1. Introduction
Phloem exudates, such as manna, represent a carbon-rich output of plant metabolic processes and may reflect underlying physiological processes related to storage, defense, and environmental stress responses [
1,
2,
3,
4]. Despite their ecological relevance as a food source for many insects, birds and mammals, [
5,
6,
7,
8,
9,
10], few recent studies have investigated the composition and intra-specific variability of these exudates in wild plant populations [
4,
11,
12].
Mannitol or mannite is a sap-like substance that occurs naturally in the phloem of most land plants as well as being produced by some bacteria, fungi, and yeast [
1,
13,
14,
15]. Mannitol production has been recorded in over 70 higher plant families including Oleaceae, Rubiaceae, Scrophulariaceae, and Apiaceae [
2,
15,
16,
17,
18]. In plants, mannitol is extruded in response to small incisions by insects and birds in the leaves and young stems of the plant, which crystallizes into small white nodules referred to as “
manna” [
7,
8,
19,
20]. Manna is a fructose-derived 6-carbon sugar alcohol (C
6H
14O
6), composed predominantly of six sugars (raffinose, stachyose, sucrose, glucose, fructose, and tetrasaccharides) in addition to trace amounts of water, pectin, and uronic acids [
13,
19]. The composition and concentration of the six sugars has been found to vary within and between plant species [
13,
19]. Manna is most commonly associated with
Fraxinus (Oleaceae) and
Eucalyptus (Myrtaceae), where it is classified as a “dried exudate” [
15,
16,
17,
20]. Sucrose and raffinose, two of the dominant sugars in manna, have been proposed as key mediators of abiotic stress and carbon storage in plants, functioning as transportable reserves or protective solutes under environmental stress [
1,
2,
3,
4,
19,
21].
Within Australia, the
Eucalyptus genus encompasses more than 800 species that occupy and dominate terrestrial habitat types, from dry deserts to tropical and temperate rainforests, and occur from sea level to the alpine tree line [
22,
23,
24,
25]. This broad occupation of various niches allows eucalypts to serve as foundation species, supporting biodiverse communities through the provision of food and habitat. Manna produced by
Eucalyptus has been identified as a crucial food source for many native birds, mammals, and insects, offering a concentrated carbohydrate source [
5,
8,
20]. Within the
Eucalyptus genus, evidence of manna production exists in 14 species [
19]. However, existing research has primarily focused on the feeding behaviors of insects and animals that rely on manna as a food source, with less emphasis on the variation in its composition [
7,
8,
19,
20]. It is established that the two dominant sugars sucrose and raffinose represent an alternate axis of quality for foragers, with sucrose being highly digestible and energy rich, whereas raffinose is indigestible [
7,
8,
20,
26]. However, knowledge gaps remain concerning how manna sugar composition, specifically in the context of these dominant sugars, varies within species and the abiotic and morphological drivers of this variation [
4,
12,
27]. Investigating this variation in
Eucalyptus species may shed light on underlying plant allocation strategies and help clarify how changes in carbohydrate composition influence ecosystem-level resource provisioning in eucalypt-dominated environments.
This study investigates patterns of variation in manna composition, quality, and quantity in the foundation tree species
Eucalyptus viminalis, a key component of dry sclerophyll forests across Tasmania [
24]. As a widespread and ecologically significant species that supports a diversity of fauna [
26,
28,
29],
E. viminalis is an ideal model for examining variation in manna. We hypothesized that manna composition, quality, and quantity would vary both temporally (seasonally and annually) and spatially (within and between trees), reflecting underlying physiological processes. At finer scales, local factors such as leaf area and branch position may also influence photosynthetic activity and carbohydrate allocation [
30,
31,
32,
33,
34]. Between trees, differences in size and canopy health are likely to affect whole-plant sugar production and storage strategies [
2,
35,
36,
37,
38]. Temporal variation in manna is also expected, given that sugar synthesis and partitioning in plants respond to seasonal changes in temperature, water availability, and photoperiod [
1,
2,
4,
12].
To investigate these hypotheses, we developed a standardized, repeatable protocol for direct manna extraction. While chemical methods exist [
16,
17,
39], we adopted a simpler technique based on simulating natural incisions made by fauna [
4,
7,
20]. Rather than applying this method in situ [
40,
41], we propose an ex situ approach using pruned branches in a controlled lab environment. This allows us to minimize variation due to external factors like insect herbivory, temperature, and humidity while maintaining consistent branch selection criteria (e.g., canopy position, aspect, and diameter). Although this design does not capture whole-plant transport dynamics, it provides a valuable baseline for assessing compositional differences in a controlled context.
Once the extraction protocol was established, we applied it to investigate tree-level and temporal drivers of manna variation. We defined composition as the relative profile of six key sugars (sucrose, raffinose, fructose, glucose, stachyose, and tetrasaccharides), quality as the ratio of the two dominant sugars, sucrose and raffinose, and quantity as the total mass of exudate produced per branch (mg). Using this approach, we aimed to (i) quantify within-population and within-tree variation in manna traits and assess how this variation is shaped by branch-level attributes; (ii) evaluate how manna composition, quality, and quantity relate to tree canopy health; and (iii) determine how these traits change across seasons and years. Our results will provide valuable information about resource provisioning within a foundation tree species,
E. viminalis, and its dependent communities [
8,
20,
26,
28,
29,
42,
43]. More broadly, it will contribute to a better understanding of how plant-level traits and temporal gradients drive functional variation in phloem exudate composition.
4. Materials and Methods
4.1. Manna Collection and Extraction
This study was conducted at the Queens Domain reserve, in southeast Tasmania (−42.87, 147.32), 80 m above sea level. This is a relatively dry, temperate site, experiencing mean annual rainfall of 571 mm, lowest temperature of 7.8 °C, and highest temperature of 17 °C (Bureau of Meteorology “Climate Data” portal 2024). Thirty adult E. viminalis trees ranging in size (diameter at breast height [DBH] 9–138 cm) were repeatedly sampled from for manna over three seasons (August = winter, November = spring, and February = summer) across three years (2021–2023). Although samples were collected across three calendar years, data were grouped into two sampling years based on seasonal sampling periods: Sampling Year 1 includes August 2021, November 2021, and February 2022; Sampling Year 2 includes August 2022, November 2022, and February 2023. This reserve land contains patches of both modified (mowed and cleared) and native vegetation. The stand in which our 30 trees were selected is classified as E. viminalis woodland, with a grassy understory containing a mix of native and exotic species. Here, E. viminalis is the dominant tree species within a medium-density mixed-age stand comprised of juvenile to established adult trees (up to 10 m tall, DBH 138 cm).
Full details on the sampling methodologies are outlined in
Appendix A.1. Briefly, a single branch ranging between 1.5 and 4.5 cm in circumference and 30 and 50 cm long was collected per
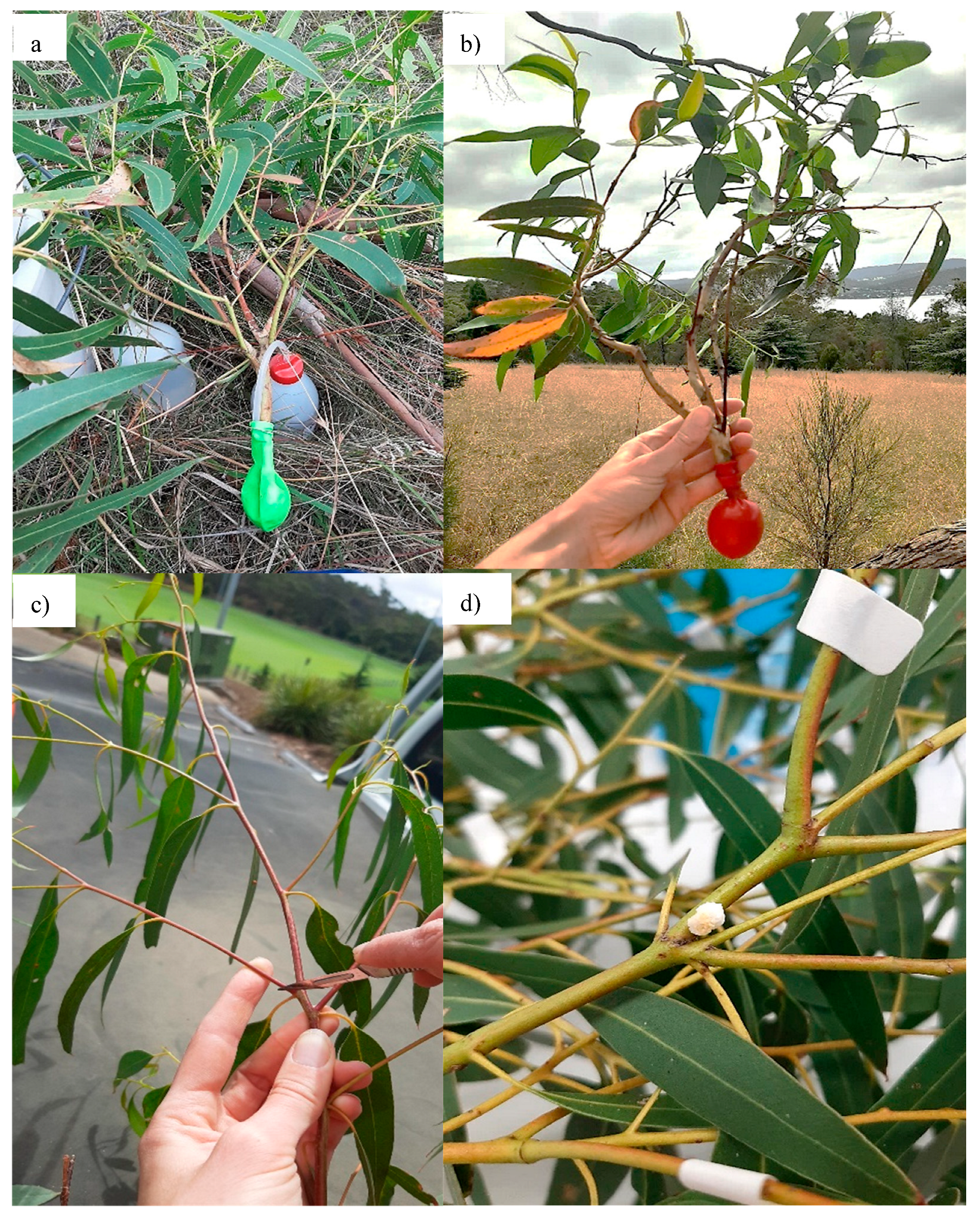
E. viminalis tree using pruning poles from between 1 and 7 m into the canopy. Upon collection, branches were placed in a balloon that was then filled with water using a safety wash bottle and sealed with a rubber band to prevent water loss (see
Figure 5). DBH was measured for each tree as a proxy for tree size.
Sampling protocols were as above for all sampling periods except for the November (spring) 2022 period. During this period, we selected 10 of our 30 trees to investigate within-tree variation in manna quantity and quality (Aim 1). Here we collected up to five branches from each tree to test whether within-tree variation is influenced by factors related to branch selection, which could be standardized in the sampling protocol. These factors included i) the approximate height of the branch within the canopy (<4 m for low, >5 m for high), ii) branch circumference (measured to the nearest 0.5 cm), and iii) aspect of the tree from which the branch was collected (N, S, E, or W)
We also collected data relating to tree health to explore the effect of tree health on manna quality and quantity (Aim 2). Here, we created an overall health score (vigor) based on established canopy damage assessment traits [
56,
57]. Specifically, vigor was calculated as the percentage of the whole tree canopy that was dead (evidence of dieback), plus the percentage of foliar damage by insects and pathogens for the branch collected. Here, a lower vigor score was considered indicative of a “healthier” tree, and a higher score as indicative of more canopy damage.
Branches were housed on campus at the University of Tasmania and kept at room temperature in shaded conditions. On arrival, balloons were removed, and branches immediately placed into pre-prepared jars of water (0.5–3 L volume). Approximately 2 cm of the stem was cut from the base of each branch to remove dysfunctional xylem cells that may have filled with air during the collection process, enabling plants to maintain hydration during manna extraction. Using a scalpel, 8–12 incisions per stem were made evenly in the leaf/stem axils, (as dictated by the number of leaves/stem axils per stem) across at least six stems of each branch (or all stems where there were less than six per branch).
After 24 h, incisions were checked for manna. If manna appeared transparent, collection was delayed, allowing time for crystallization up to a maximum of 76 h, (
Figure 5d). If manna was not present 24 h after collection, an additional 6–10 incisions were made per branch, and sample collection was delayed for another 24 h (48 h in total). Once crystalized, manna was collected from each branch by gently scraping crystallized secretions, using a scalpel, into a 10 mL vial labeled with the corresponding branch identification number (see Appendixes A.1 and B.1 for method development details). The quantity of manna produced per branch was determined using a Mettler Toledo (XPE105DR) precision balance (Mettler-Toledo, Greifensee, Switzerland) as total amount (weight in milligrams to four decimal places) produced over a 48 h period.
4.2. Manna Chemical Analysis
Once weighed, manna quality was quantified using Liquid Chromatography–Mass Spectrometry (LC-MS). To prepare samples for LC-MS analysis a subsample of manna (0.01 mg min–2.00 mg max) was placed into a 2 mL Autosampler vial (Waters, Milford, MA, USA). Manna was then dissolved with equal proportions of distilled water (e.g., 1 mg of manna with 1 mL of distilled water), and the vial was vortexed for 20 s until manna was dissolved. All vials were stored in −20 °C conditions to limit deterioration until analysis.
LC-MS was conducted at the University of Tasmania’s Central Science Laboratory using a Waters Acquity H-Class UPLC instrument coupled to a Waters Xevo TQ triple quadrupole mass spectrometer to determine manna composition and concentration of sugars present in each sample as per Wing [
41]. Briefly, the mass spectrometer was operated under negative ion electrospray mode with a needle voltage of 2.7 kV. Analytes were detected using single-ion monitoring with [M-H]-deprotonated molecular species. Data were processed using MassLynx software version 4.2 (Waters, USA). Individual sugars (raffinose, stachyose, glucose, fructose, and sucrose) were identified and quantified (into parts per million, PPM) using the external calibration approach. Unidentified tetrasaccharides were grouped and quantified as “Tetrasaccharides” (see
Appendix C.1).
4.3. Data Analysis
All analyses were performed using R version 4.4.0 [
58], and figures were generated using base R and ggplot2 [
59].
4.3.1. Characterizing Manna Composition and Quality
Manna composition is defined here as the profile of the six major sugars present in Eucalyptus viminalis manna (sucrose, raffinose, fructose, glucose, stachyose, and tetrasaccharides), characterized by their absolute concentrations (PPM), proportional contributions to total sugar content, and patterns of variation in the multivariate space (PCA).
To analyze manna composition, we first conducted bivariate outlier tests to visualize the distributions of the six manna sugars and identify potential outliers. We then calculated the Mahalanobis distance for each observation and assessed whether each distance value was statistically extreme by calculating
p values for each point based on a chi-squared distribution, using the “qchisq” function [
58]. A significance level of 0.001 was chosen to identify extreme outliers while retaining moderate variability in the dataset for subsequent analyses. Identified outliers were removed from further analysis (see
Appendix D.1). The mean absolute concentration (PPM) and proportional contributions to the total sugar content for the six manna sugars are reported (
Table 1).
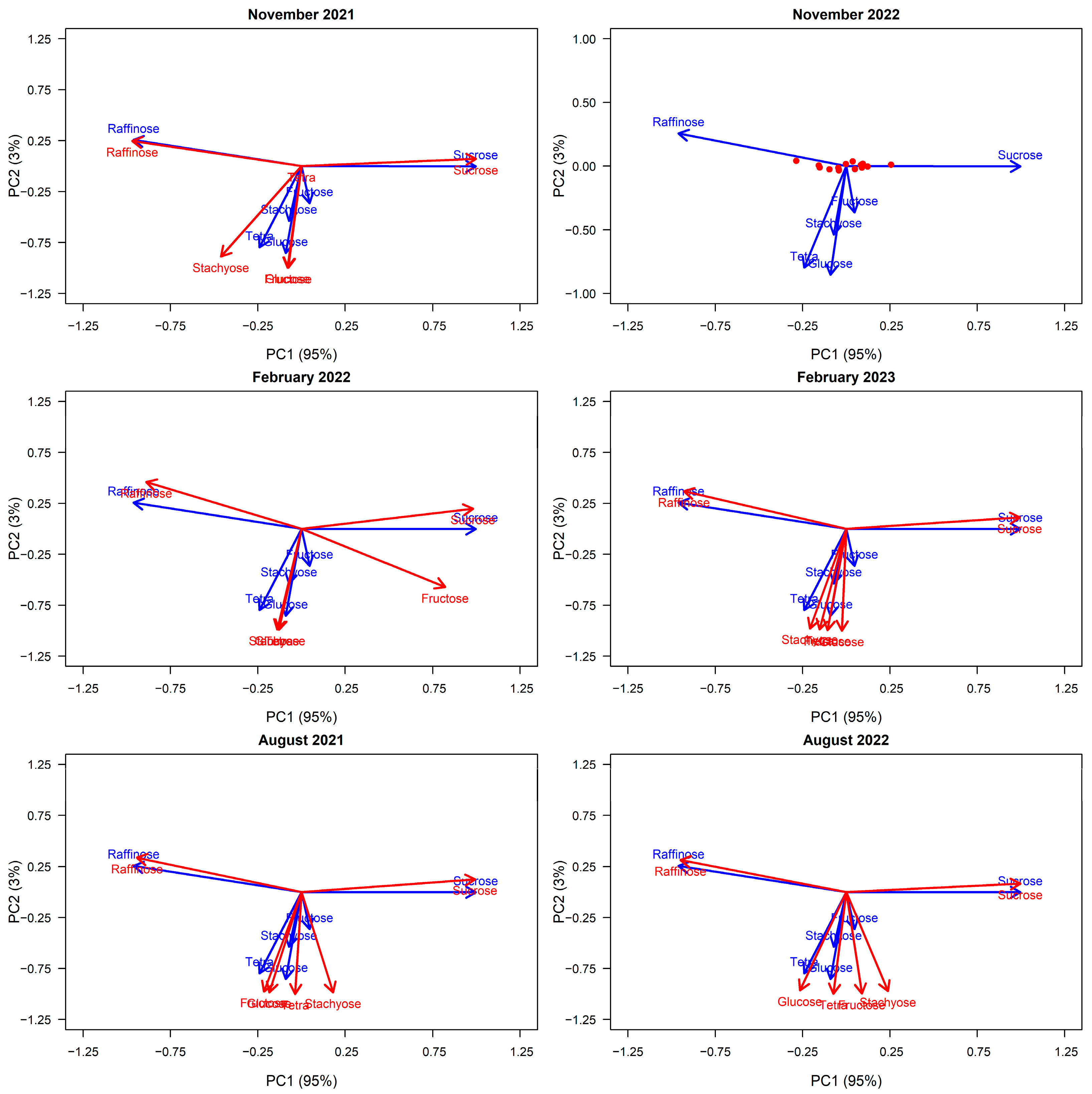
We used principal component analysis (PCA) using the “prcomp” function [
58] to reduce the complexity of the six correlated manna sugars into two informative axes that capture the main patterns of variation in manna composition within the 30
E. viminalis trees sampled. This multivariate approach allowed us to summarize how the six sugars co-vary and identify dominant sugar groupings. We used PCA exclusively on the six sugars to explore variation in manna composition, not to identify environmental or tree-level drivers, which were tested separately.
To prepare sugar data for the PCA, data were first transformed to reflect the proportion contribution to the overall sample concentration to account for volumetric variance, (e.g., influence of sample volume on sugar concentration and composition). As proportional data do not meet the assumptions of linearity required for principal component analysis, we applied an arc-sine transformation [
60]. We first ran PCA on the November 2022 manna data, as this corresponded to the sampling period that included our data for within-tree variation and health (see Results,
Figure 1).
To explore how correlations between the six key sugars—raffinose, sucrose, fructose, glucose, stachyose, and tetrasaccharides—and the principal component axes varied across sampling periods, we used the “vegan” package [
61] to vector fit each sampling event, grouped by season and year, into the PCA space defined by the November/spring 2022 data (PC axes 1 and 2). We then performed Procrustes pairwise tests within seasons (e.g., August 2021/August 2022) and in comparison to the reference period (November 2022) to consider the degree of variation in the spatial configuration of the sugars predicted into the multivariate space (see
Appendix E.1).
Across the experimental period, each manna sample was mostly comprised of two key sugars, sucrose and raffinose (see Results). These sugars have been proposed to reflect differing roles in plant carbohydrate storage and allocation and may also influence how exudates are utilized by foraging fauna [
7,
8,
20,
21,
26,
47]. To confirm that the ratio of these two dominant sugars captures the variation in manna quality generated from our PCA, we conducted a Pearson’s correlation test between our first two principal components and the ratio of sucrose to raffinose using the “corr.test” function [
58]. Given that sucrose and raffinose are equally represented by PC1, show a significant correlation with this axis, and are known to make up ~90% of manna composition [
19], we hereafter used the ratio of sucrose/raffinose as our indicator of manna quality. Here, a higher ratio value indicates a greater proportion of sucrose in a sample, indicative of increased quality.
4.3.2. Variation in Manna Quality and Quantity
Histograms were generated to visualize the distribution of manna quality (sucrose ratio) and quantity (weight in mg). Outliers were removed where the sucrose/raffinose exceeded 1 and manna weight exceeded 1000 mg. While these data points were genuine observations, their values fell beyond three standard deviations for quality (0.3316, ± 0.1348) and quantity (143.46 mg ± 205.49 mg) in the full dataset, rendering them unrepresentative of the overall trends (see
Appendix D.1).
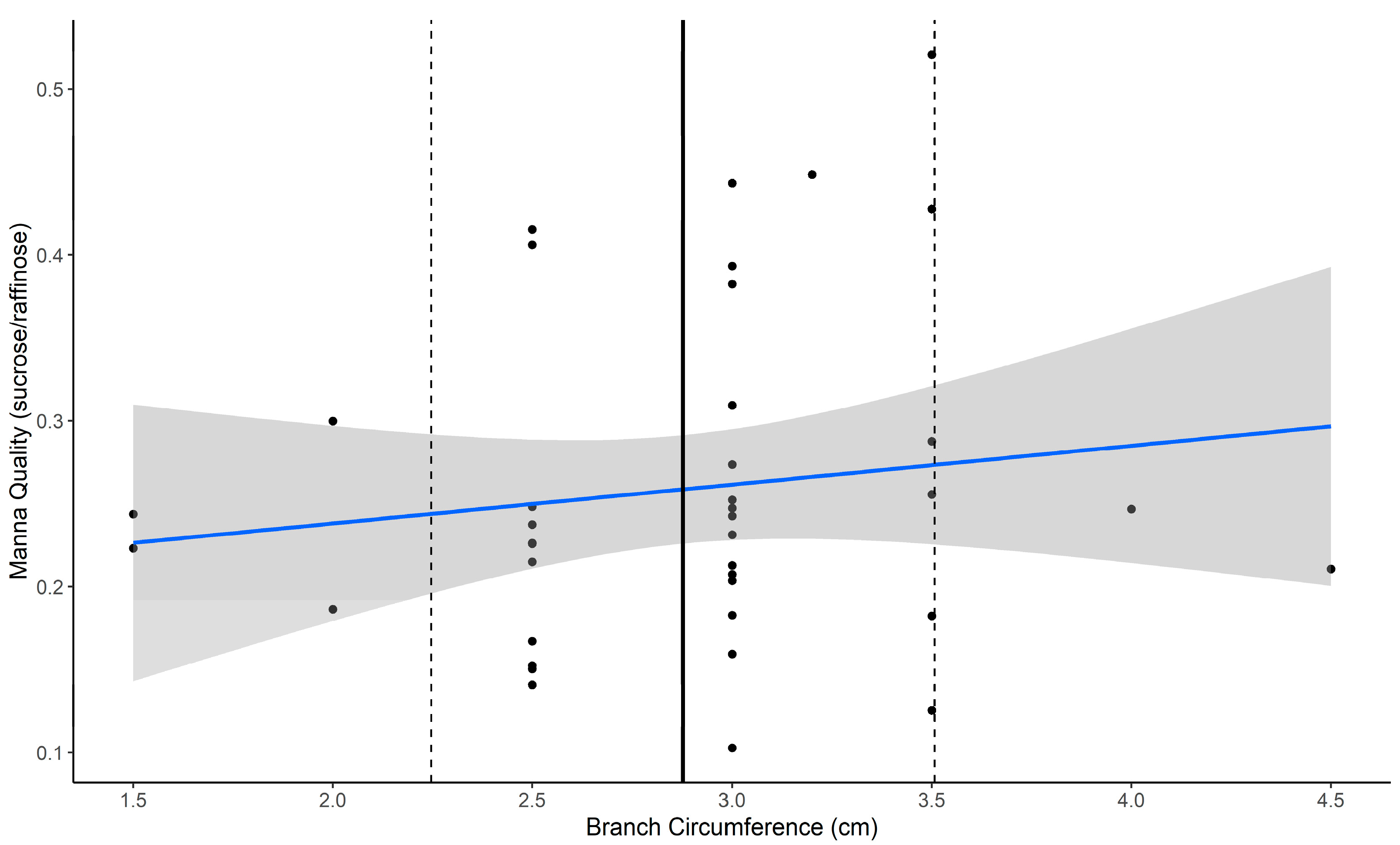
Models exploring the within- and between-tree variation in manna utilized the November/spring 2022 datasets relating to branch sampling traits (aspect, height, and branch circumference) and tree health (vigor). First, using a subset of trees for which we had data from multiple branches across the tree, we explored whether manna quality and quantity varied within a tree as a function of key branch traits. To achieve this, we ran a linear mixed-effects model using the “glmmtmb” package [
62], with manna quality or quantity as the response variable and branch aspect, branch height, and branch circumference as predictor variables, where tree ID was retained as a random variable. Manna quantity data (weight) was log transformed to normalize data and family set to “gaussian”. As the data for manna quality (sucrose/raffinose) were proportional and beta distributed, the “beta” family with the “logit” link was applied. Restricted maximum likelihood (REML) was set to true for both models to account for the unbalanced replication in branch number. The “DHARMa” package was used to test model assumptions and fit (manna quality and quantity) using the simulateResidual and testDisperson functions [
63]. Type III Wald chi-square tests were calculated for model fixed effects using car: Anova [
64]. Variation attributed to random effects was approximated using Wald likelihood ratio tests (LRTs), comparing models with and without the random effect. This modeling approach was applied for all other models detailed below.
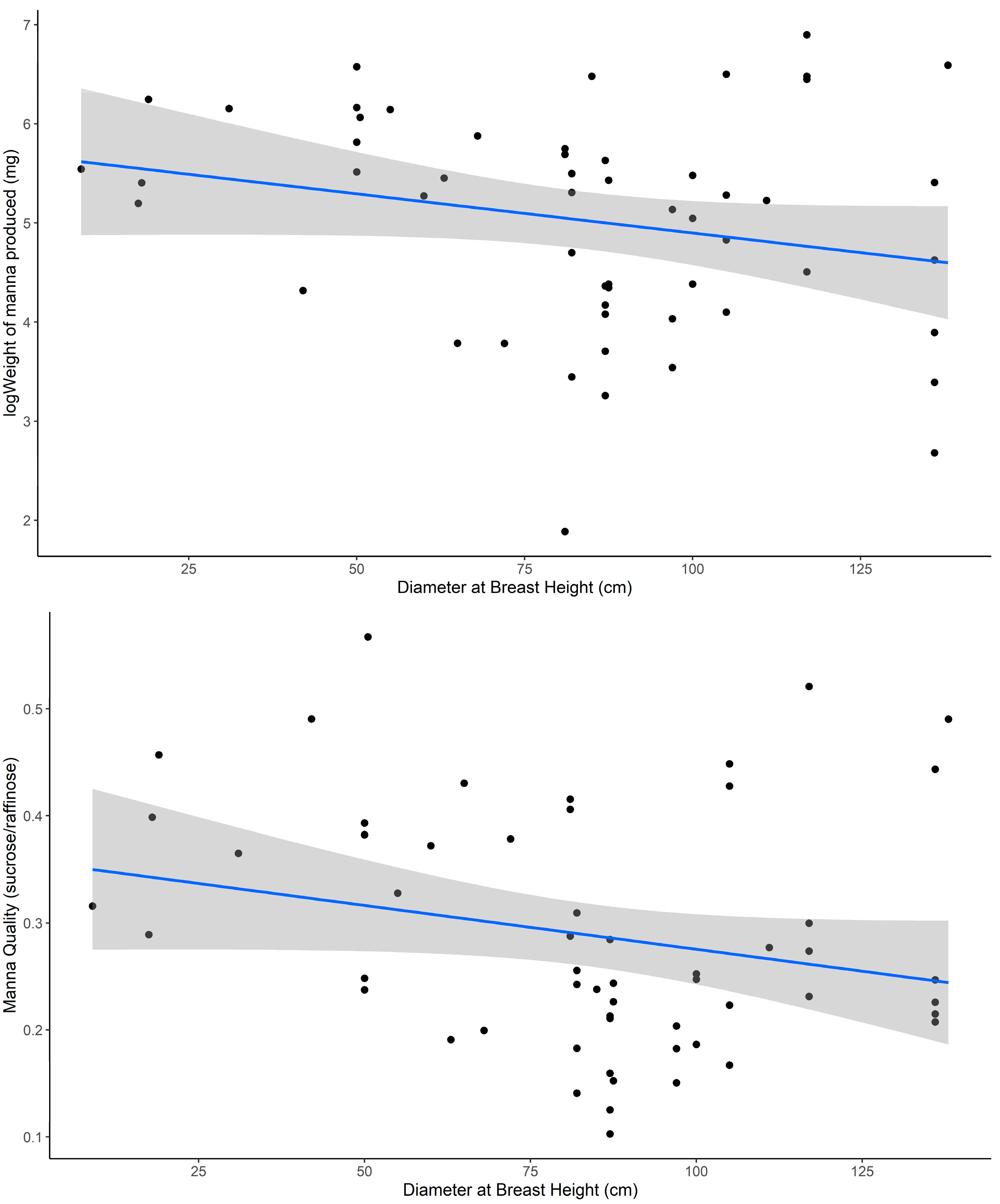
Second, we explored whether manna quality and quantity varied between trees as a function of tree health. To achieve this, we ran a linear mixed-effects model with manna quality or quantity as the response variable and tree vigor score and tree size (measured as DBH) as predictor variables. Here, branch ID was nested within-tree ID as a random effect to account for the unequal replication of branches sampled per tree.
4.3.3. Seasonal Variation in Manna Quality and Quantity
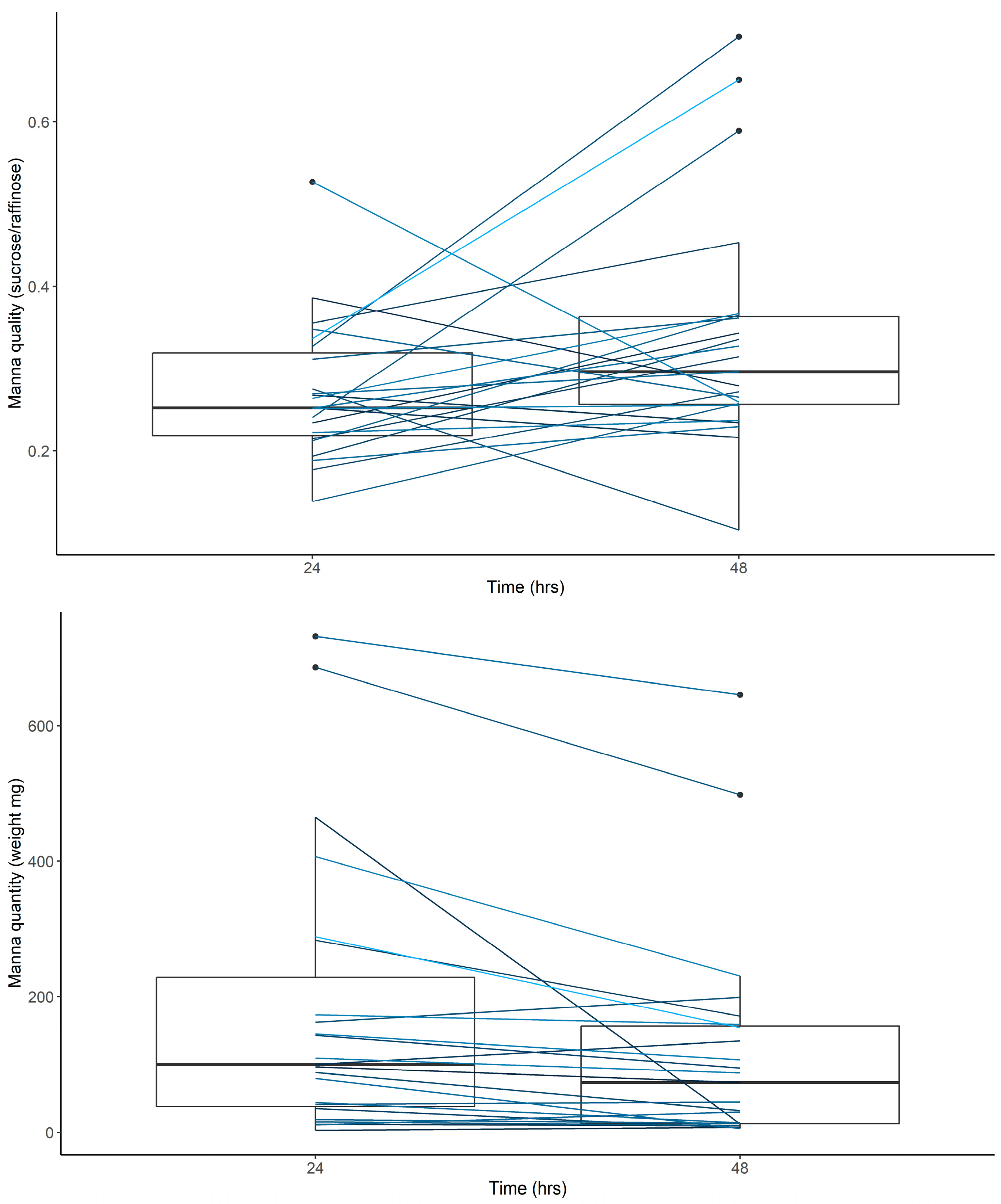
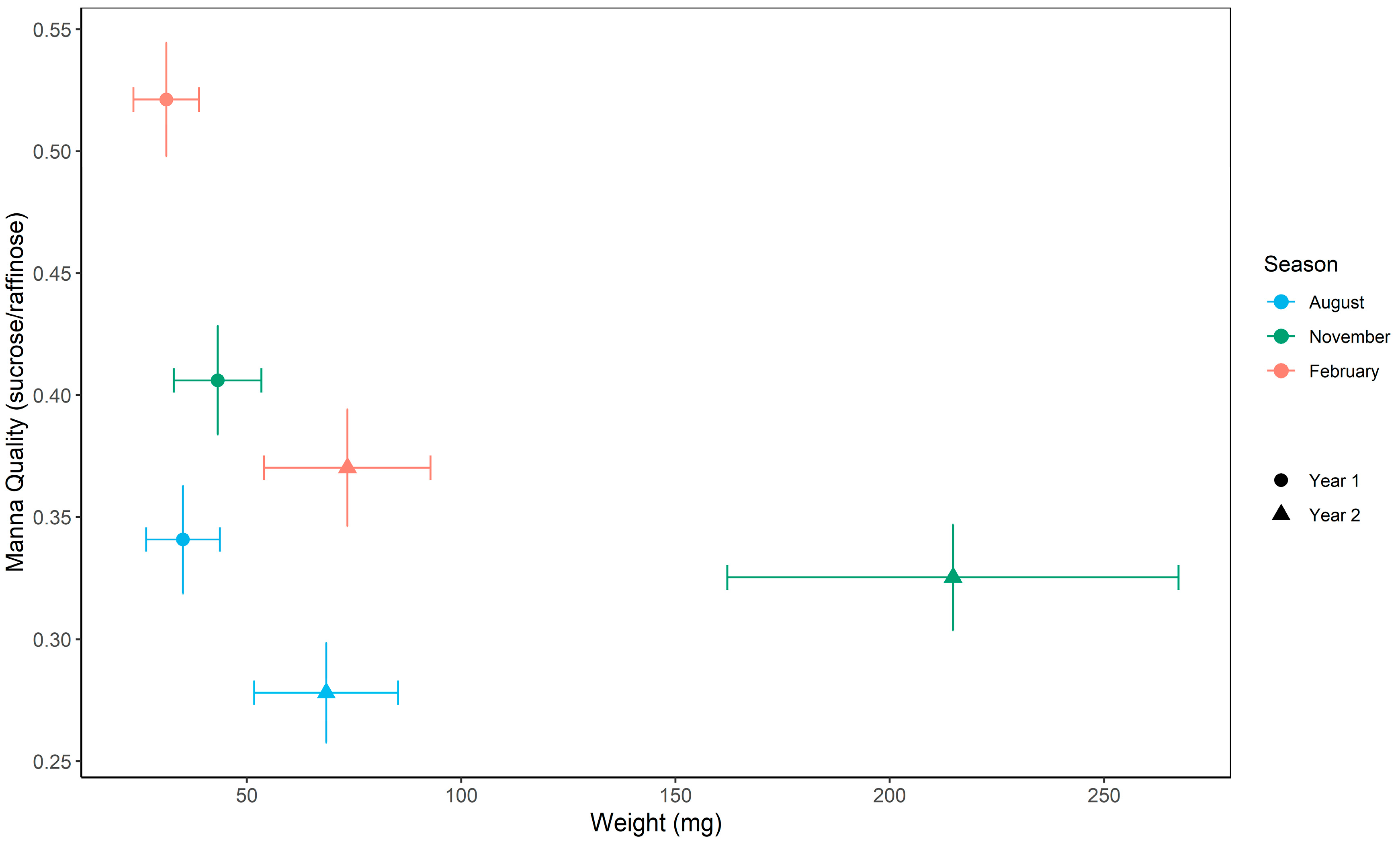
To investigate the extent of seasonal and annual variation in manna we ran linear mixed-effects models with manna quality (sucrose/raffinose) and quantity (weight mg) as response variables, sampling season (August, November, and February), year, and their interaction as fixed effects, and tree ID as a random variable. To account for the fact that we sampled multiple branches per tree within some sampling periods, we generated estimates of the mean manna quality and quantity per tree per sampling period.
The model for manna quality showed a non-significant within-group deviation with Levene’s test for homogeneity. Further investigations identified a single individual (tree 18) as the cause of this deviation. We then ran the model with and without tree 18 and found no difference in the significance or interpretation of the relationship; thus, the dataset inclusive of tree 18 was utilized.
To explore whether the seasonal variation we observed was related to climatic variation, we calculated a proxy for aridity based on the annual heat moisture index (AHM). The AHM has previously been used to examine climate-based variation in
Eucalyptus and is known to show associations with plant traits such as height and DBH [
43,
65,
66].
Here, we calculated a monthly heat moisture index per sampling period using the mean rainfall (mm) and mean highest temperature (°C) [TempH] for the month of sampling, extracted from the Bureau of Meteorology “Climate Data” portal (Commonwealth of Australia 2022). The monthly heat moisture index was calculated as:
To explore seasonal variation in manna, we correlated the monthly heat moisture index against manna quality and quantity by performing a Kendall’s rank correlation using the corr.test function. Given the sample size (six sampling events across two years), the results should be interpreted with caution.
Least-squares means (LSMs) and standard errors were extracted from the final model using the emmeans package in R [
44] and used to visualize seasonal and annual trends in manna quality and quantity, (see Results,
Figure 4).