1. Introduction
Aquilaria sinensis (Lour.) Gilg, a member of the Thymelaeaceae family within the
Aquilaria genus, is endemic to southern China’s subtropical regions, primarily inhabiting low-altitude evergreen broad-leaved forests in Guangdong, Hainan, Guangxi, Fujian, and Yunnan provinces. As the sole botanical origin of Chinese agarwood, this endemic species represents a pharmaceutically significant resource [
1]. The formation of agarwood occurs as a defense response to biotic/abiotic stressors, where traumatic parenchyma cells synthesize oleoresin deposits—a complex mixture of sesquiterpenoids and phenylpropanoids—gradually developing into dark-brown to ebony aromatic resin [
2]. Pharmacological studies have demonstrated its bioactivities, including anti-inflammatory (via modulation of NF-kB signaling pathways) [
3], antitumor [
4], and antidepressant [
5]. The resin’s quality correlates with chromatic evolution [
6], driven by progressive accumulation of sesquiterpenoids (e.g., agarospirol) and chromones (e.g., 6-hydroxy-2-(2-phenylethyl)chromone). Ecological surveys indicate that <7% of wild
A. sinensis populations naturally develop commercially viable agarwood, exacerbated by unsustainable harvesting practices and habitat fragmentation. This ecological crisis has prompted China to classify it as a Grade II protected species. While artificial induction techniques (e.g., chemical stimulation, fungal inoculation) have reduced production timelines from decades to 12–24 months, persistent quality discrepancies in sesquiterpene diversity and chromone content compared to wild specimens highlight critical technological gaps.
Fungal induction has become a prominent method for agarwood production due to its operational efficiency and environmental compatibility. Current techniques primarily involve two approaches: (1) drilling holes in
A. sinensis trunks to implant fungus-colonized media [
7], or (2) infusing fungal suspensions into the xylem via perfusion systems [
8]. Early studies by Tunstall (1929) demonstrated agarwood formation within 3–6 months using
Aspergillus spp.,
Fusarium spp., and
Penicillium spp., though the resin quality remained suboptimal [
9]. Recent advancements identified specialized fungal strains with enhanced efficacy: Chen et al. isolated endophytic
Fusarium spp. and
Lasiodiplodia spp. from
A. sinensis, confirming their superior induction capacity [
10], while Subehan et al. achieved pharmacopeia-compliant agarwood within 12 months using
Fusarium spp. [
11]. These optimized strains enable standardized agarwood production within 1–2 years, delivering significantly improved yield while meeting quality benchmarks.
Through the study of the chemical constituents of agarwood samples at home and abroad, it was found that agarotetrol, 2-(2-phenylethyl) chromone, 2-[2-(4-methoxyphenyl) ethyl] chromone, and 2-(2-phenylethyl) chromone were the markers for the identification of agarwood [
12,
13]. The chemical constituents of agarwood mainly include sesquiterpenes, 2-(2-phenylethyl) chromones, fatty acid compounds, aromatic compounds, and other components [
14]. The chemical constituents of agarwood obtained from different regions [
15] or different tree species are quite different. Agarotetrol is a natural terpenoid compound, which is an active ingredient in agarwood medicinal materials. It can be dissolved in organic solvents such as methanol and has antibacterial, sedative, and anti-asthmatic effects. Many of the 2-(2-phenylethyl) chromone derivatives have potential pharmacological activities [
16], and their content mainly reflects the difference in the quality of agarwood [
17]. At present, the biosynthesis and regulation pathways of agarotetrol and 2-(2-phenylethyl) chromone are not clear enough. The question of how to induce the production of agarotetrol and 2-(2-phenylethyl) chromone in agarwood trees has become a key factor limiting the improvement of agarwood quality. In addition, the amount of ethanol extract is also one of the important indicators of the quality of agarwood. They may be related to the formation time and lipid content of agarwood [
18]. The minimum content of ethanol extract in agarwood in national standards should be ≥10%. In the agarwood induced by fungi, the content of the above indicators is generally lower than that of wild agarwood. It is necessary to further screen the dominant strains with the function of inducing agarwood formation and improve the quality of the obtained agarwood.
This study aims to comprehensively evaluate the agarwood-inducing efficacy of a
Fusarium strain (isolated from diseased
Macadamia integrifolia in Yunnan, China) in
A. sinensis, with specific focus on characterizing resin biosynthesis dynamics through the temporal quantification of volatile organic compounds and agarotetrol (2/4 months post-induction), validating the histochemical and physicochemical properties of matured agarwood against the National Standard LY/T 3223-2020 [
19] via one-year induction trials, and benchmarking critical quality parameters—particularly ethanol extractives (≥17.69%), 2-[2-(4-methoxyphenyl)ethyl] chromone (≥2.13%), and 2-(2-phenylethyl) chromone—for pharmacological compliance. The integrated assessment seeks to establish mechanistic foundations for fungal induction while advancing standardized production protocols.
2. Materials and Methods
2.1. Materials
Plant material of A. sinensis was collected from cultivated plantations (10+ years old) in the Nabanhe River Watershed National Nature Reserve, Jinghong City, Xishuangbanna Prefecture, Yunnan Province, China (100°40′32″ E, 22°07′53″ N). Selected trees exhibited no prior resin formation, with trunk diameters exceeding 25 cm at a height of 1.3 m. The taxonomic identification was confirmed by Prof. Sima Yongkang (Yunnan Academy of Forestry and Grassland Sciences), with voucher specimens deposited in the institution’s herbarium (Accession No. YAF00011258). The experimental fungal strain Fusarium sp. YB-1, originally isolated from anthracnose-infected leaves of Macadamia integrifolia (Baoshan, Yunnan, 2018), was obtained from the Microbial Culture Collection of the Yunnan Academy of Forestry and Grassland Sciences. Reference agarwood samples (Chinese agarwood) (Chinese Pharmacopoeia Grade) were procured from Kunming Sheng’ai Traditional Chinese Medicine Museum.
2.2. Fungal Activation and Culture
Prior to activation, PDA medium was prepared by dissolving 23 g commercial PDA powder in 500 mL of distilled water within a 500 mL conical flask. After sealing with aluminum foil, the mixture was autoclaved at 121 °C (103 kPa) for 20 min, cooled to 50 °C, and aseptically dispensed into disposable plastic Petri dishes under laminar airflow. Active Fusarium YB-1 colonies exhibiting vigorous marginal growth were then aseptically transferred to fresh PDA plates using sterile bamboo tips in a laminar flow hood. Inoculated plates were sealed with parafilm and incubated at 25 °C under dark conditions until complete mycelial coverage was achieved (5–7 days).
2.3. Morphological and Molecular Identification
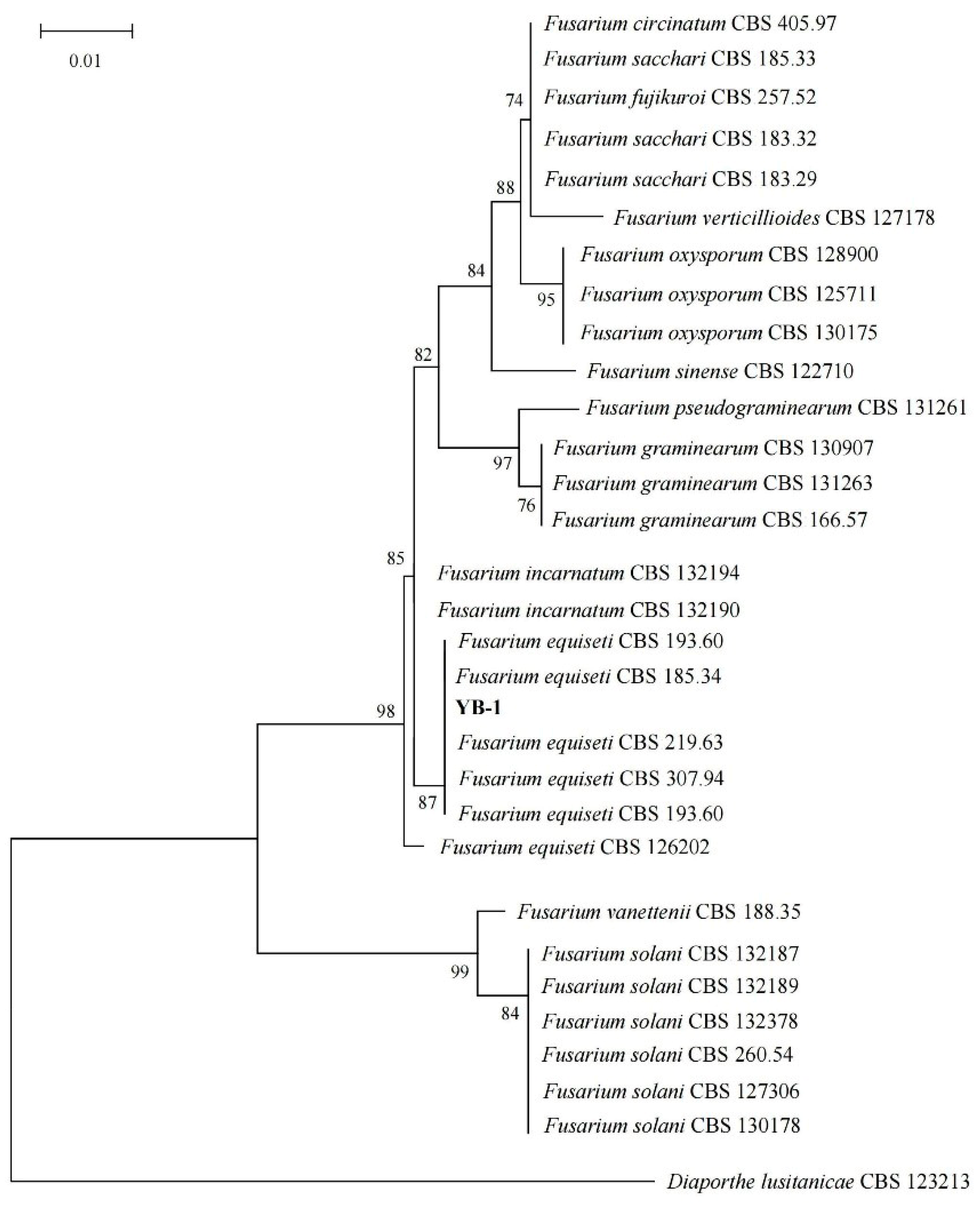
According to the classification system of Lester et al. [
20], and according to the growth of strain YB-1 on PDA medium (25 °C constant temperature), the mycelial morphology, colony characteristics, spore morphology, and sporogenous cell structure were observed. The spore size was calculated using Image Pro Plus 6.0 software, and the preliminary identification was made. After identification, the above-mentioned strains were sent to the Chinese Typical Culture Center of Yunnan Agricultural University and Wuhan University for identification. The fungal species were identified using ITS (Internal Transcribed Spacer, ITS), LSU (Large Subunit of Ribosome, LSU), SSU (Sensory Specific Satiety Units, SSU), TUB (Tubby Bipartite Transcription Factor, TUB), TEF (Transcriptional Enhancer Factor, TEF), 28S (28S Ribosomal RNA, 28S), 18S (18S Ribosomal RNA, 18S) and seven fragments. The fungal gene sequence obtained using sequencing technology was compared with the reference gene sequence in the National Center for Biotechnology Information (NCBI) database using BLAST (v2.15.0+). The top 10 were screened for data analysis, and the representative strains were combined with the reference gene using MEGA11.0 software. The ML method was used to construct a phylogenetic tree [
21].
2.4. Artificial Induction of A. sinensis
Healthy A. sinensis trees (≥10 years old, trunk diameter > 25 cm) were inoculated using a standardized drilling protocol: nine vertical rows of holes (1 cm diameter, 3–4 cm depth, 15 cm row spacing) were drilled 50 cm aboveground, with four radially distributed holes per row (7 cm spacing). Fungal inoculum (Fusarium YB-1 cultured on PDA) was aseptically inserted into pre-drilled holes using sterile bamboo skewers, sealed with parafilm, and replenished at 6-month intervals. Four treatments were implemented: (1) control (no drilling), (2) cold drill (drilling only), (3) PDA medium (sterile substrate), and (4) YB-1 inoculation. Resin formation was monitored through systematic sampling using a 15 cm sterile trephine at 2, 4, 6, and 12 months post-inoculation, collecting three radial core samples per tree across all xylem layers, with non-resinous control tissues sampled equivalently for comparative analysis.
2.5. Detection of Volatile Components in Processed Samples
Post-inoculation samples (2 months/4 months harvest) were air-dried, homogenized using a cryogenic grinder (Retsch MM400, 30 Hz, 2 min) with a 2 mm sieve, and composite aliquots (5 g) prepared for headspace solid-phase microextraction (HS-SPME) coupled with GC-MS analysis (Agilent 7890B/5977B MSD) via Yunnan Tongchuang Detection Technology Co., Ltd. (Kunming, China) Volatile extraction was performed by sealing 1.0 g of the sample in a 20 mL HS vial, equilibrating at 38 °C in a thermostatic water bath, and adsorbing compounds onto an SPME fiber for 30 min, followed by thermal desorption in the GC injection port for 2 min. Chromatographic separation employed a DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm) with a temperature program from 50 °C to 300 °C at 5 °C/min. Compound identifications were confirmed through dual criteria: (1) mass spectral similarity >85% against the NIST/EPA/NIH 2020 database, and (2) experimental retention indices (RIs) calibrated with a C
8–C
40 n-alkane standard mixture. For compounds eluting before C
8 (RI < 800), RIs were determined through extrapolation using the logarithmic retention time relationship between C
8 and C
9 alkanes according to the Kovats index system. All experimental RIs were required to be within ±15 units of literature values normalized to 5 °C/min using the correction formula: RI corr = RI ref − 4.5 × (5 − r ref), where r ref is the source ramp rate [
22,
23,
24], with RI validation strictly following ISO 11024-2:1998 guidelines [
25] for sesquiterpenes/chromones. Relative abundances were quantified via peak area normalization (signal/noise > 10:1), excluding compounds failing RI verification [
26].
2.6. Determination of Agarotetrol Content
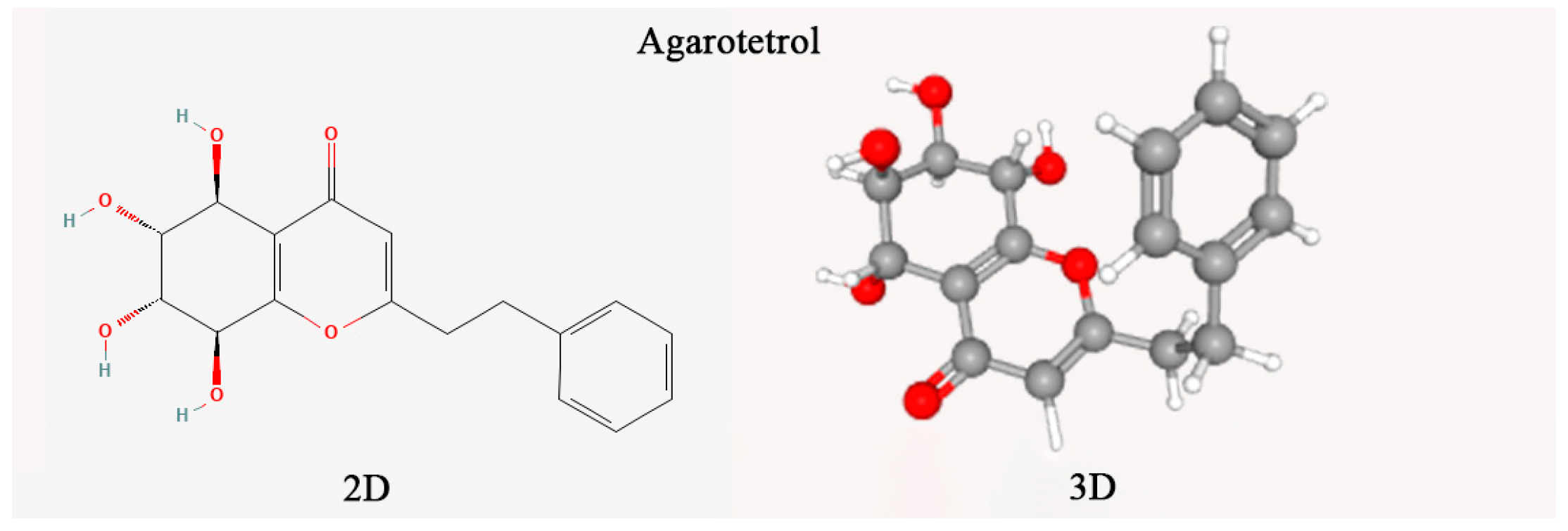
The agarotetrol content (chemical structure in
Figure 1) was determined according to the 2020 edition of the Chinese Pharmacopoeia (Part I) [
1]. A reference standard, 20 μg/mL of anhydrous ethanol (Lemeitian Pharmaceutical, CAS: 104834-43-1) was used. Agarwood samples were pulverized (<100 μm particle size) using a cryogenic mill. Precisely 0.2 g aliquots were ultrasonicated (Branson 5800, 250 W, 35 kHz) in 10 mL of ethanol (25 ± 2 °C, 60 min) with mass-loss compensation. Extracts were vacuum-filtered (Whatman No. 1) and further clarified through a 0.45 μm PVDF membrane to remove particulate matter. Chromatographic separation used an Agilent ZORBAX SB-C18 column (4.6 × 250 mm, 5 μm) with gradient elution: mobile phase A (acetonitrile) and B (0.1% formic acid) programmed as per
Table 1 (0.7 mL/min flow rate, 30 °C). Detection at 252 nm (DAD) enabled quantification via external calibration (5-point curve, R
2 = 0.9993). Method validation confirmed intra-day precision (RSD < 1.8%, n = 6) and recovery rates (98.2–102.4%). Data were analyzed using OpenLAB CDS ChemStation (v3.3).
2.7. Observation on Organizational Structure of Processed Samples
Sample preparation followed GB/T 29894-2013 wood identification standards [
27]. Six-month specimens (Control, YB-1-treated, and pharmacopeial agarwood) were fixed in FAA solution (formalin:acetic acid:70% ethanol = 5:5:90
v/
v) for 24 h, then sectioned into 1 cm
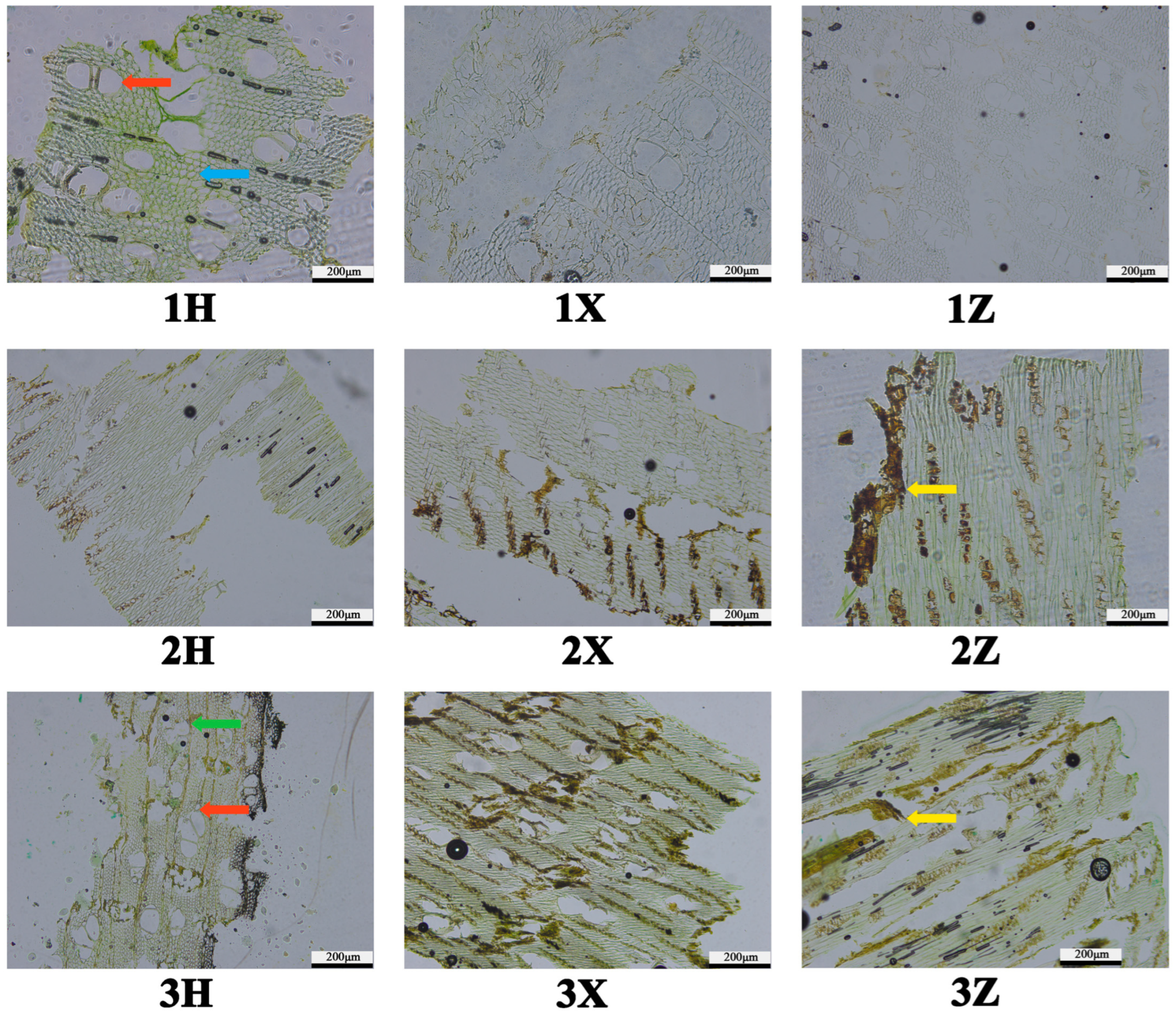
3 cubes for triplanar microtomy (transverse/radial/tangential). Dehydration employed an ethanol–xylene gradient series: 50% ethanol (1% safranin O) → 70% → 80% → 95% → absolute ethanol (2 h/step), followed by xylene infiltration (ethanol–xylene ratios 4:1→3:2→2:3→1:4→pure xylene, 2 h/step). Paraffin embedding (HistoStar™) preceded sectioning on a rotary microtome (Leica RM2235, 5° blade angle) at 20 μm thickness. Selected sections were mounted on adhesive slides, deparaffinized with xylene, and counterstained with 1% fast green FCF in ethanol–xylene (1 min). Permanent mounts were prepared using neutral balsam (Sigma–Aldrich, St. Louis, MO, USA) under controlled humidity (40 ± 5% RH). Brightfield microscopy (Nikon Eclipse Ni-U) with DS-Fi3 camera captured resin deposition patterns at 40–400× magnification, analyzing oleoresin distribution within axial parenchyma and intervascular spaces.
2.8. Determination of the Content of Ethanol Extract of Agarwood
The agarwood samples obtained after 12 months were analyzed according to the provisions of the standard LY/T 2904-2017 [
28], and 2 g of the crushed samples were weighed. The sample was placed in a 250 mL conical flask, and 100 mL of 95% ethanol was added to the pipette, sealed, weighed, and stood for 1 h. After that, the reflux condensation tube was connected, heated to boil, and kept for 1 h. After cooling, take off the conical bottle, close the plug, weigh again, use 95% ethanol to make up for the lost weight, shake well, and filter with filter paper. Next, 25 mL of the filtrate was taken with a pipette and placed in an evaporating dish that had been dried to a constant weight. After evaporation in a water bath, it was dried in an oven at 103 °C for 3 h and placed in a dryer. Cool for 30 min, weigh quickly, and calculate the content of ethanol extract in the test substance with the dry product.
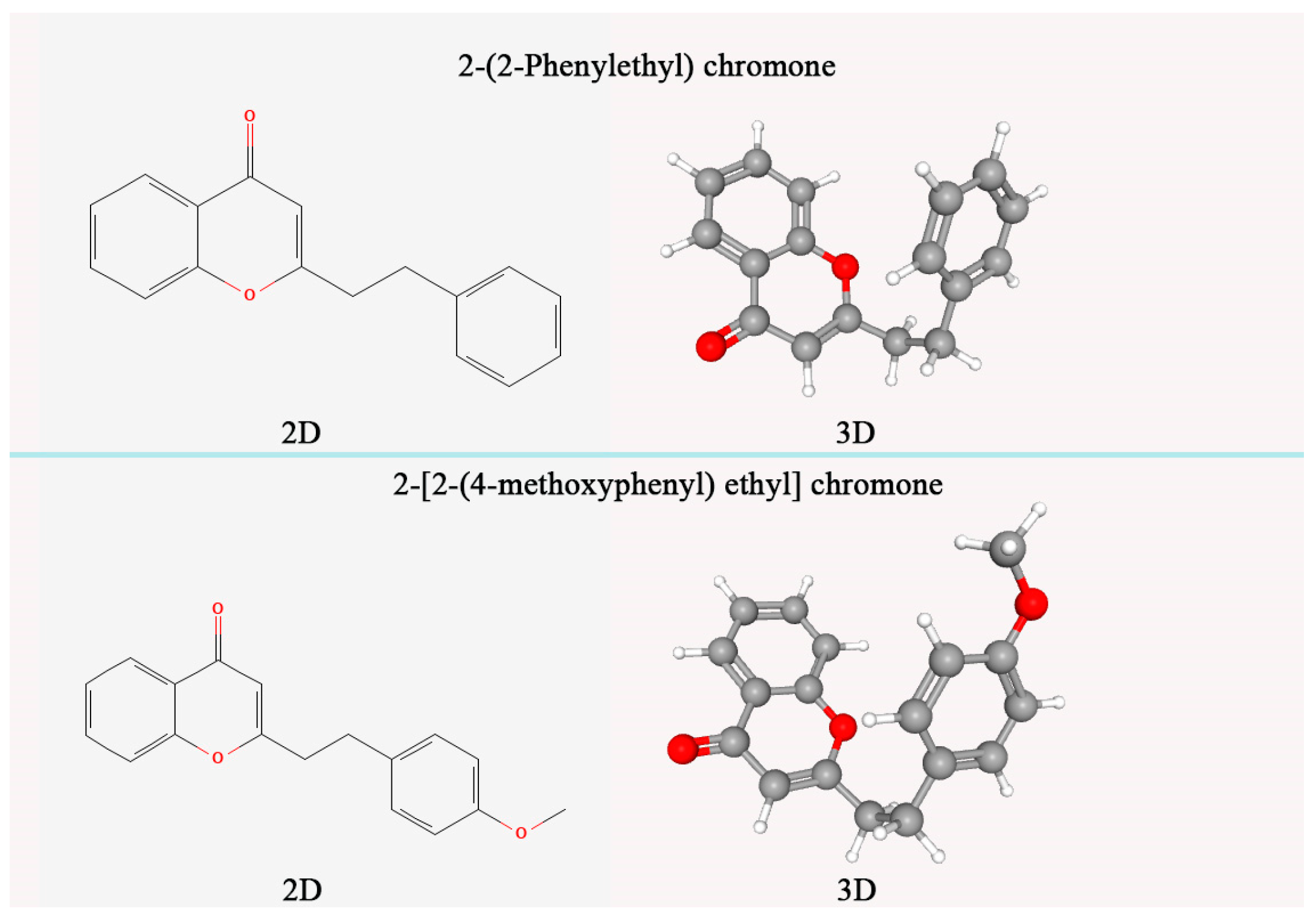
2.9. Determination of Relative Content of 2-[2-(4-Methoxy)phenylethyl] Chromone and 2-(2-Phenylethyl) Chromone
The preparation of agarwood reference sample and sample solution was carried out according to the provisions of LY/T 2904-2017 [
28], in which the sample solution was determined by ethanol extract. Appropriate amounts of 2-[2-(4-methoxy) phenylethyl] chromone and 2-(2-phenylethyl) chromone standards (chemical structure in
Figure 2) were accurately weighed (accurate to 0.0001 g), and 95% ethanol (analytically pure) was added to prepare a solution of 50 μg/mL. The filler was octadecylsilane-bonded silica gel (chromatographic column was Diamonsil C18, column length was 25 cm, inner diameter was 4.6 mm, particle size was 5 μm), mobile phase A was acetonitrile solution, mobile phase B was 0.1% formic acid solution, and gradient elution was performed according to the specified time (
Table 2). The flow rate of the mobile phase was 1.2 mL/min. The column temperature was 31 °C. Detection wavelength at 252 nm.
4. Discussion
In nature, fungi are easy to obtain and culture. Many fungi can continuously produce chemical components highly similar to natural agarwood when inducing agarwood plants, which has the advantages of high efficiency, environmental protection, and low cost. However, while the diversity of fungal species is immense, it is well-established that certain fungi can act as pathogens, potentially causing decay or death in plants. This inherent risk underscores the critical importance of screening for effective fungi capable of inducing agarwood formation without causing significant harm. At present, some fungal species have been proven to promote agarwood formation, such as
Aspergillus spp.,
Botryodyplodia spp.,
Fusarium spp., and
Penicillium spp. [
7,
10,
29,
30]. Tabata et al. [
31] found that five different species of
Fusarium sp. were inoculated into
A. sinensis, and after a period of observation, agarwood was produced. Huang Qiuwei et al. [
32] isolated 11 strains of endophytic fungi from
A. sinensis, of which the better fungi were
Fusarium oxysporum,
Fusarium fujikuroi,
Fusarium proliferatum,
Fusarium solani,
Lasiodiplodia pseudotheobromae, and
Lasiodiplodia theobromae.
Botryosphaeria sp. was isolated from
A. sinensis by Zhang Yao et al. [
29,
30]. In vitro experiments showed that
Botryosphaeria sp. could induce
A. sinensis branches to produce agarwood in vitro. In this study, we focused specifically on identifying and evaluating beneficial fungi for agarwood induction.
A. sinensis induced by
Fusarium equiseti not only promoted agarwood formation, but also the content of ethanol extract, 2-[2-(4-methoxyphenylethyl)] chromone, and 2-(2-phenylethyl) chromone in agarwood met the basic requirements of the national standard for agarwood grading at 12 months. This study reports for the first time that
Fusarium equiseti promotes agarwood formation in
A. sinensis. Importantly, during our experimental period (up to 12 months post-inoculation), the trees inoculated with
F. equiseti showed no signs of decay or mortality attributable to the fungal treatment, indicating its potential suitability under these conditions. It should be noted that the primary objective of this work was to screen for effective inducing fungi, and we did not conduct long-term studies specifically designed to assess the pathogenic potential or decay-causing capabilities of various fungi on mature agarwood trees over multiple years.
Most endophytes synthesize bioactive compounds conferring plant pathogen defense, with some possessing pharmacological potential [
33,
34].
Fusarium spp., common endophytes, produce terpenoids, polyketides, nonribosomal peptides, and anthraquinones exhibiting antibacterial, anti-inflammatory, and cytotoxic activities [
35,
36,
37,
38]. Although
Fusarium equiseti is a known pathogen causing crop decay in
Averrhoa carambola [
39],
Actinidia chinensis [
40], and
Astragalus membranaceus [
41], studies report its antibacterial properties [
42], disease suppression [
43], plant growth promotion, and L-asparaginase production (an antitumor agent) in
Potentilla anserina rhizosphere [
44]. These functional traits may underlie the agarwood induction mechanism in
A. sinensis, warranting further investigation.
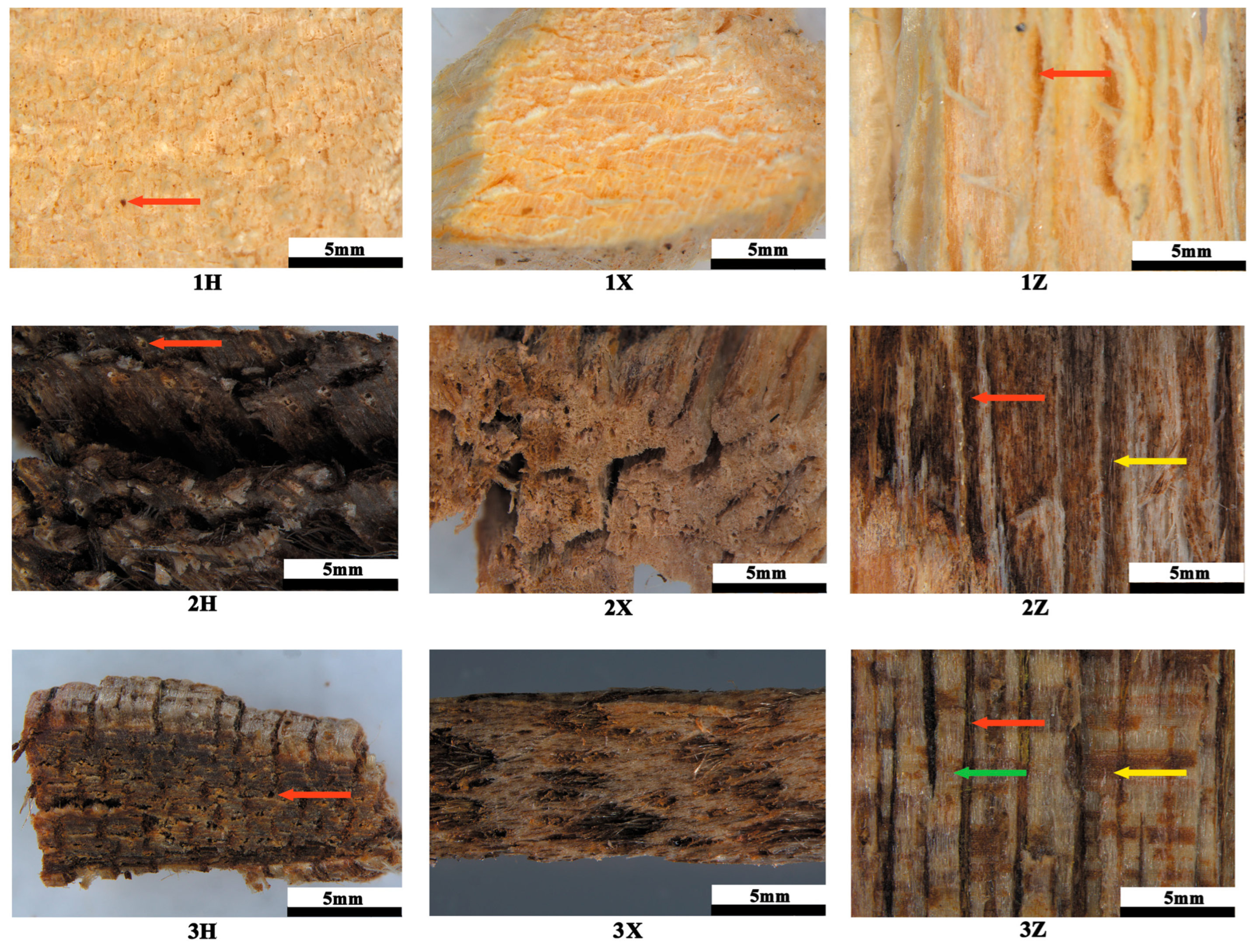
Macroscopic and anatomical analyses revealed characteristic agarwood features in induced
A. sinensis. The wood exhibits a diffuse-porous structure with large-diameter thin-walled vessels, inconspicuous axial parenchyma, and abundant inner phloem. A key macroscopic indicator of fungal induction efficacy is the extent of xylem discoloration [
45]. Agarwood deposition occurs exclusively in phloem and wood ray parenchyma following external injury or stress, manifesting as dark brown tissue with a distinct aroma [
46]. This process correlates with impaired vessel transport function [
47]. When damaged by external environmental factors, starch degradation initiates [
48], organic transport is disrupted, and wound repair triggers agarwood deposition. The weakly acidic, humid wood interior with rich carbohydrates facilitates fungal colonization, accelerating resin accumulation [
49,
50]. In this study, fungal inoculation induced progressive expansion and darkening of xylem discoloration around inoculation sites over 12 months.
F. equiseti parasitizes
A. sinensis xylem, provoking host defense responses that generate oleoresin-containing agarwood. The differential xylem colonization areas observed among fungi under identical conditions suggest variable infectivity, supporting xylem discoloration extent as a potential proxy for evaluating fungal induction efficacy in artificial inoculation systems.
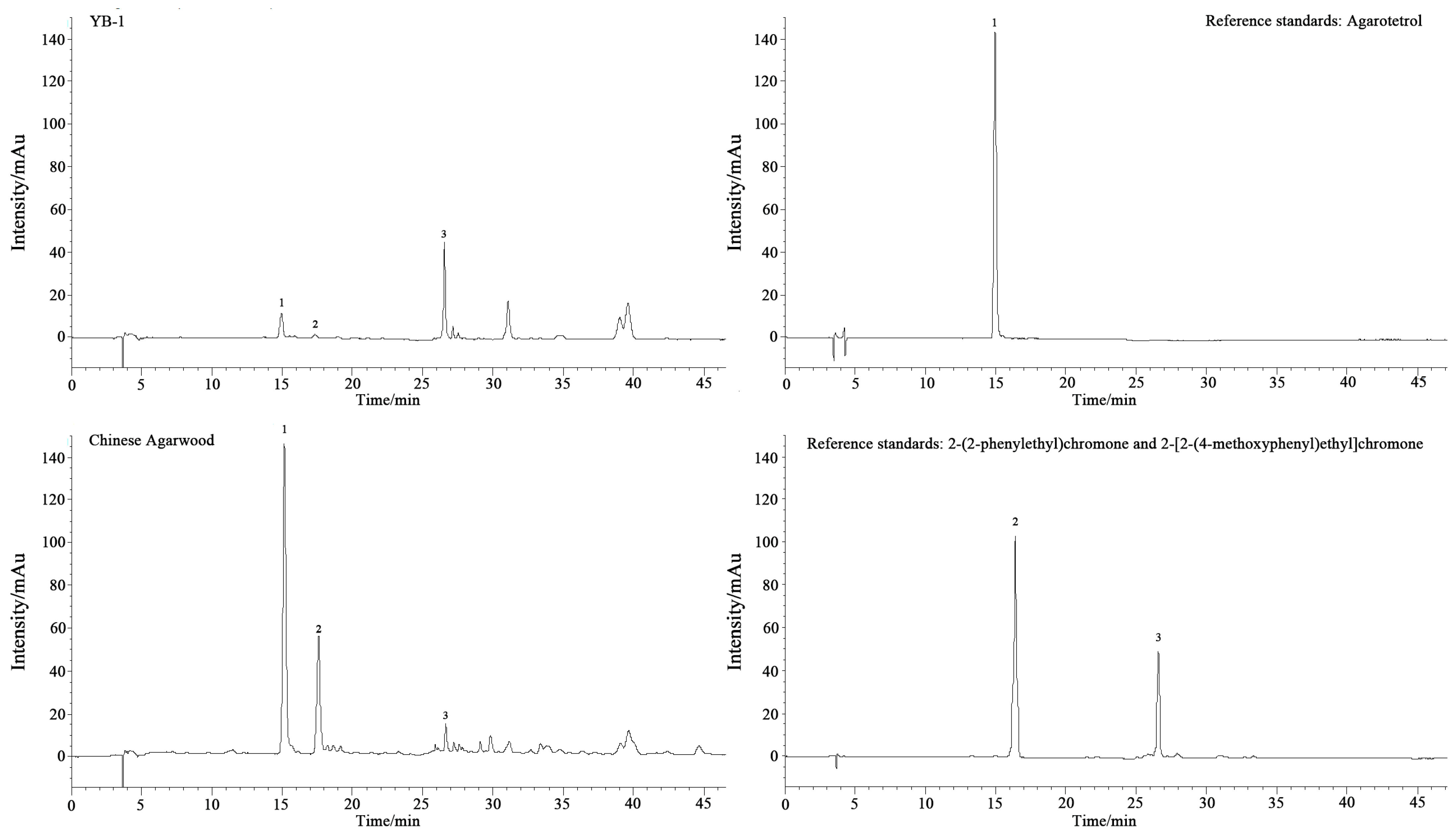
Chemical analysis confirmed the efficacy of
F. equiseti induction. Sesquiterpenes, key aromatic and bioactive constituents of agarwood with anticancer and neuropharmacological potential [
12], increased progressively over time, consistent with trends observed in chemically [
51] and fungally [
52] induced
Aquilaria species. Concurrent rises in aromatic hydrocarbons and aldehydes likely contribute to odor development. Crucially,
F. equiseti-treated samples exhibited significantly higher agarotetrol accumulation across all timepoints compared to other methods. Furthermore, ethanol extraction (a standard resin quantifier [
53]) yielded 17.69% after only 12 months, surpassing values from mixed-liquid induction (13.61% at 20 months) [
54] and fire-induced methods (7.49% at 24 months) [
55]. Although it does not match wild agarwood, this yield meets medicinal standards with markedly shorter induction time, lower cost, and stable efficacy. These results demonstrate that
F. equiseti induces stable and effective agarwood formation. We propose that extended induction periods hold potential for yielding higher-quality resin, offering a sustainable and scalable production alternative for
A. sinensis that enhances yield while conserving natural resources.