang, M.X.; Rana, S.; Li, C.Z.; Zhang, X.X.; Tian, K.X.; Liu, Z.; Li, Z.; Dai, L.; Geng, X.D.; Wang, Y.M. Transcriptome analysis reveals the role of temperature in seed germination of Idesia polycarpa Maxim through the integration of phytohormones and sugar metabolism. Braz. J. Bot. 2024, 47, 963–979. [Google Scholar] [CrossRef]
- Wang, S.S.; Rana, S.; Zhang, T.; Wang, Y.M.; Liu, Z.; Cai, Q.F.; Geng, X.D.; Yuan, Q.P.; Yang, Y.; Miao, C.; et al. Influence of Varied Phosphorus Fertilizer Ratios on the Rhizosphere Soil Microbial Community in Idesia polycarpa Seedlings. Forests 2024, 15, 1686. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, Z.; Cai, Q.F.; Wang, Y.M.; Geng, X.D.; Li, S.F.; Fang, L.S.; Yao, S.Y.; Li, H.Y.; Liu, Z. Mutant Bisexual and Wild Male Flowers Were Compared by Integrated Proteome and Transcriptome Analyses to Provide Insight into the Sex Conversion of Idesia polycarpa Maxim. Forests 2023, 14, 1737. [Google Scholar] [CrossRef]
- Xie, H.D.; Xu, Z.H.; Tao, Y.H.; Zhou, Y.F.; Yue, J.F.; Zhang, Z.Z.; Geng, X.D.; Cai, Q.F.; Liu, Z. Changes in oil accumulation and nutrient content during fruit development of Idesia polycarpa. Econ. For. Res. 2025, 43, 94–103. [Google Scholar]
- Cinosi, N.; Moriconi, F.; Farinelli, D.; Marchionni, D.; Lodolini, E.M.; Rosati, A.; Famiani, F. Effects of summer pruning on the water status and physiology of olive trees and on fruit characteristics and oil quality. Sci. Hortic. 2024, 324, 112612. [Google Scholar] [CrossRef]
- Jiang, S.Q. Effects of Soybean Isoflavones on Production Performance, Meat Quality and Antioxidant Mechanism of Lingnan Yellow-Feathered Broilers. Doctoral Thesis, Zhejiang University, Hangzhou, China, 2007. [Google Scholar]
- Xiang, X.W.; Wen, L.Y.; Wang, Z.R.; Yang, G.; Mao, J.M.; An, X.F.; Kan, J.Q. A comprehensive study on physicochemical properties, bioactive compounds, and emulsified lipid digestion characteristics of Idesia polycarpa var. Vestita Diels fruits oil. Food Chem. 2023, 404, 134634. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, R.R.; Guo, G.Z.; Deng, T.; Liang, Y.S. Evaluating Phenotypic Traits of Fruits on Single Tree of Idesia polycarpa in Southwestern Guizhou. Guizhou For. Sci. Technol. 2025, 53, 65–72. [Google Scholar]
- Liu, Y.; Wang, S.; Wang, H.M.; Liu, Z. Variation patterns of fruit traits and selection of good single plants in Idesia polycarpa. J. For. Environ. 2022, 42, 536–543. [Google Scholar]
- Cai, Q.S. Plant Physiology Experiment; China Agricultural University Press: Beijing, China, 2013; pp. 74–78. [Google Scholar]
| Seed Source | Developmental Stage | Cluster Length (cm) | Cluster Width (%) | Width/Length Ratio (%) | Fresh Weight per Cluster (g) | Fresh Weight of Fruit Stalks per Cluster (g) | Fruit Weight per Cluster (g) |
|---|---|---|---|---|---|---|---|
| SH | M1 | 21.73 ± 1.03Db | 8.10 ± 0.40Ba | 2.69 ± 0.11Ba | 22.46 ± 6.22Ca | 2.49 ± 0.71Cb | 19.97 ± 5.53Ca |
| M2 | 27.30 ± 1.85Ba | 9.87 ± 1.40Aa | 2.83 ± 0.24ABa | 36.47 ± 7.46Ba | 4.36 ± 1.03Bab | 32.11 ± 6.47Ba | |
| M3 | 26.83 ± 0.95BCa | 9.20 ± 0.38Ba | 2.93 ± 0.22ABa | 49.35 ± 11.06Ca | 5.35 ± 0.65BCab | 44.01 ± 10.41Ca | |
| M4 | 29.07 ± 1.65Ba | 10.20 ± 0.29ABa | 2.85 ± 0.08Ba | 49.82 ± 8.31Ba | 5.93 ± 0.94Ba | 43.89 ± 7.56Ba | |
| SG1 | M1 | 23.56 ± 1.20CDab | 8.37 ± 0.15Ba | 2.82 ± 0.17Ba | 30.50 ± 0.87BCa | 3.47 ± 0.20Ca | 27.03 ± 0.89BCa |
| M2 | 21.33 ± 2.14Cb | 9.03 ± 0.78Aa | 2.36 ± 0.09Bb | 32.19 ± 5.52Ba | 2.96 ± 0.64Ba | 29.23 ± 4.89Ba | |
| M3 | 21.77 ± 1.91Cab | 8.47 ± 0.52Ba | 2.57 ± 0.13Bab | 42.85 ± 15.31Ca | 4.31 ± 1.70Ca | 38.54 ± 13.61Ca | |
| M4 | 27.20 ± 1.21Ba | 10.00 ± 0.23ABa | 2.72 ± 0.06Bab | 55.82 ± 3.85Ba | 6.01 ± 0.57Ba | 49.81 ± 3.29Ba | |
| GG | M1 | 34.93 ± 0.49Ab | 9.70 ± 0.10Ab | 3.60 ± 0.06Aab | 63.33 ± 10.08Ac | 8.00 ± 1.47Ab | 55.33 ± 8.63Ac |
| M2 | 37.17 ± 1.14Ab | 11.43 ± 0.35Aa | 3.25 ± 0.13Ab | 76.73 ± 2.45Abc | 7.12 ± 1.55ABb | 69.61 ± 2.02Abc | |
| M3 | 39.20 ± 2.69Aab | 11.40 ± 1.04Aa | 3.44 ± 0.10Ab | 100.05 ± 13.70ABb | 9.78 ± 1.75Ab | 90.26 ± 11.96ABb | |
| M4 | 43.87 ± 0.88Aa | 11.27 ± 0.92Aa | 3.90 ± 0.11Aa | 155.56 ± 7.70Aa | 15.97 ± 0.66Aa | 139.60 ± 7.23Aa | |
| HX | M1 | 28.13 ± 2.28BCa | 8.17 ± 0.50Ba | 3.44 ± 0.09Aa | 53.43 ± 12.48ABb | 4.81 ± 1.05BCa | 48.62 ± 11.45ABb |
| M2 | 27.43 ± 2.45Ba | 10.00 ± 0.95Aa | 2.75 ± 0.04ABa | 85.58 ± 21.92Aab | 6.80 ± 1.86ABa | 78.79 ± 20.09Aab | |
| M3 | 32.10 ± 1.31Ba | 10.33 ± 1.01ABa | 3.18 ± 0.39ABa | 110.01 ± 14.23Aa | 7.55 ± 0.57ABCa | 102.46 ± 13.75Aa | |
| M4 | 29.17 ± 2.06Ba | 9.07 ± 0.43Ba | 3.23 ± 0.28ABa | 56.77 ± 7.10Bb | 4.84 ± 0.31Ba | 51.94 ± 6.84Bb | |
| SG2 | M1 | 31.07 ± 1.76ABa | 8.33 ± 0.43Ba | 3.73 ± 0.03Aa | 56.56 ± 4.52ABa | 7.82 ± 0.88ABa | 48.74 ± 3.65ABa |
| M2 | 30.47 ± 0.23Ba | 10.13 ± 0.78Aa | 3.05 ± 0.25Aab | 63.91 ± 7.78ABa | 8.81 ± 0.95Aa | 55.10 ± 6.94ABa | |
| M3 | 26.10 ± 1.81BCa | 9.90 ± 0.15ABa | 2.64 ± 0.22Bb | 65.36 ± 4.13BCa | 8.58 ± 0.75ABa | 56.78 ± 3.66BCa | |
| M4 | 30.00 ± 2.67Ba | 9.70 ± 0.72ABa | 3.17 ± 0.51ABab | 48.46 ± 7.15Ba | 7.84 ± 1.68Ba | 40.62 ± 5.53Ba |
| Seed Source | Developmental Stage | Number of Fruits per Cluster | Seed Weight per Thousand Grains (g) | Fruit Width (mm) | Fruit Length (mm) | Fruit Shape Index | Fruit Mass (g) |
|---|---|---|---|---|---|---|---|
| SH | M1 | 88 ± 23Ca | 0.30 ± 0.03Bab | 7.28 ± 0.14Ca | 7.95 ± 0.03Ba | 1.09 ± 0.02Aa | 0.25 ± 0.03Ba |
| M2 | 141 ± 22BCa | 0.29 ± 0.00Bb | 7.23 ± 0.28Ba | 8.01 ± 0.15Ba | 1.11 ± 0.03Aa | 0.23 ± 0.02Ba | |
| M3 | 149 ± 29BCa | 0.34 ± 0.02Aab | 7.93 ± 0.42Ca | 8.51 ± 0.21Ba | 1.08 ± 0.04Aa | 0.30 ± 0.03Ba | |
| M4 | 156 ± 21Ba | 0.36 ± 0.00Ba | 7.89 ± 0.39Ca | 8.38 ± 0.33Ba | 1.06 ± 0.01Aa | 0.30 ± 0.04Ba | |
| SG1 | M1 | 75 ± 3Ca | 0.40 ± 0.02Ab | 8.64 ± 0.18Ab | 8.67 ± 0.06Ab | 1.00 ± 0.01Ba | 0.38 ± 0.01Ab |
| M2 | 71 ± 10Ca | 0.43 ± 0.02Aab | 9.18 ± 0.37Aab | 9.17 ± 0.30Aab | 1.00 ± 0.01Ba | 0.44 ± 0.05Aab | |
| M3 | 82 ± 25Ca | 0.37 ± 0.02Ab | 9.35 ± 0.34ABab | 9.08 ± 0.27ABab | 0.97 ± 0.01Ba | 0.45 ± 0.04Aab | |
| M4 | 96 ± 5.5Ba | 0.48 ± 0.00Aa | 9.82 ± 0.03ABa | 9.54 ± 0.00Aa | 0.97 ± 0.00Ba | 0.51 ± 0.00Aa | |
| GG | M1 | 228 ± 33Ab | 0.36 ± 0.00ABb | 7.84 ± 0.19Bb | 7.25 ± 0.11Cc | 0.93 ± 0.02CDa | 0.24 ± 0.02Bb |
| M2 | 263 ± 15Ab | 0.40 ± 0.00Aa | 7.93 ± 0.17Bb | 7.34 ± 0.11Cc | 0.93 ± 0.01Ca | 0.26 ± 0.02Bb | |
| M3 | 319 ± 41Ab | 0.37 ± 0.01Ab | 8.14 ± 0.08Cb | 7.71 ± 0.05Cb | 0.95 ± 0.00Ba | 0.29 ± 0.01Bb | |
| M4 | 449 ± 27Aa | 0.38 ± 0.01Bb | 8.64 ± 0.08Ca | 8.17 ± 0.12Ba | 0.94 ± 0.00Ca | 0.35 ± 0.02Ba | |
| HX | M1 | 139 ± 34BCa | 0.38 ± 0.02Aa | 9.11 ± 0.23Aa | 8.76 ± 0.14Ab | 0.96 ± 0.01BCab | 0.38 ± 0.03Ab |
| M2 | 199 ± 48ABa | 0.39 ± 0.01Aa | 9.04 ± 0.20Aa | 8.64 ± 0.12Ab | 0.96 ± 0.01BCab | 0.39 ± 0.02Ab | |
| M3 | 205 ± 33Ba | 0.39 ± 0.02Aa | 9.97 ± 0.11Aa | 9.38 ± 0.12Aab | 0.94 ± 0.00Bb | 0.51 ± 0.02Aab | |
| M4 | 115 ± 26Ba | 0.37 ± 0.04Ba | 9.98 ± 0.52Aa | 9.68 ± 0.45Aa | 0.97 ± 0.01Ba | 0.53 ± 0.07Aa | |
| SG2 | M1 | 208 ± 18ABa | 0.32 ± 0.01Ba | 7.89 ± 0.06Bb | 7.18 ± 0.11Cb | 0.91 ± 0.01Da | 0.24 ± 0.01Bb |
| M2 | 198 ± 27ABa | 0.33 ± 0.02Ba | 8.03 ± 0.14Bab | 7.41 ± 0.07Cab | 0.92 ± 0.01Ca | 0.27 ± 0.01Bb | |
| M3 | 185 ± 24Ba | 0.24 ± 0.03Bb | 8.50 ± 0.29BCab | 7.69 ± 0.27Cab | 0.90 ± 0.01Ba | 0.31 ± 0.04Bab | |
| M4 | 118 ± 9Bb | 0.34 ± 0.02Ba | 8.83 ± 0.35BCa | 7.99 ± 0.29Ba | 0.90 ± 0.00Da | 0.38 ± 0.03Ba |
| Seed Source | Developmental Stage | Water Content (%) | Oil Content (%) | POD (U/g) | SOD (U/g) | Soluble Protein (mg·g−1) | Soluble Protein (mg·g−1) |
|---|---|---|---|---|---|---|---|
| SH | M1 | 62.7 ± 3.5Aab | 9.1 ± 0.1Bc | 1605.81 ± 337.60ABa | 238.50 ± 28.00ABb | 5.48 ± 0.13Ba | 12.16 ± 1.71Ad |
| M2 | 63.3 ± 1.2Aa | 19.4 ± 1.1BCb | 1684.40 ± 82.83Aa | 276.88 ± 5.65Ab | 5.78 ± 0.14Ba | 15.86 ± 0.433Ac | |
| M3 | 61.3 ± 0.3Aab | 24.7 ± 1.1Bab | 392.95 ± 42.56Ab | 291.99 ± 13.25Aab | 6.67 ± 0.70Aa | 21.61 ± 0.64Bb | |
| M4 | 56.7 ± 0.7Bb | 28.0 ± 3.7Ba | 214.23 ± 5.70Ab | 337.37 ± 6.50Aa | 2.72 ± 0.13BCb | 26.60 ± 0.04BCa | |
| SG1 | M1 | 68.6 ± 0.1Aa | 9.3 ± 0.5Bc | 2267.21 ± 69.84Aa | 224.44 ± 4.64Bb | 7.57 ± 0.37Ad | 16.57 ± 2.50Ab |
| M2 | 62.2 ± 1.0ABbc | 16.0 ± 0.8Cb | 1300.60 ± 273.35Ab | 245.31 ± 15.90Bb | 6.47 ± 0.10Bc | 15.97 ± 1.11Ab | |
| M3 | 62.8 ± 1.5Ab | 19.3 ± 0.8Cab | 290.46 ± 14.30Ac | 253.29 ± 20.16Aab | 7.02 ± 0.17Ab | 20.33 ± 0.25BCab | |
| M4 | 59.2 ± 0.8Ac | 19.8 ± 1.8Ca | 119.49 ± 11.11Bc | 291.12 ± 6.42BCa | 3.84 ± 0.17Aa | 21.49 ± 0.56Da | |
| GG | M1 | 66.2 ± 1.6Aa | 15.5 ± 0.7Ad | 1981.07 ± 47.30AaB | 223.42 ± 5.05Bc | 7.53 ± 0.18Aa | 14.49 ± 0.69Ac |
| M2 | 63.2 ± 0.7Aab | 19.6 ± 0.7ABcC | 1646.45 ± 613.70Aa | 175.13 ± 8.24Dd | 6.97 ± 0.41Ba | 17.23 ± 0.96Abc | |
| M3 | 61.6 ± 0.2Ab | 21.7 ± 0.4Cb | 325.64 ± 95.32Ab | 289.93 ± 6.86Ab | 7.09 ± 0.23AAa | 18.13 ± 1.27Cb | |
| M4 | 58.3 ± 0.8ABc | 28.7 ± 0.6Ba | 125.93 ± 4.28Bb | 315.53 ± 5.96ABa | 3.54 ± 0.02Ab | 24.91 ± 0.68Ca | |
| HX | M1 | 66.2 ± 0.4Aa | 15.6 ± 0.1Ac | 1820.46 ± 391.1ABa | 279.68 ± 1.17Aa | 5.86 ± 0.80Ba | 17.13 ± 1.03Ad |
| M2 | 63.3 ± 0.3Ab | 23.9 ± 1.9Aab | 1591.15 ± 256.69Aa | 222.48 ± 7.97BCb | 6.07 ± 0.54Ba | 11.22 ± 0.06Bc | |
| M3 | 61.7 ± 0.2Ac | 25.6 ± 0.9Ba | 276.27 ± 7.02Ab | 287.87 ± 12.11Aa | 6.28 ± 0.29Aa | 19.79 ± 0.33BCb | |
| M4 | 59.3 ± 0.7Ad | 20.5 ± 0.9Cb | 59.26 ± 8.55Cb | 278.18 ± 7.80Ca | 2.40 ± 0.22Cb | 27.50 ± 0.09Ba | |
| SG2 | M1 | 63.5 ± 0.6Aa | 15.1 ± 0.7Ac | 1342.72 ± 177.45Ba | 242.55 ± 6.32ABab | 5.93 ± 0.10Bc | 16.72 ± 0.28Ac |
| M2 | 60.6 ± 1.0Bb | 20.6 ± 1.1ABb | 1433.07 ± 333.15Aa | 211.56 ± 4.60Cb | 9.34 ± 0.65Aa | 18.51 ± 1.21Ac | |
| M3 | 61.2 ± 0.6Aab | 24.0 ± 1.3Bab | 278.85 ± 16.65Ab | 276.53 ± 8.07Aa | 7.40 ± 0.18Ab | 25.79 ± 1.15Ab | |
| M4 | 58.7 ± 0.9ABb | 27.3 ± 1.3Ba | 123.13 ± 33.82Bb | 280.24 ± 19.40Ca | 2.96 ± 0.21Bd | 31.88 ± 0.82Aa |
| Principal Components | Eigenvalues | Contribution Rate (%) | Cumulative Contribution Rate (%) |
|---|---|---|---|
| F1 | 6.258 | 52.1 | 52.15 |
| F2 | 2.921 | 24.3 | 76.49 |
| F3 | 0.987 | 8.2 | 84.71 |
| F4 | 0.601 | 5.0 | 89.72 |
| F5 | 0.455 | 3.8 | 93.51 |
| F6 | 0.303 | 2.5 | 96.03 |
| F7 | 0.191 | 1.6 | 97.63 |
| F8 | 0.121 | 1.0 | 98.63 |
| F9 | 0.081 | 0.7 | 99.31 |
| F10 | 0.064 | 0.5 | 99.85 |
| F11 | 0.018 | 0.2 | 100 |
| F12 | 5.524 × 10−5 | 0 | 100 |
| Indicator | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Oil content | 0.47 | 0.813 | 0.04 | 0.098 |
| Cluster length | 0.924 | 0.048 | −0.163 | −0.112 |
| Cluster width | 0.787 | 0.317 | 0.065 | 0.311 |
| Number of fruits per cluster | 0.975 | −0.005 | −0.066 | 0.002 |
| Fresh weight of fruit stalks per cluster | 0.878 | 0.239 | −0.284 | −0.038 |
| Fruit weight per cluster | 0.931 | 0.172 | −0.106 | −0.032 |
| Cluster weight | 0.941 | 0.181 | −0.124 | −0.036 |
| Fruit shape index | −0.361 | −0.06 | 0.904 | −0.116 |
| POD | −0.161 | −0.909 | 0.036 | 0.147 |
| SOD | 0.141 | 0.697 | 0.366 | −0.452 |
| Soluble protein | 0.004 | 0.869 | −0.267 | −0.268 |
| Soluble sugar | −0.007 | −0.495 | −0.122 | 0.779 |
| Seed Source | F1 | F2 | F3 | F4 | F (Full) | F_abridged | Rank (Full) |
|---|---|---|---|---|---|---|---|
| SH | 1.006 | 0.857 | 0.842 | −0.009 | 0.801 | 0.732 | 4 |
| SG1 | 0.701 | 0.419 | 0.335 | 0.223 | 0.496 | 0.467 | 5 |
| GG | 3.827 | 1.110 | −0.224 | 0.354 | 2.247 | 2.264 | 1 |
| HX | 2.090 | 1.005 | 0.113 | 0.088 | 1.343 | 1.333 | 3 |
| SG2 | 2.115 | 1.124 | −0.218 | 0.246 | 1.358 | 1.375 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Miao, C.; Yuan, Q.; Zhong, W.; Hu, Z.; Chen, C.; Liu, Z.; Wang, Y.; Geng, X.; Cai, Q.; et al. Changes in the Quality of Idesia polycarpa Maxim Fruits from Different Ecotypes During the Growth Process. Plants 2025, 14, 2324. https://doi.org/10.3390/plants14152324
Yang Y, Miao C, Yuan Q, Zhong W, Hu Z, Chen C, Liu Z, Wang Y, Geng X, Cai Q, et al. Changes in the Quality of Idesia polycarpa Maxim Fruits from Different Ecotypes During the Growth Process. Plants. 2025; 14(15):2324. https://doi.org/10.3390/plants14152324
Chicago/Turabian StyleYang, Yi, Chao Miao, Qiupeng Yuan, Wenwen Zhong, Zuwei Hu, Chen Chen, Zhen Liu, Yanmei Wang, Xiaodong Geng, Qifei Cai, and et al. 2025. "Changes in the Quality of Idesia polycarpa Maxim Fruits from Different Ecotypes During the Growth Process" Plants 14, no. 15: 2324. https://doi.org/10.3390/plants14152324
APA StyleYang, Y., Miao, C., Yuan, Q., Zhong, W., Hu, Z., Chen, C., Liu, Z., Wang, Y., Geng, X., Cai, Q., Dai, L., Wang, J., Ren, Y., Liu, F., Lu, H., Zhong, T., & Li, Z. (2025). Changes in the Quality of Idesia polycarpa Maxim Fruits from Different Ecotypes During the Growth Process. Plants, 14(15), 2324. https://doi.org/10.3390/plants14152324







