Blue–Red LED Light Modulates Morphophysiological and Metabolic Responses in the Medicinal Plant Nepeta nuda
Abstract
1. Introduction
2. Results
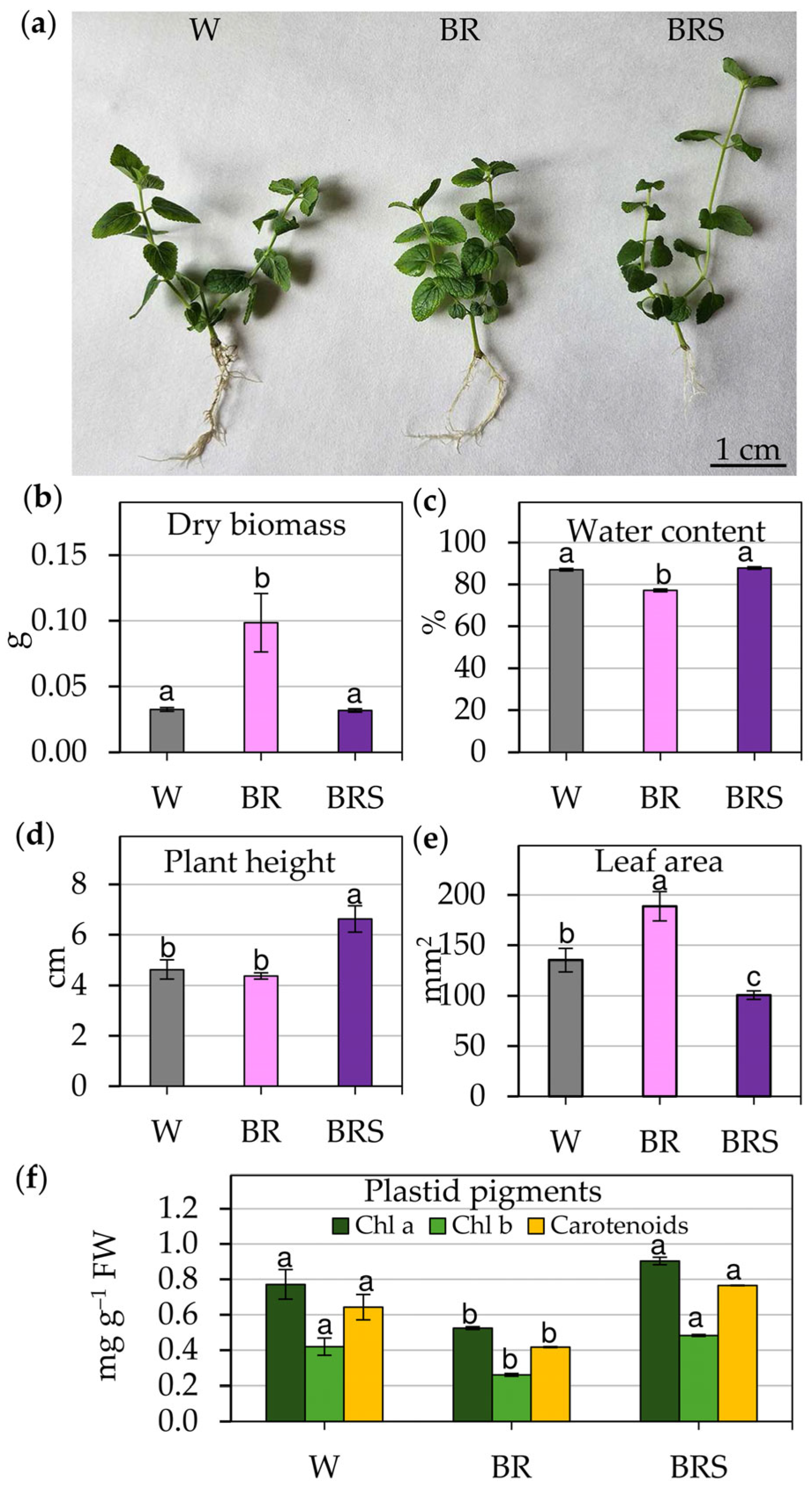
2.1. Influence of Varied Light Regimes on Growth Parameters of In Vitro Cultivated Plants
2.2. Evaluation of Physiological Changes in In Vitro Cultivated Plants Under Different Light Regimes
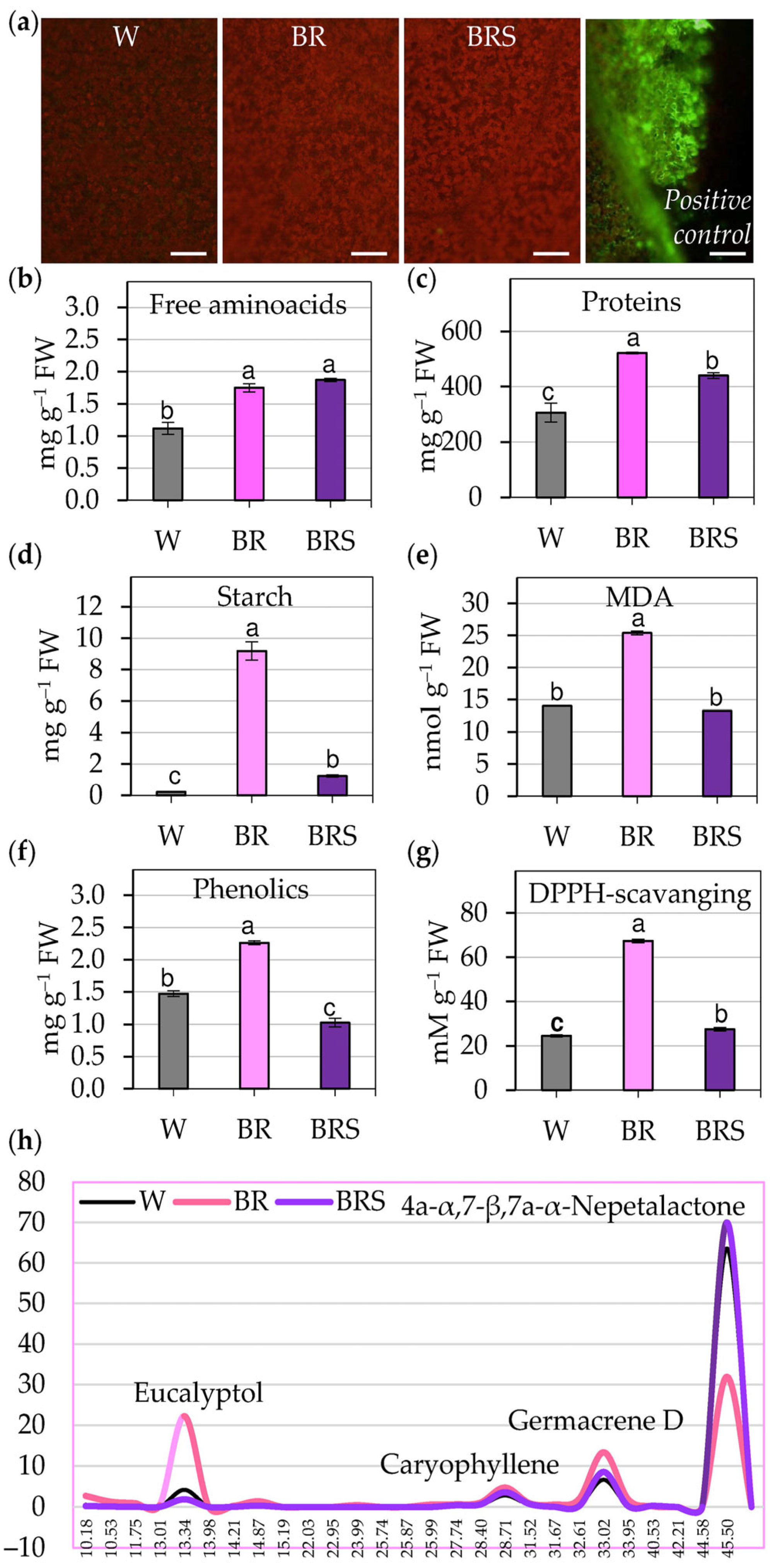
2.2.1. Oxidative State and Metabolic Content
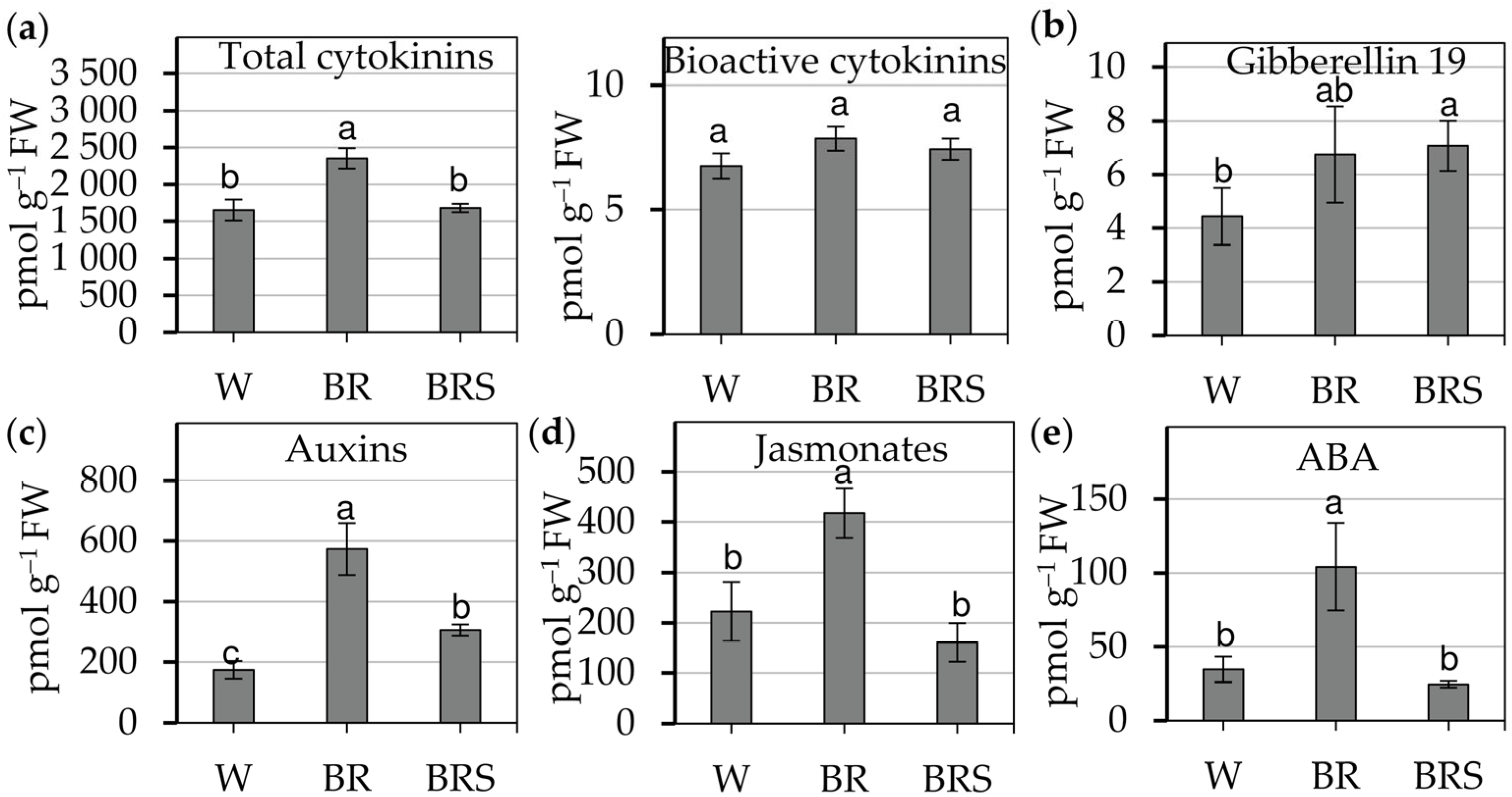
2.2.2. Light-Induced Changes in Endogenous Phytohormones
2.3. Potential for Ex Vitro Adaptation
2.3.1. Leaf Histological Parameters
2.3.2. Leaf Epidermis
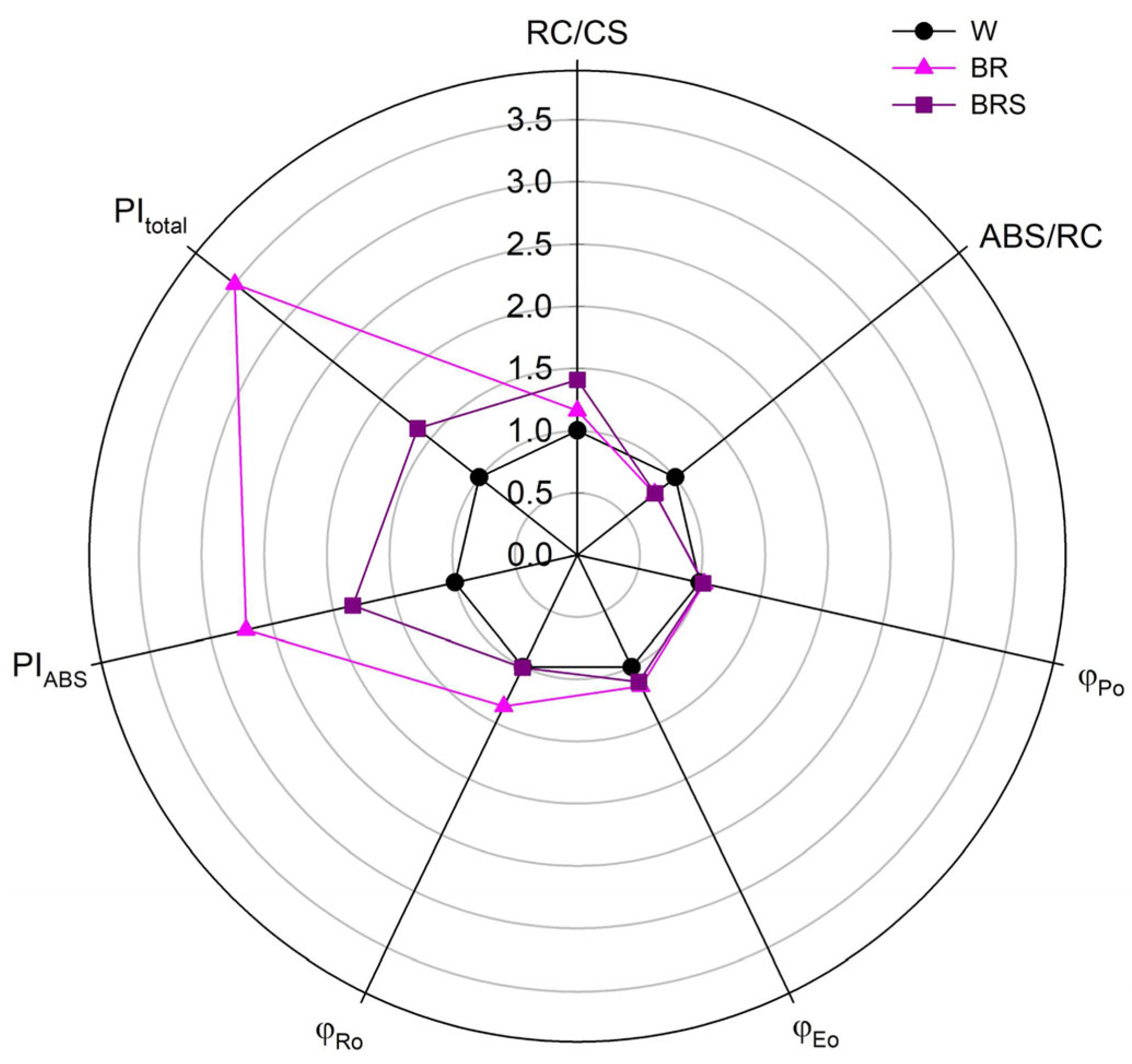
2.3.3. Photosynthetic Efficiency
3. Discussion
3.1. Oxidative Stress Caused by BR Light Triggers Antioxidant Response of N. nuda
3.2. Phytochemical Adjustment to Blue–Red Light
3.3. Blue–Red Light Modulates Leaf Structure and Enhances N. nuda Resilience to Stress
4. Materials and Methods
4.1. Plant Material
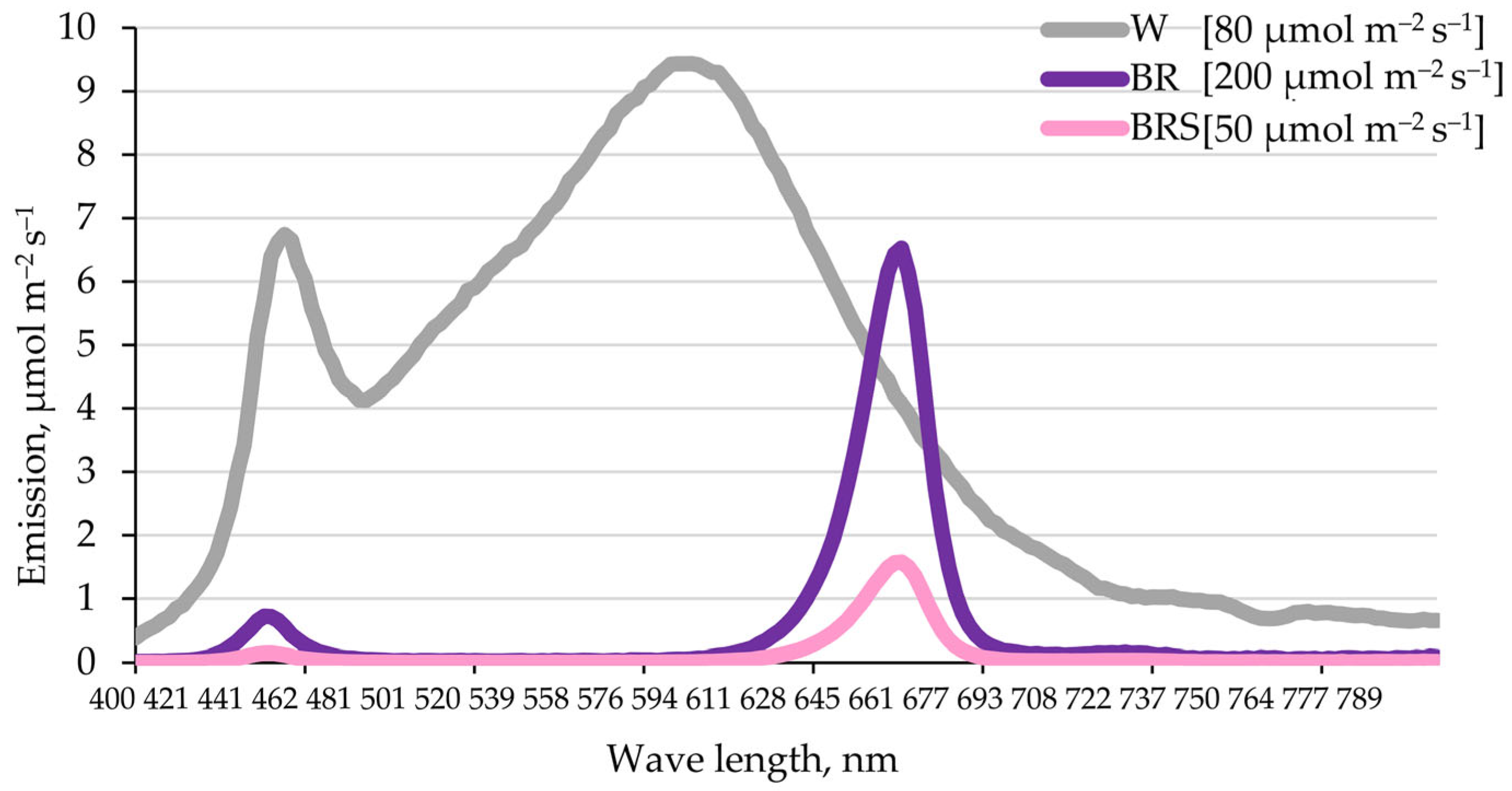
4.2. Light Regimes and Treatment
4.3. Morphometric Parameters
4.4. Localization of ROS in Plants
4.5. Simultaneous Extraction and Analysis of Metabolites
4.5.1. Plastid Pigments
4.5.2. Amino Acids
4.5.3. Proteins
4.5.4. Soluble Sugars
4.5.5. Starch
4.5.6. MDA
4.5.7. Total Phenolics
4.5.8. Antioxidant Activity
4.6. GC-MS Analysis of Volatile Compounds
4.7. Plant Hormone Profiling
4.8. Light Anatomy and Epidermis
4.9. Ex Vitro Adaptation
4.10. Chlorophyll a Fluorescence
4.11. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| BR | Blue–red light with high intensity |
| BRS | Blue–red light with low intensity (“BR with shadow”) |
| DCF-DA | Dichlorodihydrofluorescein diacetate |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| GC/MS | Gas chromatography/mass spectrometry |
| LED | Light-emitting diode |
| MDA | Malondialdehyde |
| PAR | Photosynthetically active radiation |
| ROS | Reactive oxygen species |
| W | White light |
References
- Kozai, T.; Fujiwara, K.; Runkle, E.S. LED Lighting for Urban Agriculture, 1st ed.; Springer: Singapore, 2016. [Google Scholar]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhiponova, M.; Paunov, M.; Anev, S.; Petrova, N.; Krumova, S.; Raycheva, A.; Goltsev, V.; Tzvetkova, N.; Taneva, S.; Sapunov, K.; et al. Special issue in honour of Prof. Reto J. Strasser—JIP-test as a tool for early diagnostics of plant growth and flowering upon selected light recipe. Photosynthetica 2020, 58, 399–408. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, X.; Feng, C.-H.; Niu, M.-X.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. Light and light signals regulate growth and development in woody plants. Forests 2024, 15, 523. [Google Scholar] [CrossRef]
- Wei, C.; Luo, G.; Jin, Z.; Li, J.; Li, Y. Physiological and structural changes in leaves of Platycrater arguta seedlings exposed to Increasing Light Intensities. Plants 2024, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A. Effects of PAR and UV-B radiation on herbal yield, bioactive compounds and their antioxidant capacity of some medicinal plants under controlled environmental conditions. Photochem. Photobiol. 2013, 89, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Schilisting, T.; Sá, A.C.S.; da Silva Filho, D.P.; da Silva, V.M.; Navroski, M.C.; de Oliveira Pereira, M.; Nascimento, B.; Moraes, C.; de Andrade, R.S.; Estopa, R.A.; et al. Developmental and Physiological Effects of the Light Source and Cultivation Environment on Mini Cuttings of Eucalyptus dunnii Maiden. Forests 2025, 16, 901. [Google Scholar] [CrossRef]
- Raffo, A.; Mozzanini, E.; Ferrari Nicoli, S.; Lupotto, E.; Cervelli, C. Effect of light intensity and water availability on plant growth, essential oil production and composition in Rosmarinus officinalis L. Eur. Food Res. Technol. 2019, 246, 167–177. [Google Scholar] [CrossRef]
- Yeddes, W.; Chalghoum, A.; Aidi-Wannes, W.; Ksouri, R.; Saidani Tounsi, M. Effect of bioclimatic area and season on phenolics and antioxidant activities of rosemary (Rosmarinus officinalis L.) leaves. J. Essent. Oil Res. 2019, 31, 432–443. [Google Scholar] [CrossRef]
- Kozłowska, W.; Matkowski, A.; Zielińska, S. Light intensity and temperature effect on Salvia yangii (B. T. Drew) Metabolic profile in vitro. Front. Plant Sci. 2022, 13, 888509. [Google Scholar] [CrossRef] [PubMed]
- Mišić, D.; Šiler, B.; Gašić, U.; Avramov, S.; Živković, S.; Živković, J.N.; Milutinović, M.; Tešić, Ž. Simultaneous UHPLC/DAD/(+/) HESI-MS/MS analysis of phenolic acids and nepetalactones in methanol extracts of Nepeta species: A possible application in chemotaxonomic studies. Phytochem. Anal. 2015, 26, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Aničić, N.; Matekalo, D.; Skorić, M.; Pećinar, I.; Brkušanin, M.; Nestorović Živković, J.; Dmitrović, S.; Dajić Stevanović, Z.; Schulz, H.; Mišić, D. Trichome-specific and developmentally regulated biosynthesis of nepetalactones in leaves of cultivated Nepeta rtanjensis plants. Ind. Crops Prod. 2018, 117, 347–358. [Google Scholar] [CrossRef]
- Nestorović, Ž.J.; Aničić, N.; Matekalo, D.; Skorić, M.; Filipović, B.; Marković, T.; Dmitrović, S. Polyethylene Glycol (PEG)-induced dehydration alters enzymatic and non-enzymatic components of the antioxidant defense system in Nepeta nervosa Royle ex Bentham. Horticulturae 2023, 9, 1277. [Google Scholar] [CrossRef]
- Petrova, D.; Gašić, U.; Yocheva, L.; Hinkov, A.; Yordanova, Z.; Chaneva, G.; Mantovska, D.; Paunov, M.; Ivanova, L.; Rogova, M.; et al. Catmint (Nepeta nuda L.) Phylogenetics and metabolic responses in variable growth conditions. Front. Plant Sci. 2022, 13, 866777. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, A.; Rusanov, K.; Rusanova, M.; Paunov, M.; Yordanova, Z.; Mantovska, D.; Tsacheva, I.; Petrova, D.; Mishev, K.; Dobrev, P.I.; et al. Uncovering the interrelation between metabolite profiles and bioactivity of in vitro- and wild-grown catmint (Nepeta nuda L.). Metabolites 2023, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Petrović, L.; Filipović, B.; Skorić, M.; Šiler, B.; Banjanac, T.; Matekalo, D.; Nestorović Živković, J.; Dmitrović, S.; Aničić, N.; Milutinović, M.; et al. Molecular background of the diverse metabolic profiles in leaves and inflorescences of naked catmint (Nepeta nuda L.). Front. Plant Sci. 2024, 15, 1452804. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.; Makowski, W.; Banasiuk, R.; Krolicka, A.; Piwowarczyk, B. Response of Dionaea muscipula J. Ellis to light stress in in vitro: Physiological study. Plant Cell Tiss. Organ Cult. 2018, 134, 65–77. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., 3rd. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Aničić, N.; Matekalo, D.; Skorić, M.; Živković, J.N.; Petrović, L.; Dragićević, M.; Dmitrović, S.; Mišić, D. Alterations in nepetalactone metabolism during polyethylene glycol (PEG)-induced dehydration stress in two Nepeta species. Phytochemistry 2020, 174, 112340. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Friends in arms: Flavonoids and the auxin/cytokinin balance in terrestrialization. Plants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of flowering time by environmental factors in plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Berman, A.; Shani, E. Plant hormone transport and localization: Signaling molecules on the move. Annu. Rev. Plant Biol. 2023, 74, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Lončar, B.; Pezo, M.; Stanković Jeremić, J.; Cvetković, M.; Rat, M.; Pezo, L. Volatile compounds of Nepeta nuda L. from Rtanj Mountain (Serbia). Horticulturae 2022, 8, 85. [Google Scholar] [CrossRef]
- Kobaisy, M.; Tellez, M.R.; Dayan, F.E.; Mamonov, L.K.; Mukanova, G.S.; Sitpaeva, G.T.; Gemejieva, N.G. Composition and phytotoxic activity of Nepeta pannonica L. essential oil. J. Essent. Oil Res. 2005, 17, 704–707. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D. Chemoinformatics approach to antibacterial studies of essential oils. Nat. Prod. Commun. 2015, 10, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C.; Doli, M.; Fietz, H.J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.J.; Hamilton, J.G.C.; Chapman, J.V.; Rhodes, M.J.C.; Hallahan, D.L. Analysis of monoterpenoids in glandular trichomes of the catmint Nepeta racemosa. Plant J. 1997, 11, 1387–1393. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Intern. 2004, 11, 36–42. [Google Scholar]
- Yakimova, E.T.; Yordanova, Z.P.; Slavov, S.; Kapchina-Toteva, V.M.; Woltering, E.J. Alternaria alternata AT toxin induces programmed cell death in tobacco. J. Phytopathol. 2009, 157, 592–601. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio Protoc. 2014, 8, e1108. Available online: https://www.bio-protocol.org/e1108 (accessed on 21 July 2025). [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The rainbow protocol: A sequential method for quantifying pigments, sugars, free amino acids, phenolics, flavonoids and MDA from a small amount of sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Chaneva, G.; Tomov, A.; Paunov, M.; Hristova, V.; Ganeva, V.; Mihaylova, N.; Anev, S.; Krumov, N.; Yordanova, Z.; Tsenov, B.; et al. Jewel orchid’s biology and physiological response to aquaponic water as a potential fertilizer. Plants 2022, 11, 3181. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Petrović, L.; Skorić, M.; Šiler, B.; Banjanac, T.; Gašić, U.; Matekalo, D.; Lukić, T.; Nestorović Živković, J.; Dmitrović, S.; Aničić, N.; et al. Patterns of genetic variation of Nepeta nuda L. from the Central Balkans: Understanding drivers of chemical diversity. Plants 2024, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
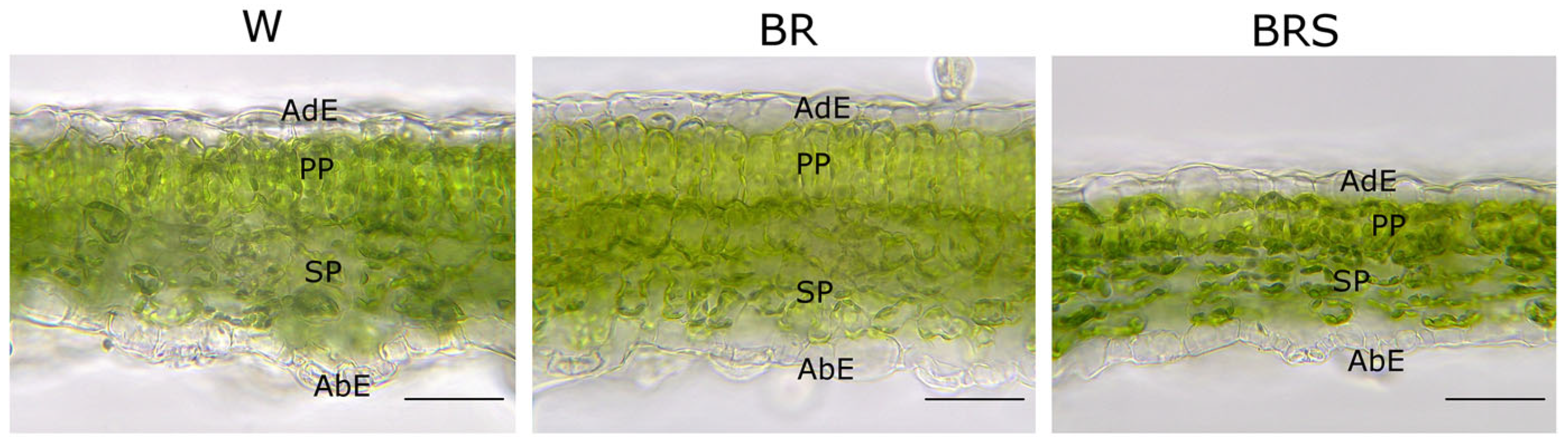
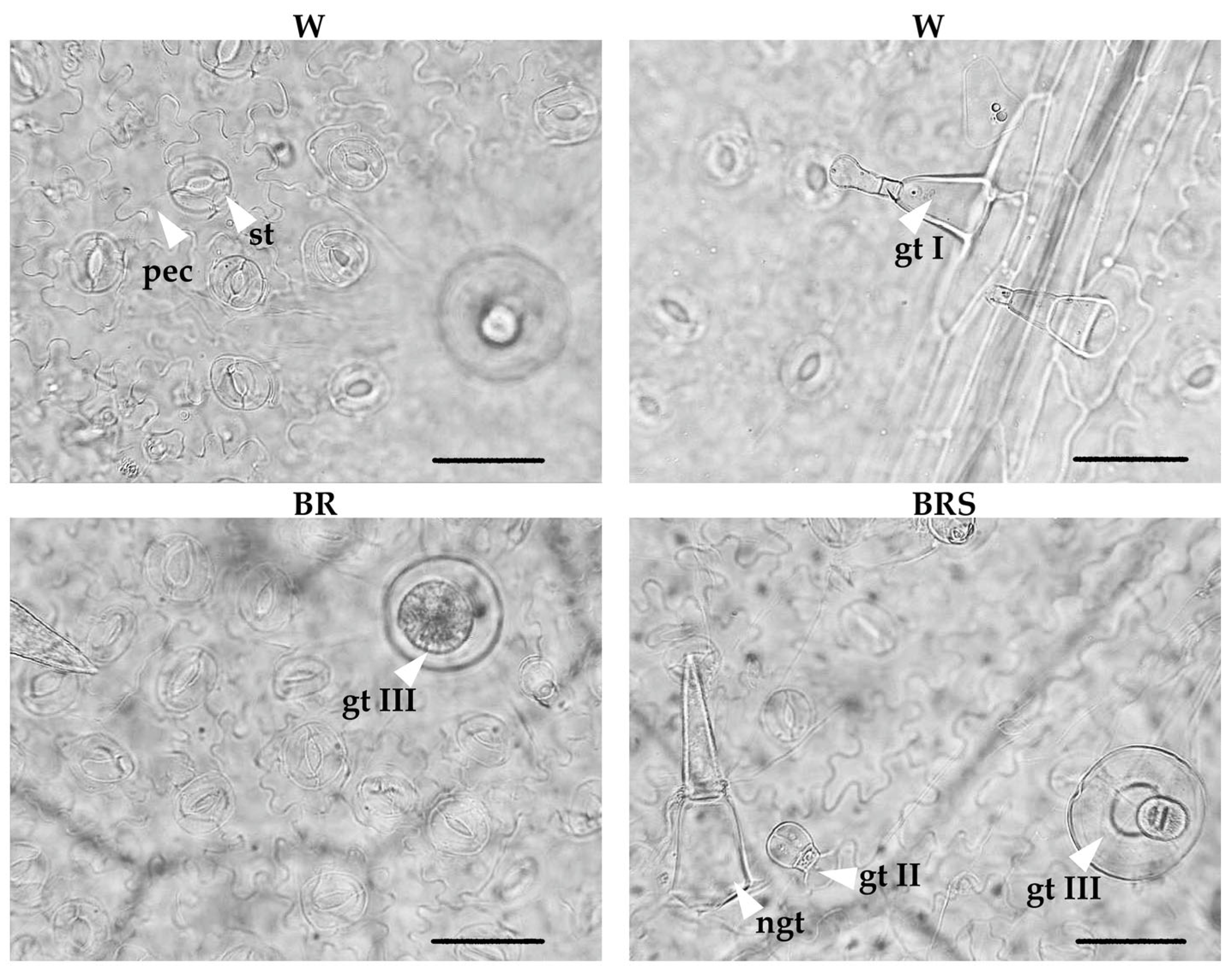
| Tissue Thickness, µm | W | BR | BRS |
|---|---|---|---|
| Leaf lamina | 97.8 ± 9.4 a | 120.8 ± 10.5 b | 82.2 ± 8.0 c |
| Mesophyll | 71.6 ± 11.3 a | 89.8 ± 11.9 b | 56.4 ± 6.8 c |
| Palisade parenchyma | 30.2 ± 5.5 a | 38.4 ± 4.1 b | 23.0 ± 4.0 c |
| Spongy parenchyma | 40.9 ± 5.3 a | 51.8 ± 8.1 b | 34.5 ± 6.2 c |
| Adaxial epidermis | 13.0 ± 4.2 a | 12.8 ± 2.7 a | 11.6 ± 3.0 a |
| Abaxial epidermis | 10.9 ± 2.1 a | 10.2 ± 2.3 a | 9.9 ± 1.8 a |
| Stomata Frequency, Number mm−2 | W | BR | BRS |
|---|---|---|---|
| Adaxial epidermis | 6.22 ± 9.56 b | 29.12 ± 20.33 a | 4.02 ± 5.56 b |
| Abaxial epidermis | 180.72 ± 31.64 b | 239.96 ± 33.60 a | 160.84 ± 30.56 b |
| Trichome Frequency, Number mm−2 | W | BR | BRS |
|---|---|---|---|
| Adaxial epidermis | |||
| Non-glandular | 5.62 ± 5.69 a | 5.82 ± 7.6 a | 5.62 ± 5.91 a |
| Glandular Type II | 9.84 ± 6.98 a | 14.66 ± 13.30 a | 9.84 ± 7.50 a |
| Glandular Type I | rare | rare | rare |
| Abaxial epidermis | |||
| Non-glandular | 5.62 ± 4.73 a | 9.24 ± 7.71 a | 7.03 ± 6.54 a |
| Glandular Type III | 4.62 ± 4.66 b | 8.23 ± 5.59 a | 4.42 ± 4.17 b |
| Glandular Type II | 13.45 ± 7.37 a | 18.67 ± 10.05 a | 14.66 ± 7.86 a |
| Glandular Type I | 3.01 ± 4.40 a | 0.60 ± 1.84 a | 1.81 ± 3.59 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhiponova, M.; Zehirov, G.; Rusanov, K.; Rusanova, M.; Stefanova, M.; Ganeva, T.; Paunov, M.; Ganeva, V.; Mishev, K.; Dobrev, P.I.; et al. Blue–Red LED Light Modulates Morphophysiological and Metabolic Responses in the Medicinal Plant Nepeta nuda. Plants 2025, 14, 2285. https://doi.org/10.3390/plants14152285
Zhiponova M, Zehirov G, Rusanov K, Rusanova M, Stefanova M, Ganeva T, Paunov M, Ganeva V, Mishev K, Dobrev PI, et al. Blue–Red LED Light Modulates Morphophysiological and Metabolic Responses in the Medicinal Plant Nepeta nuda. Plants. 2025; 14(15):2285. https://doi.org/10.3390/plants14152285
Chicago/Turabian StyleZhiponova, Miroslava, Grigor Zehirov, Krasimir Rusanov, Mila Rusanova, Miroslava Stefanova, Tsveta Ganeva, Momchil Paunov, Valentina Ganeva, Kiril Mishev, Petre I. Dobrev, and et al. 2025. "Blue–Red LED Light Modulates Morphophysiological and Metabolic Responses in the Medicinal Plant Nepeta nuda" Plants 14, no. 15: 2285. https://doi.org/10.3390/plants14152285
APA StyleZhiponova, M., Zehirov, G., Rusanov, K., Rusanova, M., Stefanova, M., Ganeva, T., Paunov, M., Ganeva, V., Mishev, K., Dobrev, P. I., Vaculíková, R., Motyka, V., Yordanova, Z., Chaneva, G., & Vassileva, V. (2025). Blue–Red LED Light Modulates Morphophysiological and Metabolic Responses in the Medicinal Plant Nepeta nuda. Plants, 14(15), 2285. https://doi.org/10.3390/plants14152285










