Molecular Mechanisms of Potato Plant–Virus–Vector Interactions
Abstract
1. Introduction
2. Characteristics of the Most Common Potato Viruses
2.1. Potato Virus Y (PVY)
2.2. Potato Leaf Roll Virus (PLRV)
2.3. Potato Virus X (PVX)
3. Viral Strategies for Successful Transmission via Vectors
4. Vector–Host Interactions in Plant Virus Transmission
5. Potato Plant Responses to Virus Infections: Defense Mechanisms and Physiological Changes
5.1. General Defense Mechanisms
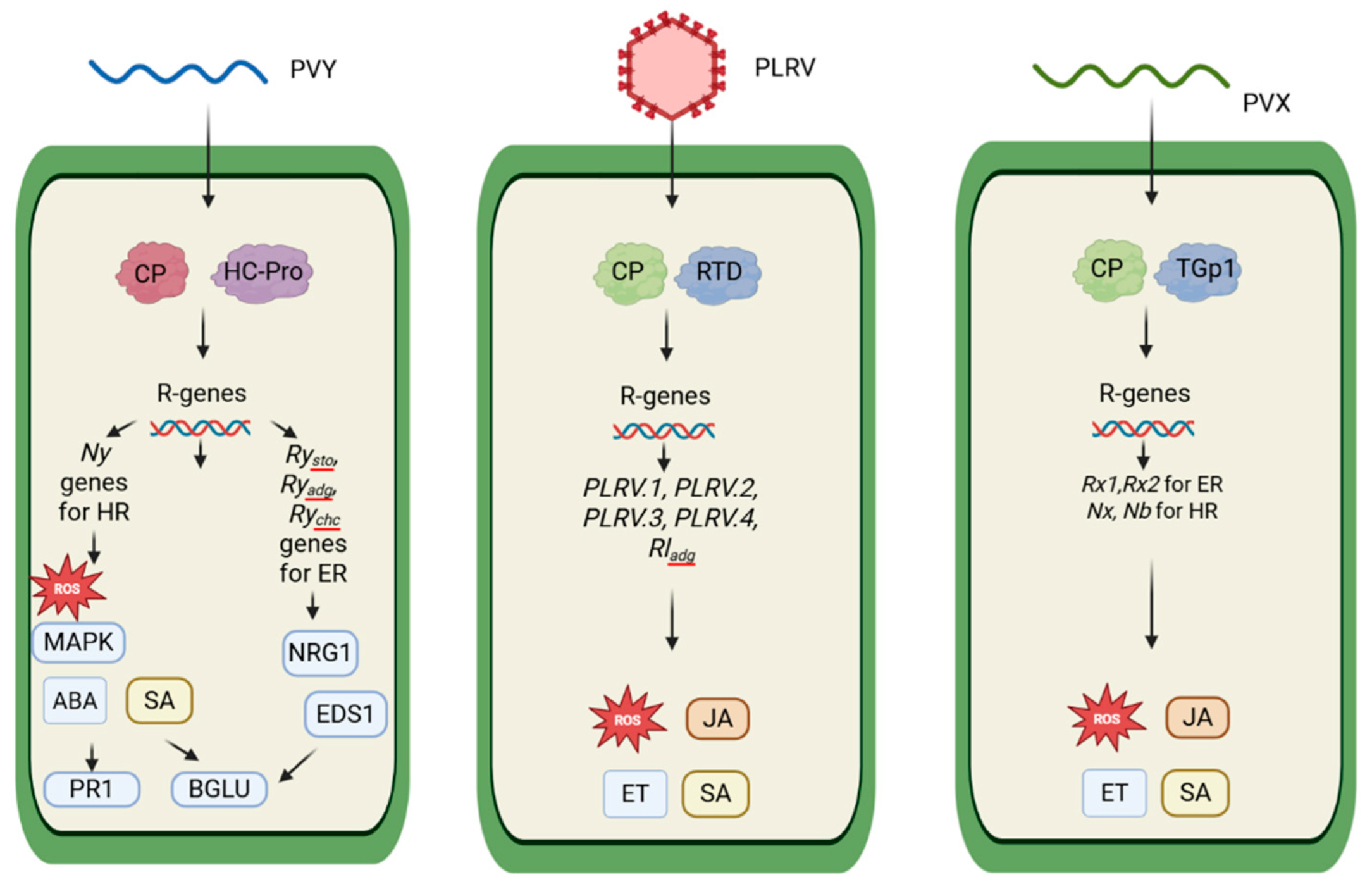
5.2. Defense Against PVY
5.3. Defense Against PLRV
5.4. Defense Against PVX
6. Future Strategies and Research Priorities in the Context of Potato Virus Management Through Manipulation of the Virus–Vector–Host System
Author Contributions
Funding
Conflicts of Interest
References
- Spooner, D.M.; McLean, K.; Ramsay, G.; Waugh, R.; Bryan, G.J. A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proc. Natl. Acad. Sci. USA 2005, 102, 14694–14699. [Google Scholar] [CrossRef] [PubMed]
- Spooner, D.M. The potato: Evolution, biodiversity and genetic resources. Am. J. Pot Res. 1990, 67, 733–735. [Google Scholar] [CrossRef]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for sustainable global food security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.P.; Petsakos, A.; Kromann, P.; Gatto, M.; Okello, J.; Suarez, V.; Hareau, G. Global food security, contributions from sustainable potato agri-food systems. In The Potato Crop, 2nd ed.; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–36. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 February 2025).
- Fox, A.; Collins, L.E.; Macarthur, R.; Blackburn, L.F.; Northing, P. New aphid vectors and efficiency of transmission of potato virus A and strains of potato virus Y in the UK. Plant Pathol. 2017, 66, 325–335. [Google Scholar] [CrossRef]
- Korkmaz, G.; Usta, M.; Güller, A.; Demirel, S. Comprehensive survey of common potato viruses in Eastern Anatolia Region of Turkey: New isolates and phylogenetic insights. Potato Res. 2025. [CrossRef]
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral diseases in potato. In The Potato Crop, 2nd ed.; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020; pp. 389–430. [Google Scholar] [CrossRef]
- Loebenstein, G.; Manadilova, A. Potatoes in the Central Asian Republics. In Virus and Virus-like Diseases of Major Crops in Developing Countries, 2nd ed.; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 113–126. [Google Scholar] [CrossRef]
- Petrov, N.M.; Stoyanova, M.I.; Gaur, R.K. Biodiversity and characterization of economically important viruses on potato cultivars. In Plant RNA Viruses, 2nd ed.; Gaur, R.K., Patil, B.L., Selvarajan, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 12; pp. 245–270. [Google Scholar] [CrossRef]
- Hameed, A.; Iqbal, Z.; Asad, S.; Mansoor, S. Detection of multiple potato viruses in the field suggests synergistic interactions among potato viruses in Pakistan. Plant Pathol. J. 2014, 30, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.F. Potato Viruses and Their Control, 3rd ed.; Intern. Potato Center (CIP): Lima, Peru, 1996; p. 214. [Google Scholar]
- Adilbayeva, K.; Moisseyev, R.; Kolchenko, M.; Kenzhebekova, R.; Khassanov, V.; Beisembina, B.; Azhimakhan, M.; Tokbergenova, Z.; Sharipova, D.; Krasavin, V.; et al. Genetic evaluation of Kazakhstani potato germplasm for pathogen and pest resistance using DNA markers. Agronomy 2024, 14, 1923. [Google Scholar] [CrossRef]
- Chikh-Ali, M.; Karasev, A.V. Virus diseases of potato and their control. In Potato Production Worldwide, 1st ed.; Caliskan, M.E., Bakhsh, A., Jabran, K., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 11; pp. 199–212. [Google Scholar] [CrossRef]
- Awasthi, L.P.; Verma, H.N. Current status of viral diseases of potato and their ecofriendly management—A critical review. Virol. Res. Rev. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Priegnitz, U.; Lommen, W.J.; van der Vlugt, R.A.; Struik, P.C. Impact of positive selection on incidence of different viruses during multiple generations of potato seed tubers in Uganda. Potato Res. 2019, 62, 1–30. [Google Scholar] [CrossRef]
- Kumar, P.; Cowan, G.H.; Squires, J.N.; Hackett, C.A.; Tobin, A.K.; Torrance, L.; Roberts, A.G. Phloem connectivity and transport are not involved in mature plant resistance (MPR) to Potato Virus Y in different potato cultivars, and MPR does not protect tubers from recombinant strains of the virus. J. Plant Physiol. 2022, 275, 153729. [Google Scholar] [CrossRef] [PubMed]
- Basky, Z.; Almási, A. Differences in aphid transmissibility and translocation between PVYN and PVYO isolates. J. Pest Sci. 2005, 78, 67–75. [Google Scholar] [CrossRef]
- Hohn, T. Plant virus transmission from the insect point of view. Proc. Natl. Acad. Sci. USA 2007, 104, 17905–17906. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Hasegawa, D.K.; Ling, K.S.; Wintermantel, W.M. Application of genomics for understanding plant virus–insect vector interactions and insect vector control. Phytopathology 2016, 106, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Dara, M.Z.N.; Abbas, A.; Temitope, A.; Li, L.; Duan, G.; Sun, W. Plant-pathogen interactions and transmissions: Unraveling the complex role of pathogen vectors in disease ecology. J. Integr. Agric. 2025. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Perry, K.L. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004, 5, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.F. Sternorrhynchous vectors of plant viruses: Virus–vector interactions and transmission mechanisms. In Advances in Virus Research, 2nd ed.; Lauffer, M.A., Maramorosch, K., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 28, pp. 113–140. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus–insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Krishnareddy, M. Impact of climate change on insect vectors and vector-borne plant viruses and phytoplasma. In Climate-Resilient Horticulture: Adaptation and Mitigation Strategies, 2nd ed.; Singh, H., Rao, N., Shivashankar, K., Eds.; Springer New Delhi: New Delhi, India, 2013; pp. 327–338. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Islam, S.S.; Adhikary, S.; Sannal, A.; Hosen, A.E.; et al. Plant disease dynamics in a changing climate: Impacts, molecular mechanisms, and climate-informed strategies for sustainable management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Ahirwar, N.K.; Pachaya, J.S. Effects of climate change on the spread and severity of Potato Virus Y: An in-depth examination. Asian J. Microbiol. Biotechnol. 2024, 9, 39–59. [Google Scholar] [CrossRef]
- Tsai, W.-A.; Brosnan, C.A.; Mitter, N.; Dietzgen, R.G. Perspectives on plant virus diseases in a climate change scenario of elevated temperatures. Stress Biol. 2022, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Amari, K.; Huang, C.; Heinlein, M. Potential impact of global warming on virus propagation in infected plants and agricultural productivity. Front. Plant Sci. 2021, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A. Future scenarios for plant virus pathogens as climate change progresses. Adv. Virus Res. 2016, 95, 87–147. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Temperature-dependent effects on the replication and transmission of arthropod-borne viruses in their insect hosts. Curr. Opin. Insect Sci. 2016, 16, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Skelsey, P. Landscape-scale patterns and predictors of potato viruses in Scotland. Plant Pathol. 2024, 73, 1553–1572. [Google Scholar] [CrossRef]
- Davis, J.A.; Radcliffe, E.B.; Ragsdale, D.W.; MacRae, I. Increasing in-row spacing enhances Potato Virus Y and Potato Leafroll Virus spread in potato. Am. J. Potato Res. 2015, 92, 497–501. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation management in potato (Solanum tuberosum L.) production: A review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
- Byarugaba, A.A.; Mukasa, S.B.; Barekye, A.; Rubaihayo, P.R. Interactive effects of Potato Virus Y and Potato Leafroll Virus infection on potato yields in Uganda. Open Agric. 2020, 5, 726–739. [Google Scholar] [CrossRef]
- McLaughlin, A.A.; Hanley-Bowdoin, L.; Kennedy, G.G.; Jacobson, A.L. Vector acquisition and co-inoculation of two plant viruses influences transmission, infection, and replication in new hosts. Sci. Rep. 2022, 12, 20355. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Bhoi, T.K.; Samal, I.; Majhi, P.K.; Komal, J.; Mahanta, D.K.; Pradhan, A.K.; Saini, V.; Raj, M.N.; Ahmad, M.A.; Behera, P.P.; et al. Insight into aphid mediated Potato Virus Y transmission: A molecular to bioinformatics prospective. Front. Microbiol. 2022, 13, 1001454. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.; Kutnjak, D.; Xu, Y.; Xu, Y.; Giovannoni, J.; Elena, S.F.; Gray, S.; Wang, A. Transmission modes affect the population structure of potato virus Y in potato. PLoS Pathog. 2020, 16, e1008608. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Talianksy, M.E. Potato Virus Y emergence and evolution from the Andes of South America to become a major destructive pathogen of potato and other Solanaceous crops worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef] [PubMed]
- Valli, A.A.; Gallo, A.; Rodamilans, B.; López-Moya, J.J.; García, J.A. The HCPro from the Potyviridae family: An enviable multitasking helper component that every virus would like to have. Mol. Plant Pathol. 2018, 19, 744–763. [Google Scholar] [CrossRef] [PubMed]
- Taliansky, M.; Mayo, M.A.; Barker, H. Potato leafroll virus: A classic pathogen shows some new tricks. Mol. Plant Pathol. 2003, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Kaplan, I.B.; Ripoll, D.R.; Liang, D.; Palukaitis, P.; Gray, S.M. A surface loop of the Potato Leafroll Virus coat protein is involved in virion assembly, systemic movement, and aphid transmission. J. Virol. 2005, 79, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.M.; Srinivasan, R. Evaluation of hairy nightshade as an inoculum source for aphid-mediated transmission of Potato Leafroll Virus. J. Econ. Entomol. 2005, 98, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.A.; Gildow, F.; Palukaitis, P.; Gray, S.M. The C terminus of the polerovirus P5 readthrough domain limits virus infection to the phloem. J. Virol. 2009, 83, 5419–5429. [Google Scholar] [CrossRef] [PubMed]
- Mayo, M.A.; Ziegler-Graff, V. Molecular biology of luteoviruses. Adv. Virus Res. 1996, 46, 413–460. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.J.; Linthorst, H.J.; Bol, J.F.; Cornelissen, B.J. The complete nucleotide sequence of Potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J. Gen. Virol. 1988, 69, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Fang, Y.; Zhang, L.; Yu, K.; Wu, X.; Cheng, X. Evaluation of Potato virus X resistance in potato cultivars and identification of an innate immunity-independent resistance phenotype. Phytopathol. Res. 2021, 3, 21. [Google Scholar] [CrossRef]
- Verchot, J. Potato virus X: A global potato-infecting virus and type member of the Potexvirus genus. Mol. Plant Pathol. 2022, 23, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato virus Y: A major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000862905.1/ (accessed on 1 February 2025).
- Howard, A.R.; Heppler, M.L.; Ju, H.-J.; Krishnamurthy, K.; Payton, M.E.; Verchot-Lubicz, J. Potato virus X TGBp1 induces plasmodesmata gating and moves between cells in several host species whereas CP moves only in N. benthamiana leaves. Virology 2004, 328, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Léonard, S.; Plante, D.; Wittmann, S.; Daigneault, N.; Fortin, M.G.; Laliberté, J.-F. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 2000, 74, 7730–7737. [Google Scholar] [CrossRef] [PubMed]
- Eskelin, K.; Hafrén, A.; Rantalainen, K.I.; Mäkinen, K. Potyviral VPg enhances viral RNA translation and inhibits reporter mRNA translation in planta. J. Virol. 2011, 85, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Olmedo-Velarde, A.; Wilson, J.R.; Stallone, M.; DeBlasio, S.L.; Chappie, J.S.; Heck, M. Potato leafroll virus molecular interactions with plants and aphids: Gaining a new tactical advantage on an old foe. Physiol. Mol. Plant Pathol. 2023, 125, 102015. [Google Scholar] [CrossRef]
- Schaad, M.C.; Jensen, P.E.; Carrington, J.C. Formation of plant RNA virus replication complexes on membranes: Role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997, 16, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-J.; Samuels, T.D.; Wang, Y.-S.; Blancaflor, E.; Payton, M.; Mitra, R.; Krishnamurthy, K.; Nelson, R.S.; Verchot-Lubicz, J. The Potato virus X TGBp2 movement protein associates with endoplasmic reticulum-derived vesicles during virus infection. Plant Physiol. 2005, 138, 1877–1895. [Google Scholar] [CrossRef] [PubMed]
- Atabekov, J.; Dobrov, E.; Karpova, O.; Rodionova, N. Potato virus X: Structure, disassembly and reconstitution. Mol. Plant Pathol. 2007, 8, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zhang, X.; Wang, Y.; Li, D.; Yu, J.; Han, C. The three essential motifs in P0 for suppression of RNA silencing activity of Potato leafroll virus are required for virus systemic infection. Viruses 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Wu, X.; Liu, J.; Fang, Y.; Luan, Y.; Cui, X.; Zhou, X.; Wang, A.; Cheng, X. P3N-PIPO interacts with P3 via the shared N-terminal domain to recruit viral replication vesicles for cell-to-cell movement. J. Virol. 2020, 94, e01898-19. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhang, C.; Hong, J.; Xiong, R.; Kasschau, K.D.; Zhou, X.; Carrington, J.C.; Wang, A.; Manchester, M. Formation of complexes at plasmodesmata for potyvirus intercellular movement is mediated by the viral protein P3N-PIPO. PLoS Pathog. 2010, 6, e1000962. [Google Scholar] [CrossRef] [PubMed]
- Vijayapalani, P.; Maeshima, M.; Nagasaki-Takekuchi, N.; Miller, W.A. Interaction of the trans-frame potyvirus protein P3N-PIPO with host protein PCaP1 facilitates potyvirus movement. PLoS Pathog. 2012, 8, e1002639. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, B. The movement of potato virus Y (PVY) in the vascular system of potato plants. Eur. J. Plant Pathol. 2017, 147, 365–373. [Google Scholar] [CrossRef]
- Xu, Y.; Da Silva, W.L.; Qian, Y.; Gray, S.M. An aromatic amino acid and associated helix in the C-terminus of the Potato leafroll virus minor capsid protein regulate systemic infection and symptom expression. PLoS Pathog. 2018, 14, e1007451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gray, S.M. Aphids and their transmitted potato viruses: A continuous challenge in potato crops. J. Integr. Agric. 2020, 19, 367–375. [Google Scholar] [CrossRef]
- Gray, S.; De Boer, S.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato virus Y: An evolving concern for potato crops in the United States and Canada. Plant Dis. 2010, 94, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, Y.; Mubeen, M.; Sajid, A.A.; Abbas, A.; Umer, M.; Usman, H.; Iqbal, S.; Moosa, A.; Anwaar, H.; Kiptoo, J. Aphid-borne Potato virus Y (PVY) is an emerging disease of potatoes in Punjab, Pakistan. J. Entomol. Zool. Stud. 2020, 8, 2427–2433. [Google Scholar]
- Patton, M.F.; Bak, A.; Sayre, J.M.; Heck, M.L.; Casteel, C.L. A polerovirus, Potato leafroll virus, alters plant–vector interactions using three viral proteins. Plant Cell Environ. 2020, 43, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.W.; Payne, R.W.; Katis, N.I. The transmission of Potato Virus Y by aphids of different vectoring abilities. Ann. Appl. Biol. 1988, 113, 35–43. [Google Scholar] [CrossRef]
- Khelifa, M. Detection and quantification of Potato virus Y genomes in single aphid stylets. Plant Dis. 2019, 103, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.; Ahmad, M.W.; Basit, A.; Ullah, S.; Mohamed, H.I.; Nisar, N.; Khan, A. Plant growth-promoting rhizobacteria and their applications and role in the management of soilborne diseases. In Nanobiotechnology for Plant Protection Bacterial Secondary Metabolites, 1st ed.; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 59–82. [Google Scholar] [CrossRef]
- Atreya, C.D.; Pirone, T.P. Mutational analysis of the helper component-proteinase gene of a potyvirus: Effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proc. Natl. Acad. Sci. USA 1993, 90, 11919–11923. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, A. Plum pox virus 6K1 protein is required for viral replication and targets the viral replication complex at the early stage of infection. J. Virol. 2016, 90, 5119–5131. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Laín, S.; García, J.A. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 1992, 73, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wu, Z.; Wang, A. The multifunctional protein CI of potyviruses plays interlinked and distinct roles in viral genome replication and intercellular movement. Virol. J. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Levay, K.; Murphy, J.F.; Klein, P.G.; Shaw, J.G.; Hunt, A.G. A potyvirus polymerase interacts with the viral coat protein and VPg in yeast cells. Virology 1995, 214, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rodamilans, B.; Shan, H.; Pasin, F.; García, J.A. Plant viral proteases: Beyond the role of peptide cutters. Front. Plant Sci. 2018, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Valdez, P.; Olvera, R.E.; Carrington, J.C. Functions of the tobacco etch virus RNA polymerase (NIb): Subcellular transport and protein–protein interaction with VPg/proteinase (NIa). J. Virol. 1997, 71, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Shi, Y.; Dai, Z.; Wang, A. The RNA-dependent RNA polymerase NIb of potyviruses plays multifunctional, contrasting roles during viral infection. Viruses 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Jia, M.; Shan, H.; Gao, W.; Jiang, L.; Cui, H.; Cheng, X.; Uzest, M.; Zhou, X.; Wang, A.; et al. Viral RNA polymerase as a SUMOylation decoy inhibits RNA quality control to promote potyvirus infection. Nat. Commun. 2025, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Gong, M.; Jiao, Y.; Li, Y.; Shen, L.; Li, B.; Wang, Y.; Wang, F.; Zhang, S.; Yang, J. Serratia marcescens-S3 inhibits Potato virus Y by activating ubiquitination of molecular chaperone proteins NbHsc70-2 in Nicotiana benthamiana. Microb. Biotechnol. 2022, 15, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Manasseh, R.; Sathuvalli, V.; Pappu, H.R. Transcriptional and functional predictors of Potato virus Y-induced tuber necrosis in potato (Solanum tuberosum). Front. Plant Sci. 2024, 15, 1369846. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Karasev, A.V.; Brown, C.J.; Lorenzen, J.H. Sequence characteristics of Potato virus Y recombinants. J. Gen. Virol. 2009, 90, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Gray, S.M. Genetic diversity of Potato virus Y complex. Am. J. Potato Res. 2013, 90, 7–13. [Google Scholar] [CrossRef]
- Shrestha, D.; Wenninger, E.J.; Hutchinson, P.J.S.; Whitworth, J.L.; Mondal, S.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Interactions among potato genotypes, growth stages, virus strains, and inoculation methods in the Potato virus Y and green peach aphid pathosystem. Environ. Entomol. 2014, 43, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Piche, L.M.; Singh, R.P.; Nie, X.; Gudmestad, N.C. Diversity among Potato virus Y isolates obtained from potatoes grown in the United States. Phytopathology 2004, 94, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Nikolaeva, O.V.; Hu, X.; Sielaff, Z.; Whitworth, J.; Lorenzen, J.H.; Gray, S.M. Serological properties of ordinary and necrotic isolates of Potato virus Y: A case study of PVYN misidentification. Am. J. Potato Res. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Dupuis, B.; Bragard, C.; Schumpp, O. Resistance of potato cultivars as a determinant factor of Potato virus Y (PVY) epidemiology. Potato Res. 2019, 62, 123–138. [Google Scholar] [CrossRef]
- Gao, L.; Tuo, D.; Shen, W.; Yan, P.; Li, X.; Zhou, P. NIa-Pro of Papaya ringspot virus interacts with Carica papaya eukaryotic translation initiation factor 3 subunit G (CpeIF3G). Virus Genes 2015, 50, 97–103. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/datasets/taxonomy/12045/ (accessed on 1 February 2025).
- Prüfer, D.; Kawchuk, L.; Monecke, M.; Nowok, S.; Fischer, R.; Rohde, W. Immunological analysis of Potato leafroll luteovirus (PLRV) P1 expression identifies a 25 kDa RNA-binding protein derived via P1 processing. Nucleic Acids Res. 1999, 27, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, M.V.; Nikonova, E.Y.; Terentiev, A.A.; Taranov, V.V.; Babakov, A.V.; Nikonov, O.S. VPg of Potato virus Y and potato cap-binding eIF4E factors: Selective interaction and its supposed mechanism. Biochemistry 2021, 86, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, T.; Li, Y.Y.; Xiang, H.Y.; Wu, Z.Y.; Wang, X.B.; Wang, Y.; Zhang, Y.L.; Li, D.W.; Yu, J.L.; Han, C.G. Amino acid sequence motifs essential for P0-mediated suppression of RNA silencing in an isolate of Potato leafroll virus from Inner Mongolia. Mol. Plant Microbe Interact. 2014, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- van der Wilk, F. Potato Leafroll Virus: Molecular Analysis and Genetically Engineered Resistance. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhao, T.-Y.; Li, Y.-Y.; Xiang, H.-Y.; Dong, S.-W.; Zhang, Z.-Y.; Wang, Y.; Li, D.-W.; Yu, J.-L.; Han, C.-G. The conserved Proline18 in the Polerovirus P3a is important for Brassica yellows virus systemic infection. Front. Microbiol. 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaundal, P.; Tiwari, R.K.; Lal, M.K.; Kumari, H.; Kumar, R.; Naga, K.C.; Kumar, A.; Singh, B.; Sagar, V.; et al. Development of reverse transcription recombinase polymerase amplification (RT-RPA): A methodology for quick diagnosis of Potato leafroll viral disease in potato. Int. J. Mol. Sci. 2023, 24, 2511. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Chen, I.H.; Hou, P.Y.; Wang, L.H.; Tsai, C.H.; Cheng, C.P. The phosphorylation of the movement protein TGBp1 regulates the accumulation of the Bamboo mosaic virus. J. Gen. Virol. 2024, 105, 001945. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.Y.; Solovyev, A.G. Triple gene block: Modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 2003, 84, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Zayakina, O.; Arkhipenko, M.; Kozlovsky, S.; Nikitin, N.; Smirnov, A.; Susi, P.; Rodionova, N.; Karpova, O.; Atabekov, J. Mutagenic analysis of Potato virus X movement protein (TGBp1) and the coat protein (CP): In vitro TGBp1-CP binding and viral RNA translation activation. Mol. Plant Pathol. 2008, 9, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Falk, B.W. Virus–vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 2006, 44, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Li, H.; Schneider, W.L.; Sherman, D.J.; Gray, S.; Smith, D.; Roossinck, M.J. Analysis of genetic bottlenecks during horizontal transmission of cucumber mosaic virus. J. Virol. 2006, 80, 8249–8258. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Moreno, A.; Fereres, A. Semipersistently transmitted, phloem-limited plant viruses are inoculated during the first subphase of intracellular stylet penetrations in phloem cells. Viruses 2021, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Shalileh, S.; Ogada, P.A.; Moualeu, D.P.; Poehling, H.M. Manipulation of Frankliniella occidentalis (Thysanoptera: Thripidae) by Tomato spotted wilt virus (Tospovirus) via the host plant nutrients to enhance its transmission and spread. Environ. Entomol. 2016, 45, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Z.; Zhang, C.; Zhou, X.; Zhang, D.; Liu, Y. The molecular mechanism of efficient transmission of plant viruses in variable virus–vector–plant interactions. Hortic. Plant J. 2021, 7, 501–508. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.-D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [PubMed]
- Boquel, S.; Giordanengo, P.; Ameline, A. Divergent effects of PVY-infected potato plant on aphids. Eur. J. Plant Pathol. 2011, 129, 507–510. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with Potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, U.N.; Nie, X.; Giguère, M.; Zhang, J.; Boquel, S.; Pelletier, Y. Aphid feeding behavior in relation to Potato virus Y (PVY) acquisition. J. Econ. Entomol. 2012, 105, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Wilson, J.; Tamborindeguy, C.; Johnson, R.S.; Pinheiro, P.V.; MacCoss, M.J.; Gray, S.M.; Heck, M. The innate immunity protein C1QBP functions as a negative regulator of circulative transmission of Potato leafroll virus by aphids. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, H.; Stephanus, A.P.; Fowles, T.M.; Wintermantel, W.M.; Trumble, J.T.; Gilbertson, R.L.; Nansen, C. Insect vector manipulation by a plant virus and simulation modeling of its potential impact on crop infection. Sci. Rep. 2022, 12, 8429. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.F.; Hansen, A.K.; Casteel, C.L. Potato leafroll virus reduces Buchnera aphidocola titer and alters vector transcriptome responses. Sci. Rep. 2021, 11, 23931. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.E.; Garzo, E.; Verbeek, M.; Vosman, B.; Dicke, M.; Tjallingii, W.F. Infection of potato plants with Potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol. Exp. Appl. 2007, 125, 135–144. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Van Loon, B.; van Loon, J.J.; Dicke, M. Insect–Plant Biology; Oxford University Press: Oxford, UK, 2005; pp. 1–440. [Google Scholar]
- Mauck, K.E.; Kenney, J.; Chesnais, Q. Progress and challenges in identifying molecular mechanisms underlying host and vector manipulation by plant viruses. Curr. Opin. Insect Sci. 2019, 33, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 2006, 63, 1658–1676. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yang, H.; Liu, S.; He, H.; Ding, W.; Qiu, L.; Li, Y. Odorant-binding protein 2 is involved in the preference of Sogatella furcifera (Hemiptera: Delphacidae) for rice plants infected with the Southern rice black-streaked dwarf virus. Fla. Entomol. 2019, 102, 353–358. [Google Scholar] [CrossRef]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. In Advances in Virus Research, 1st ed.; Malmstrom, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 189–250. [Google Scholar]
- Chesnais, Q.; Mauck, K.E.; Bogaert, F.; Bamière, A.; Catterou, M.; Spicher, F.; Brault, V.; Tepfer, M.; Ameline, A. Virus effects on plant quality and vector behavior are species specific and do not depend on host physiological phenotype. J. Pest Sci. 2019, 92, 791–804. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Safari, M.; Ferrari, M.J.; Roossinck, M.J. Manipulation of aphid behavior by a persistent plant virus. J. Virol. 2019, 93, e01781-18. [Google Scholar] [CrossRef] [PubMed]
- Czosnek, H.; Ghanim, M. The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—Insights from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 2005, 140, 215–231. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef]
- Clemente-Orta, G.; Cabello, Á.; Garzo, E.; Moreno, A.; Fereres, A. Aphidius colemani behavior changes depending on volatile organic compounds emitted by plants infected with viruses with different modes of transmission. Insects 2024, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Crowder, D.W.; Li, J.; Borer, E.T.; Finke, D.L.; Sharon, R.; Pattemore, D.E.; Medlock, J. Species interactions affect the spread of vector-borne plant pathogens independent of transmission mode. Ecology 2019, 100, e02782. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Okuno, T. Hijacking of host cellular components as proviral factors by plant-infecting viruses. Adv. Virus Res. 2020, 107, 37–86. [Google Scholar] [CrossRef] [PubMed]
- Ragunathan, D.; Prakash, V.; Kumar, R.V. Molecular biology of antiviral arms race between plants and viruses. In Plant Virus–Host Interaction, 2nd ed.; Gaur, R.K., Khurana, S.M.P., Sharma, P., Hohn, T., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 331–358. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Valli, A.; García, J.A.; Zhou, X.; Cheng, X. The tug-of-war between plants and viruses: Great progress and many remaining questions. Viruses 2019, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Calil, I.P.; Fontes, E.P.B. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lukan, T.; Baebler, Š.; Pompe-Novak, M.; Guček, K.; Zagorščak, M.; Coll, A.; Gruden, K. Cell death is not sufficient for the restriction of Potato virus Y spread in hypersensitive response-conferred resistance in potato. Front. Plant Sci. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Kirgizova, I.V.; Kalashnikova, E.A.; Turpanova, R.M.; Gadzhimuradova, A.M.; Silaev, D.V. Environmental monitoring and assessment of agricultural land using remote sensing data. IOP Conf. Ser. Earth Environ. Sci. 2023, 1154, 012033. [Google Scholar] [CrossRef]
- Osei, R.; Boamah, S.; Boakye, T.A.; Wei, L.; Jin, M.; Gyasi Santo, K.; Takyi, I.; Yang, C. In vitro application of proline in potato tubers under newly emerging bacteria Lelliottia amnigena infection. Microb. Pathog. 2023, 178, 106053. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Kondrák, M.; Bánfalvi, Z. Molecular mechanisms of resistance to Potato virus X and Y in potato. Acta Phytopathol. Entomol. Hung. 2015, 50, 151–160. [Google Scholar] [CrossRef]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 genes conferring hypersensitive response to Potato virus Y (PVY) in cultivated potatoes: Mapping and marker-assisted selection validation for PVY resistance in potato breeding. Mol. Breed. 2014, 34, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Grech-Baran, M.; Witek, K.; Szajko, K.; Witek, A.I.; Morgiewicz, K.; Wasilewicz-Flis, I.; Jakuczun, H.; Marczewski, W.; Jones, J.D.G.; Hennig, J. Extreme resistance to Potato virus Y in potato carrying the Rysto gene is mediated by a TIR-NLR immune receptor. Plant Biotechnol. J. 2020, 18, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Comparison of leaf proteomes of potato (Solanum tuberosum L.) genotypes with ER- and HR-mediated resistance to PVY infection. Eur. J. Plant Pathol. 2018, 150, 375–385. [Google Scholar] [CrossRef]
- del Toro, F.J.; Donaire, L.; Aguilar, E.; Chung, B.; Tenllado, F.; Canto, T. Potato virus Y HCPro suppression of antiviral silencing in Nicotiana benthamiana plants correlates with its ability to bind in vivo to 21- and 22-nucleotide small RNAs of viral sequence. J. Virol. 2017, 91, e00367-17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Du, Z.; Zhang, G.; Wang, T.; Jin, G. Advances in RNA-silencing-related resistance against viruses in potato. Genes 2022, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, R.R.; Das, D.K.; Mohanty, A.; Rajani, K.; Kumari, N.; Kumar, V.; Kumar, S.; Kumbhar, B.V.; Ranjan, T. Knockdown of capsid protein encoding novel ATPase domain inhibits genome packaging in Potato leafroll virus. 3 Biotech 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Necira, K.; Contreras, L.; Kamargiakis, E.; Kamoun, M.S.; Canto, T.; Tenllado, F. Comparative analysis of RNA interference and pattern-triggered immunity induced by dsRNA reveals different efficiencies in the antiviral response to Potato virus X. Mol. Plant Pathol. 2024, 25, e70008. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kasai, A.; Maoka, T.; Masuta, C.; Sano, T.; Nakahara, K.S. RNA silencing-related genes contribute to tolerance of infection with Potato virus X and Y in a susceptible tomato plant. Virol. J. 2020, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.; Almendral, D.; Allende, L.; Pacheco, R.; Chung, B.N.; Canto, T.; Tenllado, F. The P25 protein of Potato virus X (PVX) is the main pathogenicity determinant responsible for systemic necrosis in PVX-associated synergisms. J. Virol. 2015, 89, e02896-14. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Li, J.; Rahman, M.A.; Xie, F.; Song, B.; Nie, B. Resistance to biotic and abiotic stress in potato: The origin of the genes and corresponding molecular markers. Phytopathol. Res. 2024, 6, 4. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Mihovilovich, E.; Bonierbale, M. Genetic characterization and mapping of major gene resistance to Potato leafroll virus in Solanum tuberosum ssp. andigena. Theor. Appl. Genet. 2007, 114, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Mihovilovich, E.; Aponte, M.; Lindqvist-Kreuze, H.; Bonierbale, M. An RGA-derived SCAR marker linked to PLRV resistance from Solanum tuberosum ssp. andigena. Plant Mol. Biol. Rep. 2014, 32, 117–128. [Google Scholar] [CrossRef]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef] [PubMed]
- Lazar, A.; Coll, A.; Dobnik, D.; Baebler, Š.; Bedina-Zavec, A.; Žel, J.; Gruden, K. Involvement of Potato (Solanum tuberosum L.) MKK6 in Response to Potato Virus Y. PLoS ONE 2014, 9, e104553. [Google Scholar] [CrossRef] [PubMed]
- El-Dougdoug, N.K. Physiological and molecular defense level in potato cultivars against Potato virus X. Ann. Agric. Sci. Moshtohor 2020, 58, 1079–1088. [Google Scholar] [CrossRef]
- González-Jara, P.; Tenllado, F.; Martínez-García, B.; Atencio, F.A.; Barajas, D.; Vargas, M.; Díaz-Ruiz, J.; Díaz-Ruíz, J.R. Host-dependent differences during synergistic infection by Potyviruses with Potato virus X. Mol. Plant Pathol. 2004, 5, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N.-S. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Tyutereva, E.V.; Fedoseeva, E.S.; Kreslavski, V.D.; Ivanov, Y.V.; Akimov, Y.A.; Mishin, I.A.; Allakhverdiev, S.I. The role of Bacillus subtilis in protecting potato plants against viral infection: Regulation of hormonal balance and photosynthetic activity. Biomolecules 2022, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.A.; El Kammar, H.F.; Saied, S.M.; Soliman, A.M. Effect of Bacillus subtilis on Potato virus Y (PVY) disease resistance and growth promotion in potato plants. Eur. J. Plant Pathol. 2023, 167, 743–758. [Google Scholar] [CrossRef]
- Baebler, Š.; Coll, A.; Gruden, K. Plant molecular responses to Potato virus Y: A continuum of outcomes from sensitivity and tolerance to resistance. Viruses 2020, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, A.; Köhn, B.A.; Dedi, C.; Baulcombe, D.C. The coat protein of Potato virus X is a strain-specific elicitor of Rx1-mediated virus resistance in potato. Plant J. 1995, 8, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Spillane, C.; Kavanagh, T.A.; Baulcombe, D.C. Elicitation of Rx-mediated resistance to PVX in potato does not require new RNA synthesis and may involve a latent hypersensitive response. Mol. Plant Microbe Interact. 1998, 11, 833–835. [Google Scholar] [CrossRef]
- Cavatorta, J.; Perez, K.W.; Gray, S.M.; Van Eck, J.; Yeam, I.; Jahn, M. Engineering virus resistance using a modified potato gene. Plant Biotechnol. J. 2011, 9, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, C.; Rosado, A.; Sauvage, C.; Gauffier, C.; German-Retana, S.; Moury, B.; Gallois, J.L. A new eIF4E1 allele characterized by RNAseq data mining is associated with resistance to Potato virus Y in tomato albeit with a low durability. J. Gen. Virol. 2016, 97, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Ruffel, S.; Gallois, J.L.; Moury, B.; Robaglia, C.; Palloix, A.; Caranta, C. Simultaneous mutations in translation initiation factors eIF4E and eIF(iso)4E are required to prevent Pepper veinal mottle virus infection of pepper. J. Gen. Virol. 2006, 87, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Mazier, M.; Flamain, F.; Nicolaï, M.; Sarnette, V.; Caranta, C. Knock-down of both eIF4E1 and eIF4E2 genes confers broad-spectrum resistance against potyviruses in tomato. PLoS ONE 2011, 6, e29595. [Google Scholar] [CrossRef] [PubMed]
- Zafirov, D.; Giovinazzo, N.; Bastet, A.; Gallois, J.L. When a knockout is an Achilles’ heel: Resistance to one potyvirus species triggers hypersusceptibility to another one in Arabidopsis thaliana. Mol. Plant Pathol. 2021, 22, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Cavatorta, J.R.; Savage, A.E.; Yeam, I.; Gray, S.M.; Jahn, M.M. Positive Darwinian selection at single amino acid sites conferring plant virus resistance. J. Mol. Evol. 2008, 67, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Krishnaswamy, S. Eukaryotic translation initiation factor 4E-mediated recessive resistance to plant viruses and its utility in crop improvement. Mol. Plant Pathol. 2012, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Bastet, A.; Zafirov, D.; Giovinazzo, N.; Guyon-Debast, A.; Nogué, F.; Robaglia, C.; Gallois, J.L. Mimicking natural polymorphism in eIF4E by CRISPR-Cas9 base editing is associated with resistance to potyviruses. Plant Biotechnol. J. 2019, 17, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Baebler, Š.; Stare, K.; Kovač, M.; Blejec, A.; Prezelj, N.; Stare, T.; Kogovšek, P.; Pompe-Novak, M.; Rosahl, S.; Ravnikar, M.; et al. Dynamics of responses in compatible potato–Potato virus Y interaction are modulated by salicylic acid. PLoS ONE 2011, 6, e29009. [Google Scholar] [CrossRef] [PubMed]
- Križnik, M.; Petek, M.; Dobnik, D.; Ramšak, Ž.; Baebler, Š.; Pollmann, S.; Kreuze, J.F.; Žel, J.; Gruden, K. Salicylic acid perturbs sRNA-gibberellin regulatory network in immune response of potato to Potato virus Y infection. Front. Plant Sci. 2017, 8, 2192. [Google Scholar] [CrossRef] [PubMed]
- Nasr-Eldin, M.; Messiha, N.; Othman, B.; Megahed, A.; Elhalag, K. Induction of potato systemic resistance against the Potato virus Y (PVYNTN), using crude filtrates of Streptomyces spp. under greenhouse conditions. Egypt. J. Biol. Pest Control 2019, 29, 62. [Google Scholar] [CrossRef]
- Stare, T.; Ramšak, Ž.; Križnik, M.; Gruden, K. Multiomics analysis of tolerant interaction of potato with Potato virus Y. Sci. Data 2019, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jia, M.A.; Yang, Y.; Zhan, L.; Cheng, X.; Cai, J.; Zhang, J.; Yang, J.; Liu, T.; Fu, Q.; et al. Integrated analysis of tobacco miRNA and mRNA expression profiles under PVY infection provides insight into tobacco–PVY interactions. Sci. Rep. 2017, 7, 4895. [Google Scholar] [CrossRef]
- Song, H.; Gao, X.; Song, L.; Jiao, Y.; Shen, L.; Yang, J.; Li, C.; Shang, J.; Wang, H.; Zhang, S.; et al. Unraveling the regulatory network of miRNA expression in Potato virus Y-infected Nicotiana benthamiana using integrated small RNA and transcriptome sequencing. Front. Genet. 2024, 14, 1290466. [Google Scholar] [CrossRef] [PubMed]
- Shiboleth, Y.M.; Haronsky, E.; Leibman, D.; Arazi, T.; Wassenegger, M.; Whitham, S.A.; Gaba, V.; Gal-On, A. The conserved FRNK box in HC-Pro, a plant viral suppressor of gene silencing, is required for small RNA binding and mediates symptom development. J. Virol. 2007, 81, 13135–13148. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, S.; Tabassum, B.; Tariq, M.; Riaz, S.; Yousaf, I.; Jabbar, B.; Khan, A.; Samuel, A.O.; Zameer, M.; Nasir, I.A. Silencing a Myzus persicae macrophage inhibitory factor by plant-mediated RNAi induces enhanced aphid mortality coupled with boosted RNAi efficacy in transgenic potato lines. Mol. Biotechnol. 2022, 64, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, S.B.; Christiaens, O.; Taning, C.N.T.; De Jonghe, K.; Smagghe, G. The cuticle protein MPCP2 is involved in Potato virus Y transmission in the green peach aphid Myzus persicae. J. Plant Dis. Prot. 2019, 126, 351–357. [Google Scholar] [CrossRef]
- Zhang, X.; Rashid, M.-O.; Zhao, T.-Y.; Li, Y.-Y.; He, M.-J.; Wang, Y.; Li, D.-W.; Yu, J.-L.; Han, C.-G. The carboxyl terminal regions of P0 protein are required for systemic infections of poleroviruses. Int. J. Mol. Sci. 2022, 23, 1945. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhuo, T.; Zhao, T.; Zhou, C.; Li, Y.; Wang, Y.; Li, D.; Yu, J.; Han, C. Functional characterization of RNA silencing suppressor P0 from Pea mild chlorosis virus. Int. J. Mol. Sci. 2020, 21, 7136. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Tsai, C.H.; Lie, M.; Havecker, E.; Baulcombe, D.C. The polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr. Biol. 2007, 17, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Derrien, B.; Baumberger, N.; Schepetilnikov, M.; Viotti, C.; De Cillia, J.; Ziegler-Graff, V.; Isono, E.; Schumacher, K.; Genschik, P. Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 15942–15946. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Clavel, M.; Lechner, E.; Viotti, C.; Wu, J.; Dubois, M.; Hacquard, T.; Derrien, B.; Izquierdo, E.; Lecorbeiller, M.; et al. The viral F-box protein P0 induces an ER-derived autophagy degradation pathway for the clearance of membrane-bound AGO1. Proc. Natl. Acad. Sci. USA 2019, 116, 22872–22883. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Miao, H.; Wang, Q.; Walling, L.L.; Liu, S.S. Virus-induced phytohormone dynamics and their effects on plant–insect interactions. New Phytol. 2021, 230, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Casteel, C.L. Effector-mediated plant–virus–vector interactions. Plant Cell 2022, 34, 1514–1531. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akanda, A.M. Effect of PLRV infected seed tuber on disease incidence, plant growth and yield parameters of potato. Bangladesh J. Agril. Res. 2010, 35, 359–366. [Google Scholar] [CrossRef]
- Anzlovar, S.; Kovač, M.; Ravnikar, M. Photosynthetic pigments in healthy and virus-infected potato plantlets (Solanum tuberosum L.) grown in vitro. Phyton 1996, 36, 221–230. [Google Scholar]
- Sánchez, G.; Gerhardt, N.; Siciliano, F.; Vojnov, A.; Malcuit, I.; Marano, M.R. Salicylic acid is involved in the Nb-mediated defense responses to Potato virus X in Solanum tuberosum. Mol. Plant Microbe Interact. 2010, 23, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Tameling, W.I.; Vossen, J.H.; Albrecht, M.; Lengauer, T.; Berden, J.A.; Haring, M.A.; Cornelissen, B.J.; Takken, F.L. Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol. 2006, 140, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Le Sourd, F.; Boulben, S.; Le Bouffant, R.; Cormier, P.; Morales, J.; Belle, R.; Mulner-Lorillon, O. eEF1B: At the dawn of the 21st century. Biochim. Biophys. Acta Gene Struct. Expr. 2006, 1759, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, J.L.; Brinton, M.A. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J. Virol. 1997, 71, 6433–6444. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.G.; Blackwell, J.L.; Shi, P.Y.; Brinton, M.A. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J. Virol. 2007, 81, 10172–10187. [Google Scholar] [CrossRef]
- Zeenko, V.V.; Ryabova, L.A.; Spirin, A.S.; Rothnie, H.M.; Hess, D.; Browning, K.S.; Hohn, T. Eukaryotic elongation factor 1A interacts with the upstream pseudoknot domain in the 3′ untranslated region of Tobacco mosaic virus RNA. J. Virol. 2002, 76, 5678–5691. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ren, R.; Peng, J.; Wang, D.; Shi, X.; Zheng, L.; Zhang, Z.; Zhu, C.; Liu, Y.; Dai, L.; et al. The Gustavus gene can regulate the fecundity of the green peach aphid, Myzus persicae (Sulzer). Front. Physiol. 2021, 11, 596392. [Google Scholar] [CrossRef] [PubMed]
- Marmonier, A.; Velt, A.; Villeroy, C.; Rustenholz, C.; Chesnais, Q.; Brault, V. Differential gene expression in aphids following virus acquisition from plants or from an artificial medium. BMC Genom. 2022, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Chesnais, Q.; Golyaev, V.; Velt, A.; Rustenholz, C.; Verdier, M.; Brault, V.; Pooggin, M.M.; Drucker, M. Transcriptome responses of the aphid vector Myzus persicae are shaped by identities of the host plant and the virus. Peer Community J. 2022, 2, e82. [Google Scholar] [CrossRef]
- MacKenzie, T.D.B.; Arju, I.; Poirier, R.; Singh, M. A genetic survey of pyrethroid insecticide resistance in aphids in New Brunswick, Canada, with particular emphasis on aphids as vectors of Potato virus Y. J. Econ. Entomol. 2018, 111, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Prasad, M. Diverse roles of phytohormonal signaling in modulating plant–virus interactions. J. Exp. Bot. 2025, 76, 1921–1940. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Devendran, R.; Ghosh, A. Perspicacious insights into plant–virus–vector interactions applying omics. BMC Genom. 2024, 25, 866. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.; Hollander, J.G.; Lefeber, A.W.; Erkelens, C.; Nuzillard, J.M.; Verpoorte, R. NMR metabolomics to revisit the Tobacco mosaic virus infection in Nicotiana tabacum leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Mascellani Bergo, A.; Leiss, K.; Havlik, J. Twenty years of 1H NMR plant metabolomics: A way forward toward assessment of plant metabolites for constitutive and inducible defenses to biotic stress. J. Agric. Food Chem. 2024, 72, 8332–8346. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.T.; Zidack, N.; McDonald, R.; Flenniken, M.L. Transcriptome and small RNA profiling of Potato virus Y infected potato cultivars, including systemically infected Russet Burbank. Viruses 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Shine, M.B.; Zhang, K.; Liu, H.; Lim, G.-H.; Xia, F.; Yu, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Phased small RNA–mediated systemic signaling in plants. Sci. Adv. 2022, 8, eabm8791. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhang, F.; Zhong, Z.; Chen, R.; Wang, Y.; Chang, L.; Bock, R.; Nie, B.; Zhang, J. Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol. J. 2019, 17, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Noureen, A.; Khan, M.Z.; Amin, I.; Zainab, T.; Mansoor, S. CRISPR/Cas9-mediated targeting of susceptibility factor eIF4E enhances resistance against Potato virus Y. Front. Genet. 2022, 13, 922019. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kancharla, N.; Javalkote, V.S.; Dasgupta, S.; Brutnell, T.P. CRISPR-Cas12a (Cpf1): A versatile tool in the plant genome editing toolbox for agricultural advancement. Front. Plant Sci. 2020, 11, 584151. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M.; Vazquez-Vilar, M.; Orzáez, D.; Daròs, J.A. CRISPR-Cas12a genome editing at the whole-plant level using two compatible RNA virus vectors. CRISPR J. 2021, 4, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mahfouz, M.M. CRISPR/Cas systems versus plant viruses: Engineering plant immunity and beyond. Plant Physiol. 2021, 186, 1770–1785. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.; Tashkandi, M.; Mansoor, S.; Mahfouz, M.M. Engineering plant immunity: Using CRISPR/Cas9 to generate virus resistance. Front. Plant Sci. 2016, 7, 1673. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Amin, I.; Hameed, A.; Mansoor, S. CRISPR-Cas13a: Prospects for plant virus resistance. Trends Biotechnol. 2018, 36, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | PVY (Potato Virus Y) | PLRV (Potato Leafroll Virus) | PVX (Potato Virus X) |
|---|---|---|---|
| Family | Potyviridae [43,44] | Solemoviridae [45,46,47,48,49] | Alphaflexiviridae [50,51,52] |
| The shape of viral particle | filamentous [53] | icosahedral [46,48] | filamentous [50,51,52] |
| Genome | +ssRNA (single-stranded and positive-sense) [53] | +ssRNA (single-stranded and positive-sense) [49] | +ssRNA (single-stranded and positive-sense) [50] |
| Genome size | 9.7 kb [53,54] | 5.3–5.7 kb [45,46,55] | 6.4 kb [50] |
| Genome end’s structure | VPg at the 5′ end and poly(A) tail [56] | VPg at 5′ and structured 3′-UTR (no polyA) [57,58] | 5′ cap and poly(A) tail [52,55] |
| Site of virus replication in plant | cytoplasm and associated with vesicular structures and endoplasmic reticulum [59] | cytoplasm of phloem cells and replication strictly limited to phloem cells [45] | cytoplasm and associated with ER membranes [60,61] |
| Replication mechanism | minus-strand RNA serves as an intermediate template for genomic plus-strand RNA synthesis; synthesis of subgenomic RNA is limited [62] | minus-strand RNA serves as an intermediate template for genomic plus-strand RNA synthesis; subgenomic RNAs are formed for gene expression [45,46,63] | minus-strand RNA serves as an intermediate template for genomic plus-strand RNA synthesis; subgenomic RNAs are formed for gene expression [52] |
| Local movement in the plant | moves through plasmodesmata using viral movement proteins (MPs) [64,65,66] | a specific RTP domain for movement through the plasmodesmata [46,48] | triple gene block (TGB) proteins facilitate transport through the plasmodesmata [55,60] |
| Systemic movement in the plant | movement through the phloem; uses HC-Pro to facilitate cell-to-cell and systemic movement [44,67] | strictly phloem-limited and movement via sieve elements (CP and RTPD proteins) [46,68] | primarily through the phloem and occasionally detected in the xylem (TGB and CP proteins) [52] |
| Modes of viral transmission | vegetative propagation, insect vectors (aphids), and mechanical contact [44,69,70,71] | vegetative propagation and insect vectors (aphids) [58,69,72] | vegetative propagation and mechanical contact [51,61] |
| Response Mechanism | Potato Virus Y (PVY) | Potato Leafroll Virus (PLRV) | Potato Virus X (PVX) |
|---|---|---|---|
| RNAi mechanism | vsiRNA production against HC-Pro and CP [146]; key host factors DCL2, DCL4, AGO1, and RDR6; HC-Pro suppresses siRNA accumulation and AGO1 activity [44]. | vsiRNA generated against CP, P1, and RTP (limited studies) [147]; host factors likely include DCL2, DCL4, AGO1, and RDR1/6; CP and/or RTP may act as suppressors (mechanism unclear) [147,148]. | vsiRNA production targets P25 and CP [51,61,104]; host factors include DCL4, AGO2, and RDR6; P25 is a potent suppressor of local and systemic silencing [149,150,151]. |
| R gene-mediated resistance | HR (Ny-1 and Ny-2) and ER (Rysto, Ryadg, and Rychc) [144,145]; Ny-1 decreases efficiency at >24 °C [142,143]. | R gene-mediated (Rladg, PLRV.1, PLRV.2, PLRV.3, and PLRV.4); SAR activation [13,152,153,154]. | HR (Nx and Nb) and ER (Rx1 and Rx2) [51,52]. ER is stable at various temperatures [142]. |
| PR gene expression | strong upregulation of PR-1b and other PR genes via SA signaling [155]; linked to SAR and MAPK activation [142,156]. | no confirmed data for PLRV. | expression of PR genes observed, especially under stress or co-infection [151,157,158]; varies by host genotype and viral load [51,149,157,158]. |
| Plant hormones response | SA increases [155] and ABA accumulation observed [156]. | SA increases and JA/ET signaling suppressed [72]. | slight increase in SA and ABA levels modulated [159]; ISR induced by endophytic Bacillus strains [160]. |
| MAPK signaling pathway activation | activated [156]; contributes to HR and PR gene expression (PR-1b) [155]. | not well characterized. | activated via P25-induced stress; contributes to ROS production and HR [149,151]. |
| Impact of endophytic bacteria | Bacillus strains induce ISR, reducing the viral load [161]. | not specifically studied | Bacillus strains induce ISR, reducing the viral load [160,161]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhebekova, R.; Pozharskiy, A.; Adilbayeva, K.; Gritsenko, D. Molecular Mechanisms of Potato Plant–Virus–Vector Interactions. Plants 2025, 14, 2282. https://doi.org/10.3390/plants14152282
Kenzhebekova R, Pozharskiy A, Adilbayeva K, Gritsenko D. Molecular Mechanisms of Potato Plant–Virus–Vector Interactions. Plants. 2025; 14(15):2282. https://doi.org/10.3390/plants14152282
Chicago/Turabian StyleKenzhebekova, Roza, Alexandr Pozharskiy, Kamila Adilbayeva, and Dilyara Gritsenko. 2025. "Molecular Mechanisms of Potato Plant–Virus–Vector Interactions" Plants 14, no. 15: 2282. https://doi.org/10.3390/plants14152282
APA StyleKenzhebekova, R., Pozharskiy, A., Adilbayeva, K., & Gritsenko, D. (2025). Molecular Mechanisms of Potato Plant–Virus–Vector Interactions. Plants, 14(15), 2282. https://doi.org/10.3390/plants14152282







