From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals
Abstract
1. Introduction
2. Research Gap
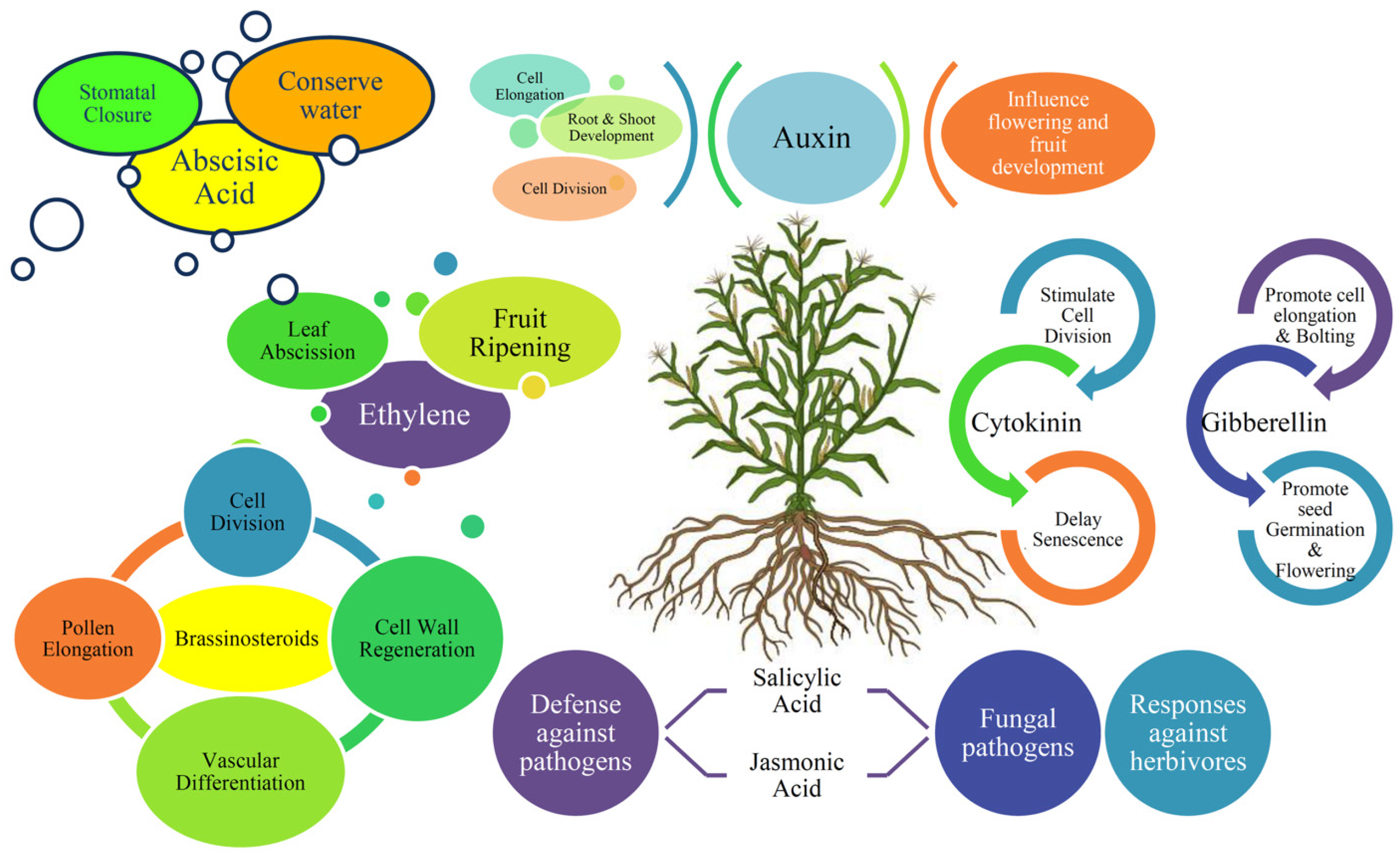
3. Phytohormones in Abiotic Stress Responses in Plants
3.1. Abscisic Acid (ABA)
3.2. Salicylic Acid (SA)
3.3. Jasmonic Acid (JA)
3.4. Ethylene
3.5. Auxins
3.6. Cytokinins
3.7. Gibberellins (GAs)
3.8. Brassinosteroids (BRs)
4. Molecular Mechanisms of Hormonal Crosstalk Under Abiotic Stress
5. Crosstalk Dynamics: Specific Hormonal Interactions
5.1. ABA–Ethylene Crosstalk
5.2. ABA–JA and JA–SA Interactions
5.3. ABA–Auxin Crosstalk
5.4. ABA–Cytokinin Crosstalk
5.5. ABA–BR and BR–GA Interactions
6. Applying Hormonal Crosstalk Research, Specifically in Relation to SDGs
7. Conclusions
8. Future Prospects
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Naik, P.M.; Al–Khayri, J.M. Abiotic and Biotic Elicitors–Role in Secondary Metabolites Production Through In Vitro Culture of Medicinal Plants; IntechOpen: London, UK, 2016. [Google Scholar]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Mangal, V.; Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Sood, S.; Kumar, D.; Bharadwaj, V.; Singh, B.; Singh, R.K.; Aftab, T. Molecular insights into the role of reactive oxygen, nitrogen and sulphur species in conferring salinity stress tolerance in plants. J. Plant Growth Regul. 2023, 42, 554–574. [Google Scholar] [CrossRef]
- Akın, B. Tissue culture techniques of medicinal and aromatic plants: History, cultivation and micropropagation. J. Sci. Rep.-A 2020, 45, 253–266. [Google Scholar]
- Lile, R.; Ocnean, M.; Balan, I.M. Challenges for Zero Hunger (SDG 2): Links with Other SDGs. In Transitioning to Zero Hunger; Kiba, D.I., Ed.; MDPI: Basel, Switzerland, 2023; Volume 2, pp. 9–66. [Google Scholar]
- Salasan, C.; Balan, I.M. The environmentally acceptable damage and the future of the EU’s rural development policy. In Economics and Engineering of Unpredictable Events; Routledge: Oxfordshire, UK, 2022; pp. 49–56. [Google Scholar]
- Balan, I.M.; Gherman, E.D.; Brad, I.; Gherman, R.; Horablaga, A.; Trasca, T.I. Metabolic food waste as food insecurity factor—Causes and preventions. Foods 2022, 11, 2179. [Google Scholar] [CrossRef]
- Dorgo, G.; Honti, G.; Abonyi, J. Automated analysis of the interactions between sustainable development goals extracted from models and texts of sustainability science. Chem. Eng. Trans. 2018, 70, 781–786. [Google Scholar]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Physiology, Biochemistry and Molecular Biology; Springer: Berlin/Heidelberg, Germany, 1995; Volume 1, pp. 1–12. [Google Scholar]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defence strategies for sustainable agriculture. Physiol. Plant 2024, 176, e14307. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. J. Plant Growth Regul. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Jangra, A.; Chaturvedi, S.; Kumar, N.; Singh, H.; Sharma, V.; Thakur, M.; Tiwari, S.; Chhokar, V. Polyamines: The gleam of next-generation plant growth regulators for growth, development, stress mitigation, and hormonal crosstalk in plants—A systematic review. J. Plant Growth Regul. 2023, 42, 5167–5191. [Google Scholar] [CrossRef]
- Talabi, A.O.; Vikram, P.; Thushar, S.; Rahman, H.; Ahmadzai, H.; Nhamo, N.; Shahid, M.; Singh, R.K. Orphan crops: A best fit for dietary enrichment and diversification in highly deteriorated marginal environments. Front. Plant Sci. 2022, 13, 839704. [Google Scholar] [CrossRef]
- Pandey, N.; Iqbal, Z.; Pandey, B.K.; Sawant, S.V. Phytohormones and drought stress: Plant responses to transcriptional regulation. In Mechanism of Plant Hormone Signaling Under Stress, II; Pandey, G.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 477–504. [Google Scholar] [CrossRef]
- Gupta, N.K.; Shavrukov, Y.; Singhal, R.; Borisjuk, N. Multiple Abiotic Stress Tolerances in Higher Plants: Addressing the Growing Challenges; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Farooq, M.; Usman, M.; Nadeem, F.; ur Rehman, H.; Wahid, A.; Basra, S.M.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Das, S.; Shil, S.; Rime, J.; Alice, A.K.; Yumkhaibam, T.; Mounika, V.; Singh, A.P.; Kundu, M.; Lalhmangaihzuali, H.; Hazarika, T.K.; et al. Phytohormonal signaling in plant resilience: Advances and strategies for enhancing abiotic stress tolerance. Plant Growth Regul. 2025, 105, 329–360. [Google Scholar] [CrossRef]
- Gul, A.; Nawaz, S. Role of phytohormones in biotic vs abiotic stresses with respect to PGPR and autophagy. In Phytohormones and Stress Responsive Secondary Metabolites; Academic Press: Cambridge, UK, 2023; pp. 41–62. [Google Scholar]
- Khan, A.; Kanwal, F.; Ullah, S.; Fahad, M.; Tariq, L.; Altaf, M.T.; Riaz, A.; Zhang, G. Plant secondary metabolites—Central regulators against abiotic and biotic stresses. Metabolites 2025, 15, 276. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef]
- Pontin, M.; Murcia, G.; Bottini, R.; Fontana, A.; Bolcato, L.; Piccoli, P. Nitric oxide and abscisic acid regulate osmoprotective and antioxidative mechanisms related to water stress tolerance of grapevines. Aust. J. Grape Wine Res. 2021, 27, 392–405. [Google Scholar] [CrossRef]
- Cabej, N.R. Plant epigenetics. In Epigenetic Principles of Evolution, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 733–781. [Google Scholar]
- Wu, W.; Cao, S.-F.; Shi, L.-Y.; Chen, W.; Yin, X.-R.; Yang, Z.-F. Abscisic acid biosynthesis, metabolism and signaling in ripening fruit. Front. Plant Sci. 2023, 14, 1279031. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Arbona, V.; Gomez-Cadenas, A.; Rodriguez-Concepcion, M.; Rodriguez-Villalon, A. A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in Arabidopsis. PLoS ONE 2014, 9, e90765. [Google Scholar] [CrossRef]
- Chen, Q.; Ya, H.; Feng, Y.; Jiao, Z. Expression of the key genes involved in ABA biosynthesis in rice implanted by ion beam. Appl. Biochem. Biotechnol. 2014, 173, 239–247. [Google Scholar] [CrossRef]
- Krochko, J.E.; Abrams, G.D.; Loewen, M.K.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol. 1998, 118, 849–860. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.-K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Long, H.; Zheng, Z.; Zhang, Y.; Xing, P.; Wan, X.; Zheng, Y.; Li, L.; Liu, J.-H. An abscisic acid (ABA) homeostasis regulated by its production, catabolism and transport in peanut leaves in response to drought stress. PLoS ONE 2019, 14, e0213963. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Y.-M.; Sun, Y.-F.; Zhang, M.; Chen, X.-J.; Huang, Y.-Y. The regulatory role of abscisic acid on cadmium uptake, accumulation and translocation in plants. Front. Plant Sci. 2022, 13, 953717. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Chen, L.; Zhao, G.; Li, S.; Zhou, H.; Dai, Y.; Sun, N.; Xie, Y.; Gao, J.; et al. Genome-wide identification and expression analysis of the NCED family in cotton (Gossypium hirsutum L.). PLoS ONE 2021, 16, e0246021. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.; Wang, D.; Ahmad, P.; Qin, M.; Li, R.; Ali, B.; Sonah, H.; Deshmukh, R.; Yadav, K.K.; et al. Silicon improves salt resistance by enhancing ABA biosynthesis and aquaporin expression in Nicotiana tabacum L. Plant Physiol. Biochem. 2024, 215, 108977. [Google Scholar]
- Choudhary, A.; Nepovimova, E.; Rajput, V.D.; Malik, T.; Choudhary, M.; Bhardwaj, N.; Peter, L.; Puri, S.; Kimta, N. Mechanistic understanding of GABA and trehalose in modulating plant response to drought stress. Plant Stress 2025, 16, 100838. [Google Scholar] [CrossRef]
- Martignago, D.; da Silveira Falavigna, V.; Coupland, G.; Conti, L.; Melzer, R. Dancing molecules: Group A bZIPs and PEBPs at the heart of plant development and stress responses. J. Exp. Bot. 2025, 76, 2081–2095. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Palta, J.A.; Liu, H.-Y.; Chen, Z.; Li, F.-M. Exogenous ABA induces osmotic adjustment, improves leaf water relations and water use efficiency, but not yield in soybean under water stress. Agronomy 2019, 9, 395. [Google Scholar] [CrossRef]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef]
- Kuromori, T.; Fujita, M.; Urano, K.; Tanabata, T.; Sugimoto, E.; Shinozaki, K. Overexpression of AtABCG25 enhances the abscisic acid signal in guard cells and improves plant water use efficiency. Plant Sci. 2016, 251, 75–81. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 597642. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Sun, L.; Sun, M.; Xu, H.; Zhou, X. Plant hormones and phenolic acids response to UV-B stress in Rhododendron chrysanthum pall. Biol. Direct 2024, 19, 40. [Google Scholar] [CrossRef]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.P.; Rodríguez, D.; Nicolás, C.; Rodríguez, P.L.; Nicolás, G.; Lorenzo, O. Negative regulation of abscisic acid signaling by the Fagus sylvatica FsPP2C1 plays a role in seed dormancy regulation and promotion of seed germination. Plant Physiol. 2003, 133, 135–144. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Fujii, H.; Verslues, P.E.; Zhu, J.-K. Identification of two protein kinases required for abscisic acid regulation of seed germination, root growth, and gene expression in Arabidopsis. Plant Cell 2007, 19, 485–494. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, S.C. Arabidopsis SnRK2. 3/SRK2I plays a positive role in seed germination under cold stress conditions. Environ. Exp. Bot. 2023, 212, 105399. [Google Scholar] [CrossRef]
- Mishra, S.; Roychowdhury, R.; Ray, S.; Hada, A.; Kumar, A.; Sarker, U.; Aftab, T.; Das, R. Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 2024, 11, 100427. [Google Scholar] [CrossRef]
- Song, W.; Shao, H.; Zheng, A.; Zhao, L.; Xu, Y. Advances in roles of salicylic acid in plant tolerance responses to biotic and abiotic stresses. Plants 2023, 12, 3475. [Google Scholar] [CrossRef]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M.T. Salicylic acid improves growth and physiological attributes and salt tolerance differentially in two bread wheat cultivars. Plants 2022, 11, 1853. [Google Scholar] [CrossRef]
- Urmi, T.A.; Islam, M.M.; Zumur, K.N.; Abedin, M.A.; Haque, M.M.; Siddiqui, M.H.; Murata, Y.; Hoque, M.A. Combined effect of salicylic acid and proline mitigates drought stress in rice (Oryza sativa L.) through the modulation of physiological attributes and antioxidant enzymes. Antioxidants 2023, 12, 1438. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef]
- Göre, M. Mitigation of salt stress in Camelina sativa by epibrassinolide and salicylic acid treatments. Sci. Rep. 2025, 15, 7965. [Google Scholar] [CrossRef]
- Bozovic, V.; Svensson, J.; Schmitt, J.; Kohn, C. Dehydrins (LTI29, LTI30, COR47) from Arabidopsis thaliana expressed in Escherichia coli protect thylakoid membrane during freezing. J. Serbian Chem. Soc. 2013, 78, 1149–1160. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef]
- Acevedo, A.F.G.; da Silva Gomes, J.W.; Avilez, A.A.; Sarria, S.D.; Broetto, F.; Vieites, R.L.; de Souza Guimarães, M.L.C. Foliar salicylic acid application to mitigate the effect of water deficiency on potato (Solanum tuberosum L.). Plant Stress 2023, 7, 100135. [Google Scholar] [CrossRef]
- Song, W.; Zhang, P.; Zhang, H.; Xue, Y.a.; Zhang, Q.; Ning, M.; Zhao, X.; Cai, W.; Liu, X.; Zhang, X.; et al. A low concentration of exogenous salicylic acid enhances cold tolerance in Hami melons (Cucumis melo var. saccharinus) by modulating salicylic acid-response CmGST genes. Postharvest Biol. Technol. 2022, 193, 112034. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Liao, W. Calmodulin-Binding Transcription Factors: Roles in Plant Response to Abiotic Stresses. Plants 2025, 14, 532. [Google Scholar] [CrossRef]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis–structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- De Ollas, C.; Arbona, V.; Gómez-Cadenas, A.; Dodd, I.C. Attenuated accumulation of jasmonates modifies stomatal responses to water deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef]
- Seo, J.S.; Joo, J.; Kim, M.J.; Kim, Y.K.; Nahm, B.H.; Song, S.I.; Cheong, J.J.; Lee, J.S.; Kim, J.K.; Choi, Y.D. OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J. 2011, 65, 907–921. [Google Scholar] [CrossRef]
- Xing, Q.; Liao, J.; Cao, S.; Li, M.; Lv, T.; Qi, H. CmLOX10 positively regulates drought tolerance through jasmonic acid-mediated stomatal closure in oriental melon (Cucumis melo var. makuwa Makino). Sci. Rep. 2020, 10, 17452. [Google Scholar] [CrossRef]
- Masri, R.; Kiss, E. The role of NAC genes in response to biotic stresses in plants. Physiol. Mol. Plant Pathol. 2023, 126, 102034. [Google Scholar] [CrossRef]
- Geng, J.; Liu, J.-H. The transcription factor CsbHLH18 of sweet orange functions in modulation of cold tolerance and homeostasis of reactive oxygen species by regulating the antioxidant gene. J. Exp. Bot. 2018, 69, 2677–2692. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Liu, Y.; Wang, Z.; Zhao, J. JAZ proteins: Key regulators of plant growth and stress response. Crop J. 2024, 12, 1505–1516. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Liu, W.; Wang, N.; Qu, C.; Jiang, S.; Fang, H.; Zhang, Z.; Chen, X. Methyl jasmonate enhances apple’cold tolerance through the JAZ–MYC2 pathway. Plant Cell Tissue Organ Cult. 2019, 136, 75–84. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; You, C.X.; Hao, Y.J. Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytol. 2021, 229, 2707–2729. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Chen, Y.; Zhu, S.; Chen, M.; Lan, X.; Chen, G.; Liao, Z. Cold stress improves the production of artemisinin depending on the increase in endogenous jasmonate. Biotechnol. Appl. Biochem. 2017, 64, 305–314. [Google Scholar] [CrossRef]
- Ming, R.; Zhang, Y.; Wang, Y.; Khan, M.; Dahro, B.; Liu, J.-H. The JA-responsive MYC2-BADH-like transcriptional regulatory module in Poncirus trifoliata contributes to cold tolerance by modulation of glycine betaine biosynthesis. New Phytol. 2021, 229, 2730–2750. [Google Scholar] [CrossRef]
- Seo, J.; Yi, G.; Lee, J.G.; Choi, J.H.; Lee, E.J. Seed browning in pepper (Capsicum annuum L.) fruit during cold storage is inhibited by methyl jasmonate or induced by methyl salicylate. Postharvest Biol. Technol. 2020, 166, 111210. [Google Scholar] [CrossRef]
- Kuo, H.-Y.; Kang, F.-C.; Wang, Y.-Y. Glucosinolate Transporter1 involves in salt-induced jasmonate signaling and alleviates the repression of lateral root growth by salt in Arabidopsis. Plant Sci. 2020, 297, 110487. [Google Scholar] [CrossRef]
- Wu, H.; Ye, H.; Yao, R.; Zhang, T.; Xiong, L. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Sci. 2015, 232, 1–12. [Google Scholar] [CrossRef]
- Zhao, G.; Song, Y.; Wang, Q.; Yao, D.; Li, D.; Qin, W.; Ge, X.; Yang, Z.; Xu, W.; Su, Z.; et al. Gossypium hirsutum salt tolerance is enhanced by overexpression of G. arboreum JAZ1. Front. Bioeng. Biotechnol. 2020, 8, 157. [Google Scholar] [CrossRef]
- Sehar, Z.; Fatma, M.; Khan, S.; Mir, I.R.; Abdi, G.; Khan, N.A. Melatonin influences methyl jasmonate-induced protection of photosynthetic activity in wheat plants against heat stress by regulating ethylene-synthesis genes and antioxidant metabolism. Sci. Rep. 2023, 13, 7468. [Google Scholar] [CrossRef]
- Yang, J.; Miao, W.; Chen, J. Roles of jasmonates and brassinosteroids in rice responses to high temperature stress—A review. Crop J. 2021, 9, 977–985. [Google Scholar] [CrossRef]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene signaling under stressful environments: Analyzing collaborative knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Naing, A.H.; Campol, J.R.; Kang, H.; Xu, J.; Chung, M.Y.; Kim, C.K. Role of ethylene biosynthesis genes in the regulation of salt stress and drought stress tolerance in petunia. Front. Plant Sci. 2022, 13, 844449. [Google Scholar] [CrossRef]
- Müller, M. Foes or friends: ABA and ethylene interaction under abiotic stress. Plants 2021, 10, 448. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Xu, J.; Naing, A.H.; Bunch, H.; Jeong, J.; Kim, H.; Kim, C.K. Enhancement of the flower longevity of petunia by CRISPR/Cas9-mediated targeted editing of ethylene biosynthesis genes. Postharvest Biol. Technol. 2021, 174, 111460. [Google Scholar] [CrossRef]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014, 10, e1004664. [Google Scholar] [CrossRef]
- Cao, Y.-R.; Chen, S.-Y.; Zhang, J.-S. Ethylene signaling regulates salt stress response: An overview. Plant Signal. Behav. 2008, 3, 761–763. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Luo, P.; Li, T.-T.; Shi, W.-M.; Ma, Q.; Di, D.-W. The roles of GRETCHEN HAGEN3 (GH3)-dependent auxin conjugation in the regulation of plant development and stress adaptation. Plants 2023, 12, 4111. [Google Scholar] [CrossRef]
- Mal, S.; Panchal, S. Drought and salt stress mitigation in crop plants using stress-tolerant auxin-producing endophytic bacteria: A futuristic approach towards sustainable agriculture. Front. Plant Sci. 2024, 15, 1422504. [Google Scholar] [CrossRef]
- Swarup, R.; Bhosale, R. Developmental roles of AUX1/LAX auxin influx carriers in plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef]
- Garrido-Vargas, F.; Godoy, T.; Tejos, R.; O’Brien, J.A. Overexpression of the auxin receptor AFB3 in Arabidopsis results in salt stress resistance and the modulation of NAC4 and SZF1. Int. J. Mol. Sci. 2020, 21, 9528. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Ramakrishnan, M.; Van Ha, C.; Zheng, B.; Bhardwaj, M.; Tran, L.-S.P. Roles of abscisic acid and auxin in plants during drought: A molecular point of view. Plant Physiol. Biochem. 2023, 204, 108129. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Song, X.; Zhao, Y.; Yang, D.; Wang, W.; Liu, W.; Wei, X. Identification of ARF gene family and functional analysis of CqARF05 under drought and salt stress in quinoa. Sci. Rep. 2025, 15, 5072. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Liu, F.; Wang, Y.; Bi, H.; Ai, X. Hydrogen sulfide improves the cold stress resistance through the CsARF5-CsDREB3 module in cucumber. Int. J. Mol. Sci. 2021, 22, 13229. [Google Scholar] [CrossRef]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 regulates microRNA-mediated cold stress response in Arabidopsis roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Mughal, N.; Shoaib, N.; Chen, J.; Li, Y.; He, Y.; Fu, M.; Li, X.; He, Y.; Guo, J.; Deng, J.; et al. Adaptive roles of cytokinins in enhancing plant resilience and yield against environmental stressors. Chemosphere 2024, 364, 143189. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef]
- Hajam, A.H.; Ali, M.S.; Singh, S.K.; Bashri, G. Understanding Cytokinin: Biosynthesis, Signal Transduction, Growth Regulation, and Phytohormonal Crosstalk under Heavy Metal Stress. Environ. Exp. Bot. 2024, 228, 106025. [Google Scholar] [CrossRef]
- Hudeček, M.; Nožková, V.; Plíhalová, L.; Plíhal, O. Plant hormone cytokinin at the crossroads of stress priming and control of photosynthesis. Front. Plant Sci. 2023, 13, 1103088. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Gao, S.; Chu, C. Gibberellin metabolism and signaling: Targets for improving agronomic performance of crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef]
- Maitra, S.; Hossain, A.; Sahu, C.; Mishra, U.N.; Banerjee, P.; Bhadra, P.; Praharaj, S.; Shankar, T.; Bhattacharya, U. The Role of Gibberellin against Abiotic Stress Tolerance in Plants. In Plant Growth Regulators for Climate-Smart Agriculture; CRC Press: Boca Raton, FL, USA, 2021; pp. 63–80. [Google Scholar]
- Xue, H.; Gao, X.; He, P.; Xiao, G. Origin, evolution, and molecular function of DELLA proteins in plants. Crop J. 2022, 10, 287–299. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J. Exp. Bot. 2020, 71, 1160–1170. [Google Scholar]
- Gou, H.; Lu, S.; Nai, G.; Ma, W.; Ren, J.; Guo, L.; Chen, B.; Mao, J. The role of gibberellin synthase gene VvGA2ox7 acts as a positive regulator to salt stress in Arabidopsis thaliana. BMC Plant Biol. 2024, 24, 1051. [Google Scholar] [CrossRef]
- Khan, T.A.; Kappachery, S.; Karumannil, S.; AlHosani, M.; Almansoori, N.; Almansoori, H.; Yusuf, M.; Tran, L.-S.P.; Gururani, M.A. Brassinosteroid signaling pathways: Insights into plant responses under abiotic stress. Int. J. Mol. Sci. 2023, 24, 17246. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Premachandran, Y. Triggered in distress: A miRNA-controlled switch for drought-induced ABA biosynthesis in rice. Plant Physiol. 2022, 189, 447–449. [Google Scholar] [CrossRef]
- Ali, A.; Pardo, J.M.; Yun, D.-J. Desensitization of ABA-signaling: The swing from activation to degradation. Front. Plant Sci. 2020, 11, 379. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, L.; Zhou, X. SnRK2s: Kinases or Substrates? Plants 2025, 14, 1171. [Google Scholar] [CrossRef]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef]
- Weigel, R.R.; Pfitzner, U.M.; Gatz, C. Interaction of NIMIN1 with NPR1 modulates PR gene expression in Arabidopsis. Plant Cell 2005, 17, 1279–1291. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Zavaliev, R.; Dong, X. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 2024, 84, 131–141. [Google Scholar] [CrossRef]
- Zeng, T.; Su, H.; Wang, M.; He, J.; Gu, L.; Wang, H.; Du, X.; Wang, C.; Zhu, B. The role of MYC2 transcription factors in plant secondary metabolism and stress response mechanisms. Plants 2025, 14, 1255. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Guo, X.; Hu, X.; Dong, D.; Li, G.; Xiong, X. Exogenously applied methyl Jasmonate induces early defense related genes in response to Phytophthora infestans infection in potato plants. Hortic. Plant J. 2022, 8, 511–526. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Chang, C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants 2015, 1, 14004. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Chiang, M.-H.; Hwang, S.-G.; Lin, P.-C. Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol. Biol. 2009, 71, 61–80. [Google Scholar] [CrossRef]
- Tiwari, M.; Kumar, R.; Subramanian, S.; Doherty, C.J.; Jagadish, S.K. Auxin–cytokinin interplay shapes root functionality under low-temperature stress. Trends Plant Sci. 2023, 28, 447–459. [Google Scholar] [CrossRef]
- Mazzoni-Putman, S.M.; Brumos, J.; Zhao, C.; Alonso, J.M.; Stepanova, A.N. Auxin interactions with other hormones in plant development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039990. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Cheng, C.; Ren, Z.; Xu, S.; Li, X. GAI functions in the plant response to dehydration stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 819. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Pédron, J.; Patrit, O.; Simond-Côte, E.; Maia-Grondard, A.; Pétriacq, P.; Gonzalez, R.; Blottière, L.; Kraepiel, Y. Manipulation of ABA content in Arabidopsis thaliana modifies sensitivity and oxidative stress response to Dickeya dadantii and influences peroxidase activity. Front. Plant Sci. 2017, 8, 456. [Google Scholar] [CrossRef]
- Li, L.; Mu, T.; Zhang, R.; Zhang, G.; Lv, J.; Liu, Z.; Luo, S.; Yu, J. The BES1/BZR1 family transcription factor as critical regulator of plant stress resilience. Plant Stress 2024, 15, 100730. [Google Scholar] [CrossRef]
- Cao, X.; Wei, Y.; Shen, B.; Liu, L.; Mao, J. Interaction of the transcription factors BES1/BZR1 in plant growth and stress response. Int. J. Mol. Sci. 2024, 25, 6836. [Google Scholar] [CrossRef]
- Murphy, A. Hormone crosstalk in plants. J. Exp. Bot. 2015, 66, 4853–4854. [Google Scholar] [CrossRef]
- Moore, S.; Zhang, X.; Mudge, A.; Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Spatiotemporal modelling of hormonal crosstalk explains the level and patterning of hormones and gene expression in Arabidopsis thaliana wild-type and mutant roots. New Phytol. 2015, 207, 1110–1122. [Google Scholar] [CrossRef]
- Nawaz, H.; Irum, A.; Nasim, W.; Hussain, N.; Usman, M.; Alam, J. Hormonal cross-talk mechanisms and plant immunity or defense: An overview. In Hormonal Cross-Talk, Plant Defense and Development: Plant Biology, Sustainability and Climate Change; Academic Press: Cambridge, UK, 2023; pp. 1–12. [Google Scholar]
- Arc, E.; Sechet, J.; Corbineau, F.; Rajjou, L.; Marion-Poll, A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci. 2013, 4, 63. [Google Scholar] [CrossRef]
- Ghassemian, M.; Nambara, E.; Cutler, S.; Kawaide, H.; Kamiya, Y.; McCourt, P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 2000, 12, 1117–1126. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Luo, X.; Zhou, W.; Shu, K. Multifaceted signaling networks mediated by abscisic acid insensitive 4. Plant Commun. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A.M. Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J. Exp. Bot. 2017, 68, 2913–2918. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef]
- Li, Z.; Huang, R.F. The reciprocal regulation of abscisic acid and ethylene biosyntheses. Plant Signal. Behav. 2011, 6, 1647–1650. [Google Scholar] [CrossRef]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Khan, N. Decoding Phytohormone Signaling in Plant Stress Physiology: Insights, Challenges, and Future Directions. Environ. Exp. Bot. 2025, 231, 106099. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef]
- Wager, A.; Browse, J. Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 2012, 3, 41. [Google Scholar] [CrossRef]
- Yoshida, R.; Umezawa, T.; Mizoguchi, T.; Takahashi, S.; Takahashi, F.; Shinozaki, K. The regulatory domain of SRK2E/OST1/SnRK2. 6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006, 281, 5310–5318. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Satoh, R.; Maruyama, K.; Parvez, M.M.; Seki, M.; Hiratsu, K.; Ohme-Takagi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 2005, 17, 3470–3488. [Google Scholar] [CrossRef]
- Hirayama, T.; Umezawa, T. The PP2C–SnRK2 complex: The central regulator of an abscisic acid signaling pathway. Plant Signal. Behav. 2010, 5, 160–163. [Google Scholar] [CrossRef]
- Van der Does, D.; Leon-Reyes, A.; Koornneef, A.; Van Verk, M.C.; Rodenburg, N.; Pauwels, L.; Goossens, A.; Körbes, A.P.; Memelink, J.; Ritsema, T.; et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 2013, 25, 744–761. [Google Scholar] [CrossRef]
- Tomaž, Š.; Gruden, K.; Coll, A. TGA transcription factors—Structural characteristics as basis for functional variability. Front. Plant Sci. 2022, 13, 935819. [Google Scholar] [CrossRef]
- Chávez-Martínez, A.I.; Ortega-Amaro, M.A.; Torres, M.; Serrano, M.; Jiménez-Bremont, J.F. Arabidopsis adc-silenced line exhibits differential defense responses to Botrytis cinerea and Pseudomonas syringae infection. Plant Physiol. Biochem. 2020, 156, 494–503. [Google Scholar] [CrossRef]
- Luo, P.; Di, D.-W. Precise regulation of the TAA1/TAR-YUCCA auxin biosynthesis pathway in plants. Int. J. Mol. Sci. 2023, 24, 8514. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef]
- Luo, X.; Xu, J.; Zheng, C.; Yang, Y.; Wang, L.; Zhang, R.; Ren, X.; Wei, S.; Aziz, U.; Du, J.; et al. Abscisic acid inhibits primary root growth by impairing ABI4-mediated cell cycle and auxin biosynthesis. Plant Physiol. 2023, 191, 265–279. [Google Scholar] [CrossRef]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal. Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef]
- Del Carmen Rodríguez-Gacio, M.; Matilla-Vázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling: The breakthrough goes on. Plant Signal. Behav. 2009, 4, 1035. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nguyen, T.Q.; Kisiala, A.B.; Emery, R.J.N. Beyond transport: Cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta 2021, 254, 45. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Chandrasekaran, U.; Luo, X.; Zheng, C.; Shu, K. ABA biosynthesis and signaling cascades under hypoxia stress. Front. Plant Sci. 2021, 12, 661228. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2023, 191, 1953–1967. [Google Scholar] [CrossRef]
- Puig, J.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Regulation of shoot and root development through mutual signaling. Mol. Plant 2012, 5, 974–983. [Google Scholar] [CrossRef]
- Tan, S.; Sha, Y.; Sun, L.; Li, Z. Abiotic stress-induced leaf senescence: Regulatory mechanisms and application. Int. J. Mol. Sci. 2023, 24, 11996. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, J.; Zhuang, Y.; Ye, L.; Li, Z.; Wang, Y.; Qi, M.; Xu, L.; Zhang, Y. Polycomb repressive complex 2 attenuates ABA-induced senescence in Arabidopsis. Plant J. 2019, 97, 368–377. [Google Scholar] [CrossRef]
- Wang, J.; Tian, C.; Zhang, C.; Shi, B.; Cao, X.; Zhang, T.-Q.; Zhao, Z.; Wang, J.-W.; Jiao, Y. Cytokinin signaling activates WUSCHEL expression during axillary meristem initiation. Plant Cell 2017, 29, 1373–1387. [Google Scholar] [CrossRef]
- Guan, C.; Wang, X.; Feng, J.; Hong, S.; Liang, Y.; Ren, B.; Zuo, J. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive5 protein in Arabidopsis. Plant Physiol. 2014, 164, 1515–1526. [Google Scholar] [CrossRef]
- Wang, H.; Tang, J.; Liu, J.; Hu, J.; Liu, J.; Chen, Y.; Cai, Z.; Wang, X. Abscisic acid signaling inhibits brassinosteroid signaling through dampening the dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant 2018, 11, 315–325. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 2014, 26, 4394–4408. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.-Y.; Li, L.; et al. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat. Commun. 2017, 8, 14573. [Google Scholar] [CrossRef]
- Tyler, L.; Thomas, S.G.; Hu, J.; Dill, A.; Alonso, J.M.; Ecker, J.R.; Sun, T.-P. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004, 135, 1008–1019. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Fu, C.; Ahmad, N.; Zhao, C.; Hou, L.; Naeem, M.; Pan, J.; Wang, X.; Zhao, S. Genome-wide identification and expression analysis of GA20ox and GA3ox genes during pod development in peanut. PeerJ 2023, 11, e16279. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.-Y.; Wang, Z.-Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Thilakarathne, A.S.; Liu, F.; Zou, Z. Plant Signaling Hormones and Transcription Factors: Key Regulators of Plant Responses to Growth, Development, and Stress. Plants 2025, 14, 1070. [Google Scholar] [CrossRef]
- Wilson, S.; Pretorius, T.; Naidoo, S. Mechanisms of systemic resistance to pathogen infection in plants and their potential application in forestry. BMC Plant Biol. 2023, 23, 404. [Google Scholar] [CrossRef]
- Kamali, S.; Singh, A. Genomic and transcriptomic approaches to developing abiotic stress-resilient crops. Agronomy 2023, 13, 2903. [Google Scholar] [CrossRef]
- Yetgin, A.; Srivastava, R.K.; Mandal, N. Insights into Plant Hormone Signaling Networks for Environmental Responses. In Mitigation and Adaptation Strategies Against Climate Change in Natural Systems; Srivastava, R.K., Chakraborty, A., Eds.; Springer: Cham, Switzerland, 2025; pp. 505–523. [Google Scholar] [CrossRef]
- Chaffai, R.; Ganesan, M.; Cherif, A. Transcriptional Regulation of Gene Expression in Plant Abiotic Stress Response. In Plant Adaptation to Abiotic Stress: From Signaling Pathways and Microbiomes to Molecular Mechanisms; Springer: Berlin/Heidelberg, Germany, 2024; pp. 303–343. [Google Scholar]
- Bano, A.; Waqar, A.; Khan, A.; Tariq, H. Phytostimulants in sustainable agriculture. Horticulturae 2022, 6, 801788. [Google Scholar] [CrossRef]

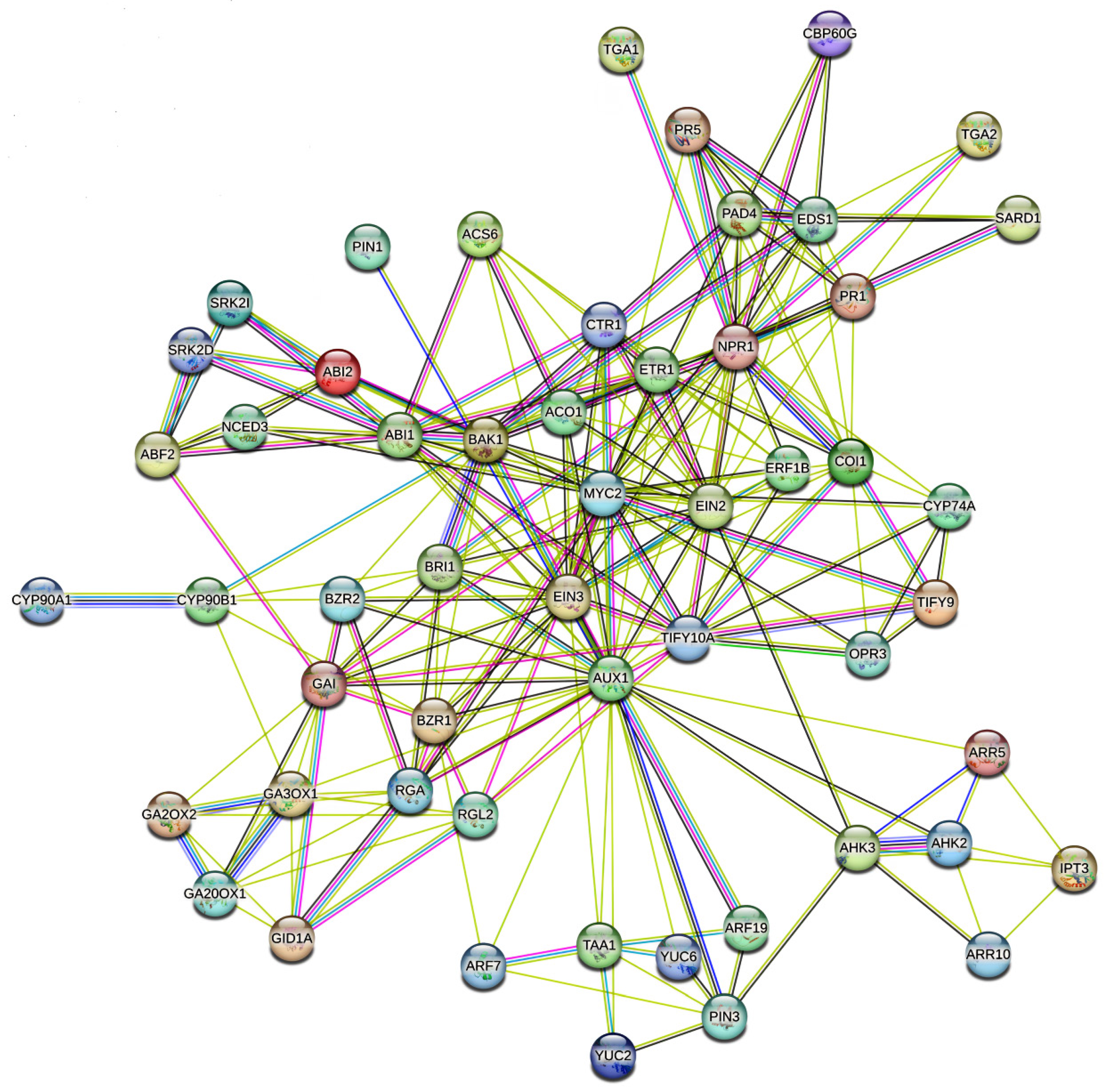
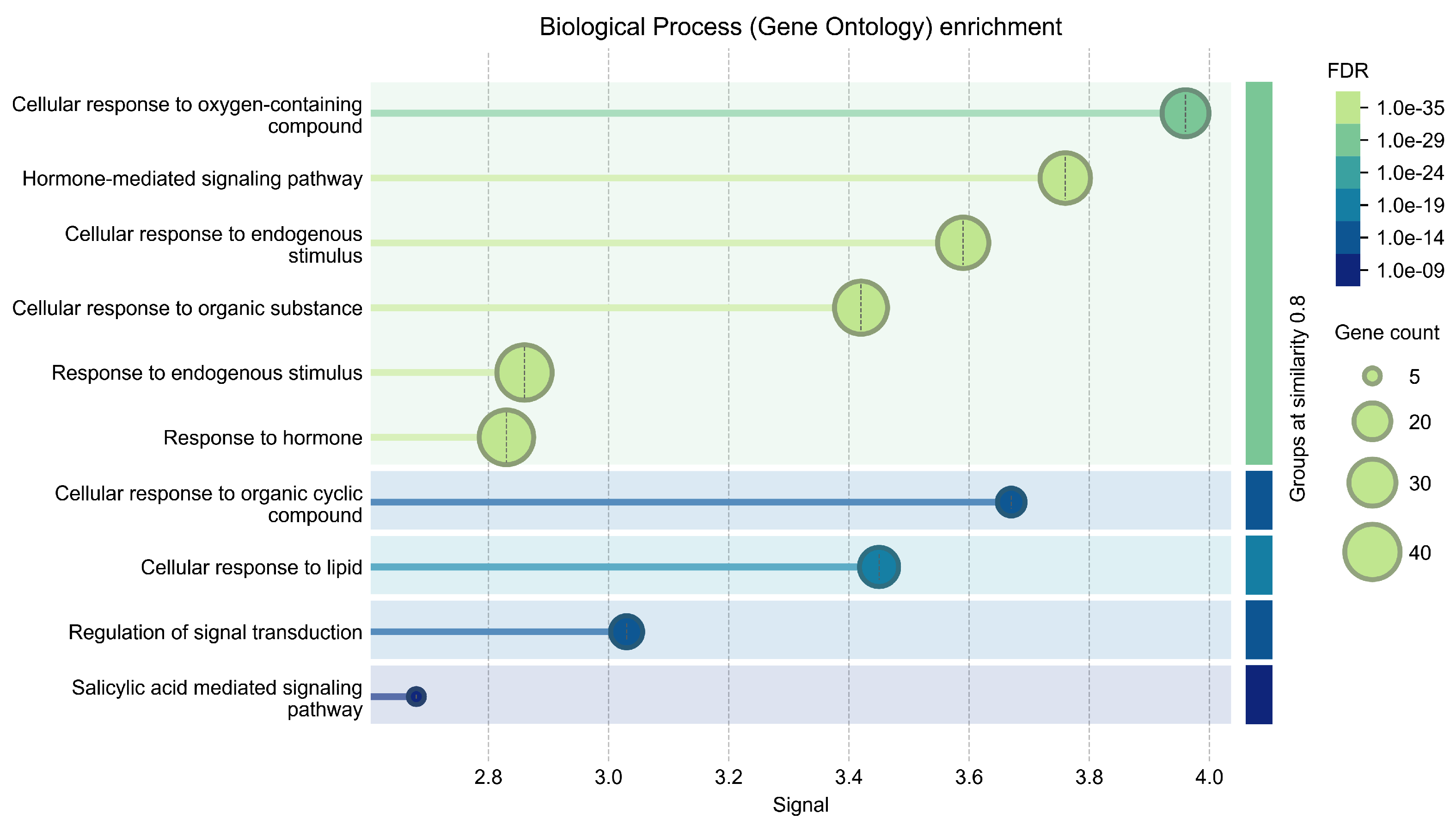

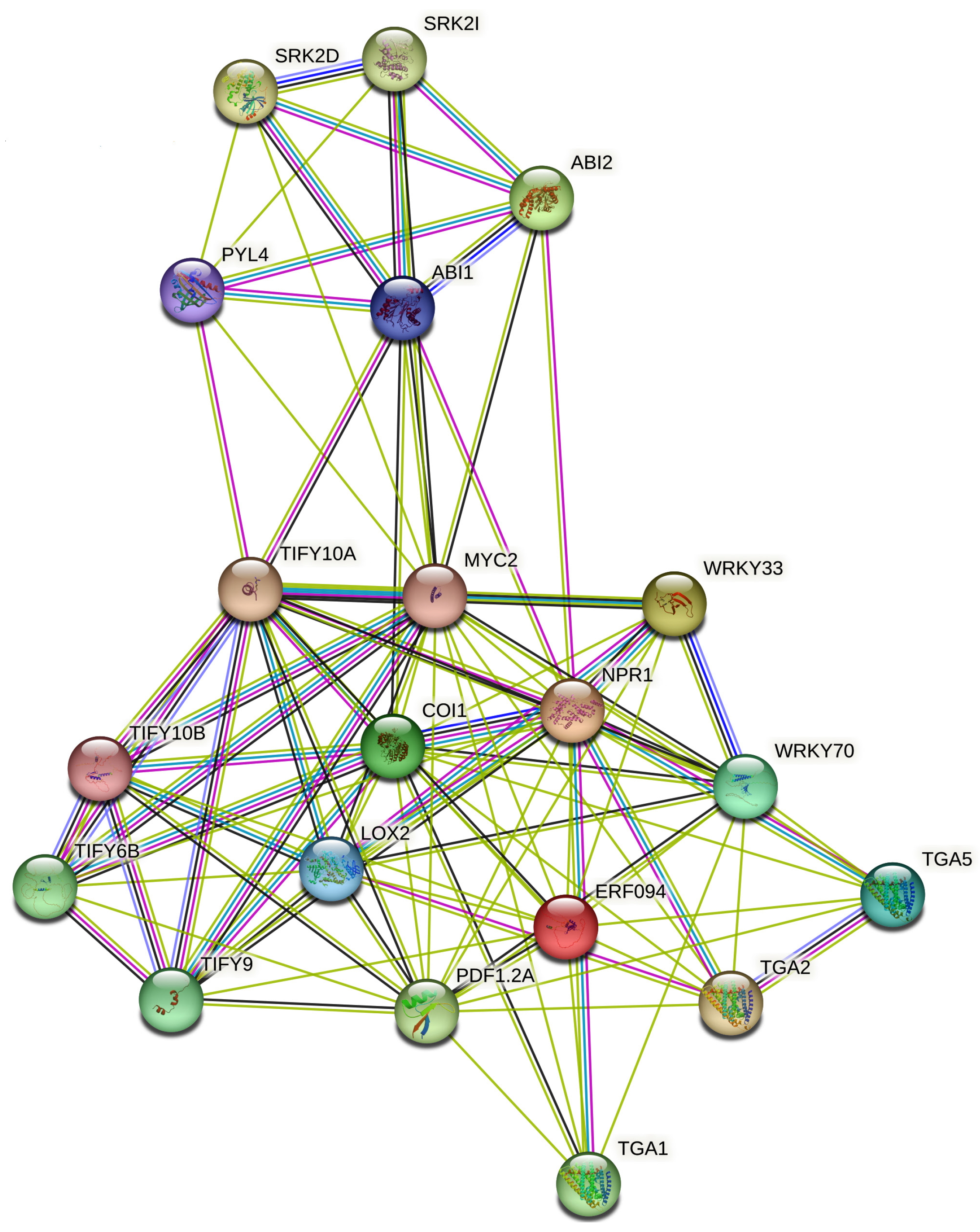
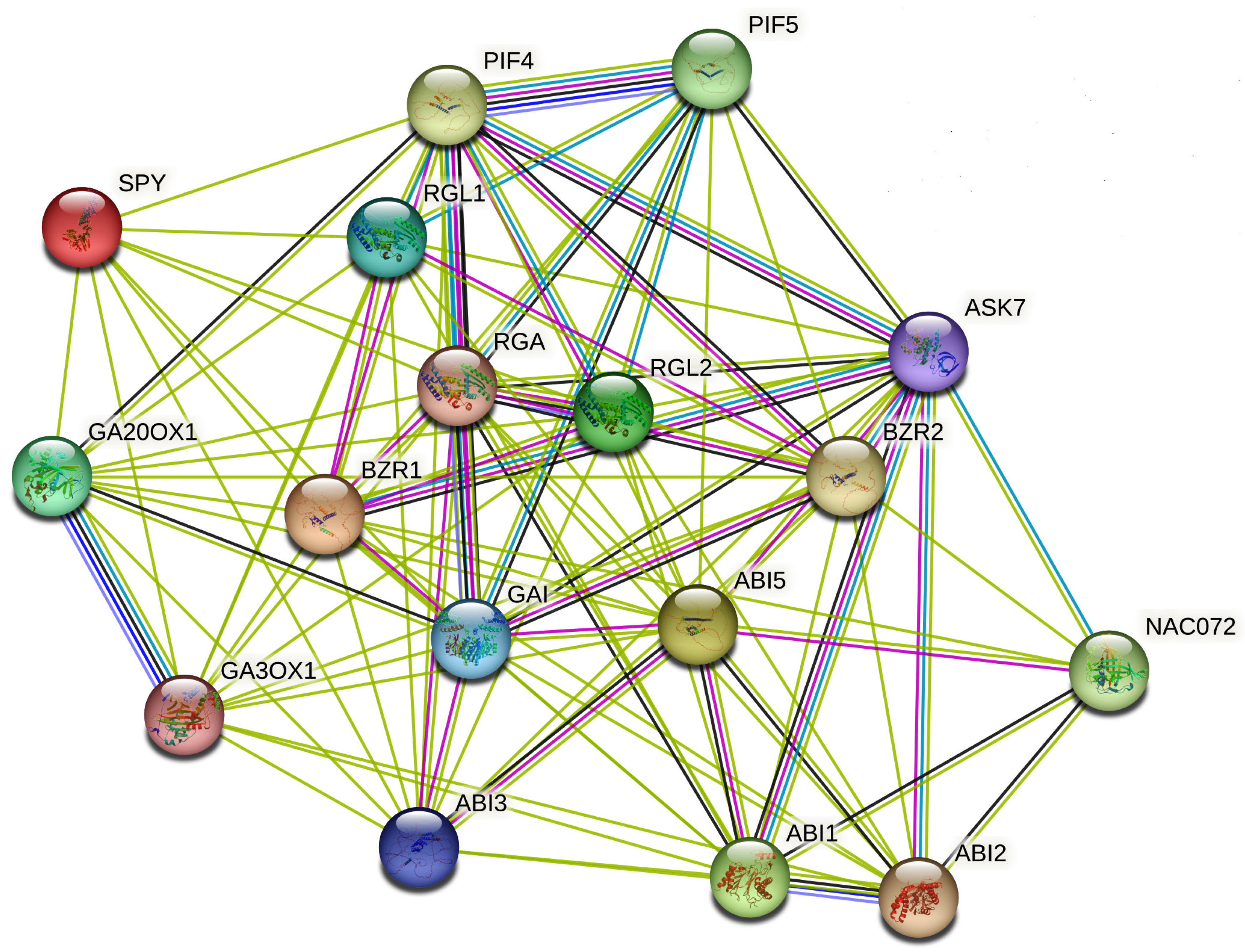
| Term ID | Term Description | Observed Gene Count | Background Gene Count | Strength | Signal | False Discovery Rate | Matching Proteins |
|---|---|---|---|---|---|---|---|
| ath04075 | Plant hormone signal transduction | 32 | 289 | 1.75 | 8.09 | 2.40 × 10−16 | COI1, ABI2, BRI1, EIN3, ARR10, TGA2, ETR1, ABI1, NPR1, ARF7, CTR1, PR1, SRK2D, SRK2I, MYC2, TGA1, RGL2, ERF1B, BZR1, TIFY9, BAK1, AUX1, AHK3, AHK2, TIFY10A, BZR2, GAI, ABF2, GID1A, EIN2, ARR5, RGA |
| ath04016 | MAPK signaling pathway-plant | 13 | 136 | 1.69 | 4 | 1.25 × 10−16 | ABI2, EIN3, ETR1, ABI1, CTR1, PR1, SRK2D, SRK2I, MYC2, ERF1B, BAK1, EIN2, ACS6 |
| ath00904 | Diterpenoid biosynthesis | 3 | 22 | 1.84 | 1.05 | 0.00072 | GA3OX1, GA20OX1, GA2OX2 |
| ath01110 | Biosynthesis of secondary metabolites | 11 | 1219 | 0.66 | 0.6 | 0.00075 | CYP90B1, GA3OX1, GA20OX1, ACO1, CYP90A1, IPT3, CYP74A, OPR3, NCED3, ACS6, GA2OX2 |
| ath00905 | Brassinosteroid biosynthesis | 2 | 8 | 2.1 | 0.81 | 0.0045 | CYP90B1, CYP90A1 |
| ath00380 | Tryptophan metabolism | 3 | 62 | 1.39 | 0.7 | 0.0064 | YUC6, TAA1, YUC2 |
| ath01100 | Metabolic pathways | 13 | 2285 | 0.46 | 0.39 | 0.0076 | CYP90B1, GA3OX1, GA20OX1, ACO1, CYP90A1, YUC6, IPT3, CYP74A, OPR3, NCED3, TAA1, ACS6, YUC2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, D.; Kashtoh, H.; Panda, J.; Rustagi, S.; Mohanta, Y.K.; Singh, N.; Baek, K.-H. From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals. Plants 2025, 14, 2322. https://doi.org/10.3390/plants14152322
Das D, Kashtoh H, Panda J, Rustagi S, Mohanta YK, Singh N, Baek K-H. From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals. Plants. 2025; 14(15):2322. https://doi.org/10.3390/plants14152322
Chicago/Turabian StyleDas, Dipayan, Hamdy Kashtoh, Jibanjyoti Panda, Sarvesh Rustagi, Yugal Kishore Mohanta, Niraj Singh, and Kwang-Hyun Baek. 2025. "From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals" Plants 14, no. 15: 2322. https://doi.org/10.3390/plants14152322
APA StyleDas, D., Kashtoh, H., Panda, J., Rustagi, S., Mohanta, Y. K., Singh, N., & Baek, K.-H. (2025). From Hormones to Harvests: A Pathway to Strengthening Plant Resilience for Achieving Sustainable Development Goals. Plants, 14(15), 2322. https://doi.org/10.3390/plants14152322









