Genome-Wide Identification and Expression Analysis of Auxin-Responsive GH3 Gene Family in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of CaGH3 Gene Family in Pepper
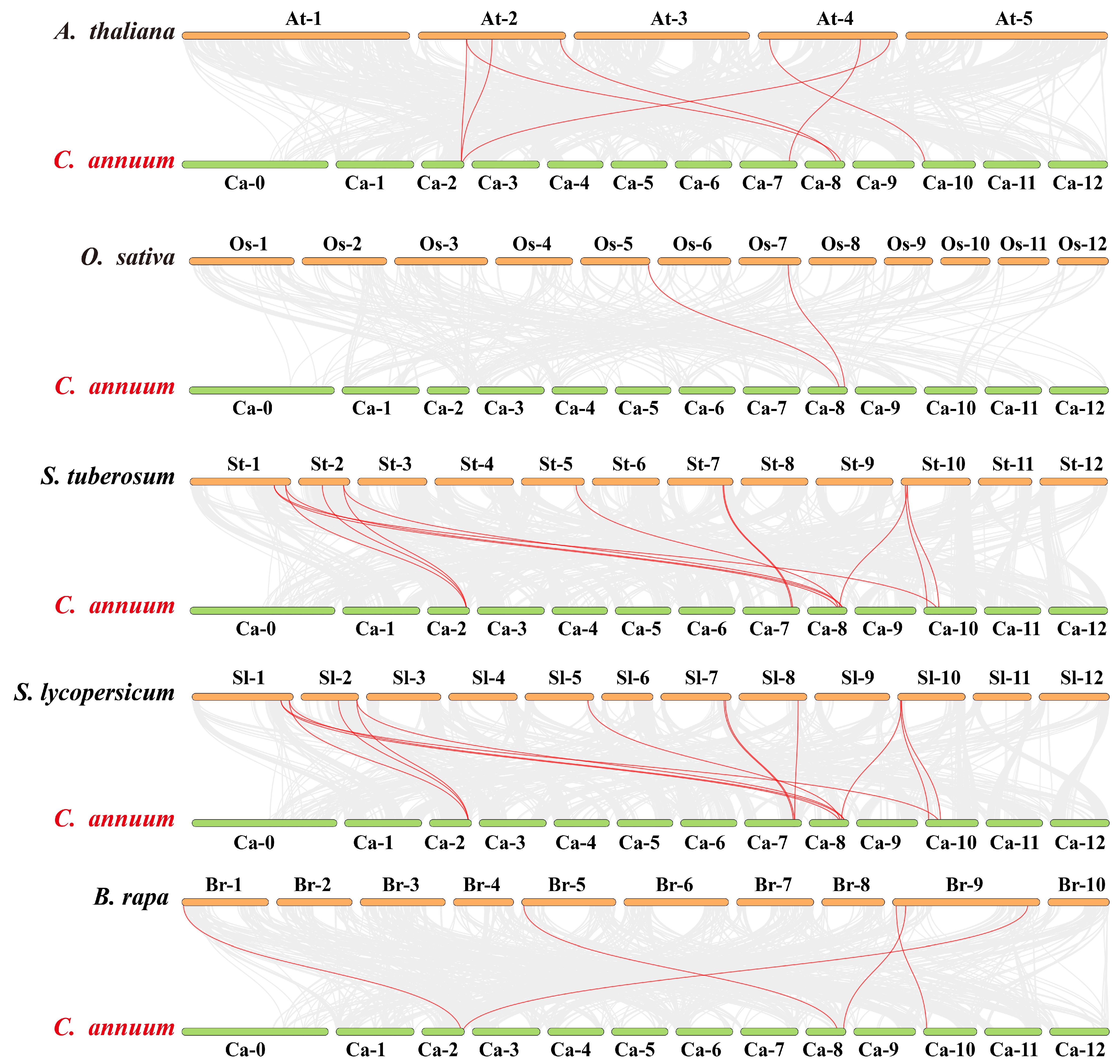
2.2. Prediction of Collinear Analysis of CaGH3 Family Genes
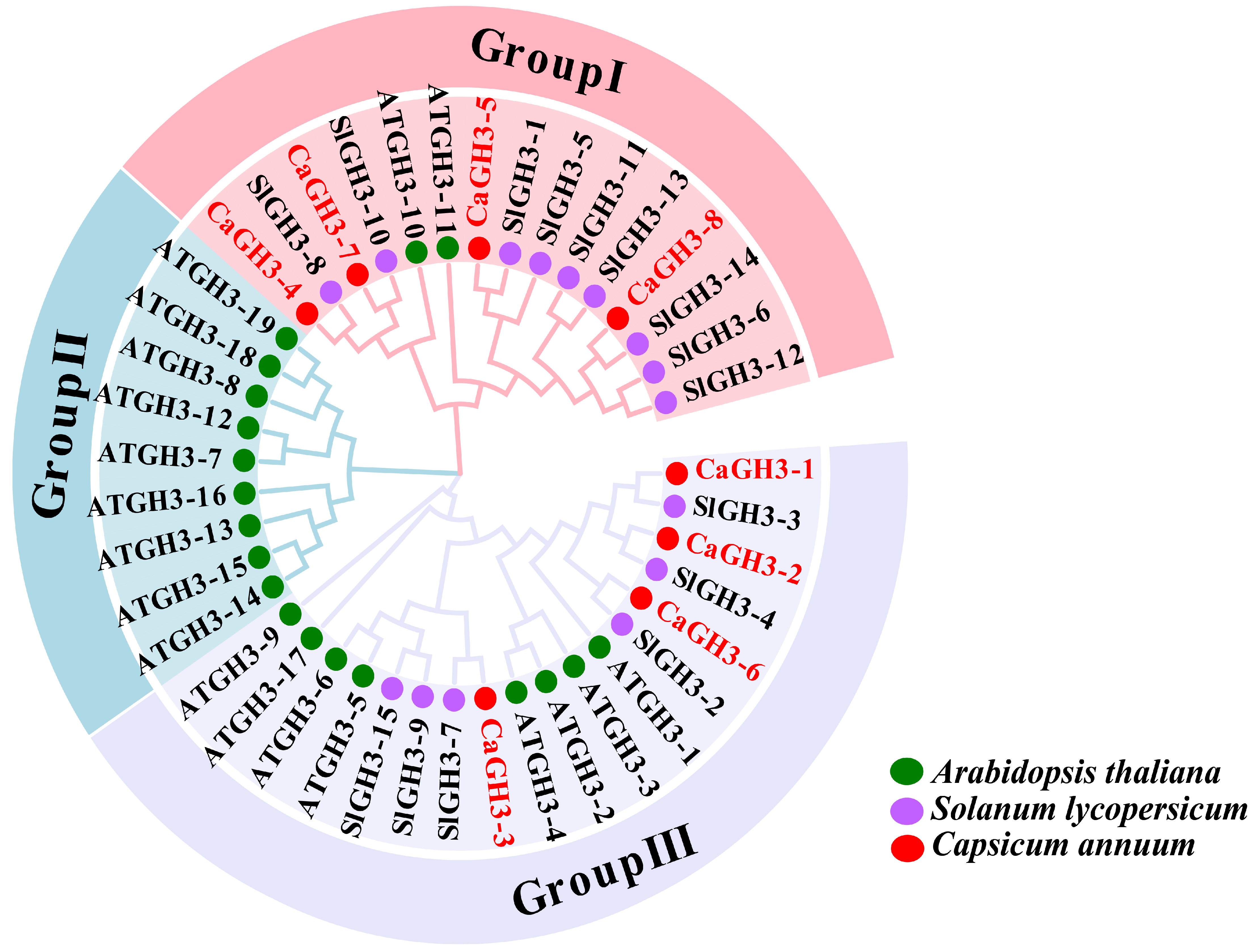
2.3. Evolutionary Analysis of CaGH3 Genes Among Multiple Species
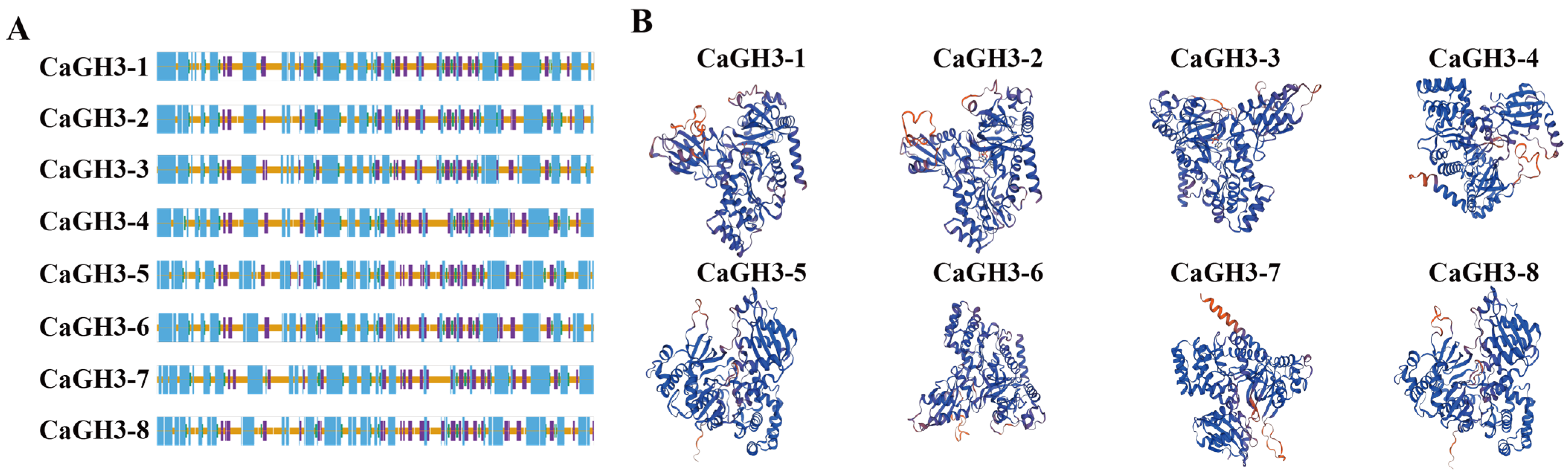
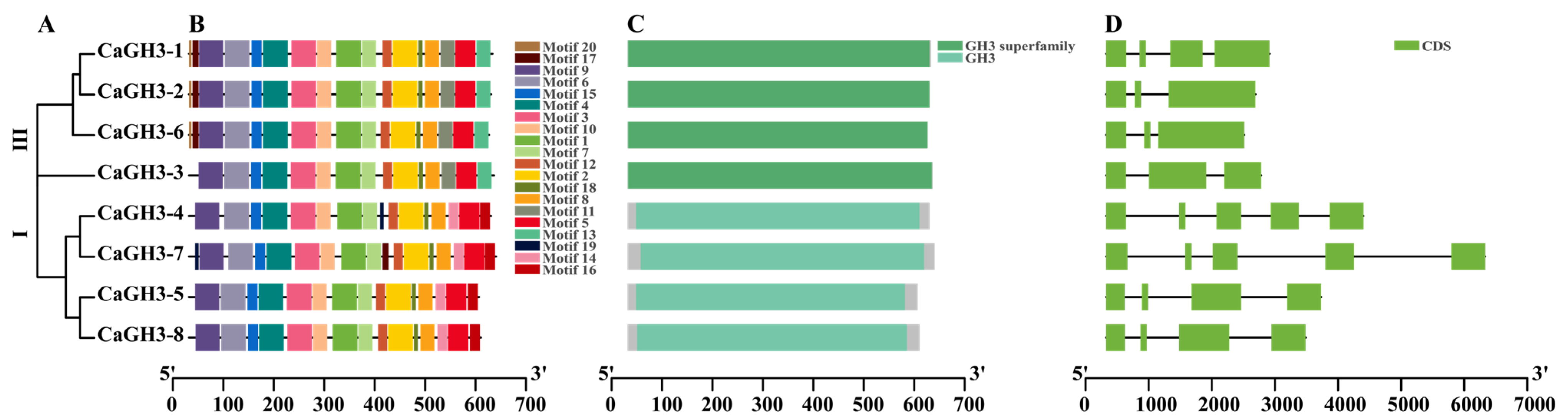
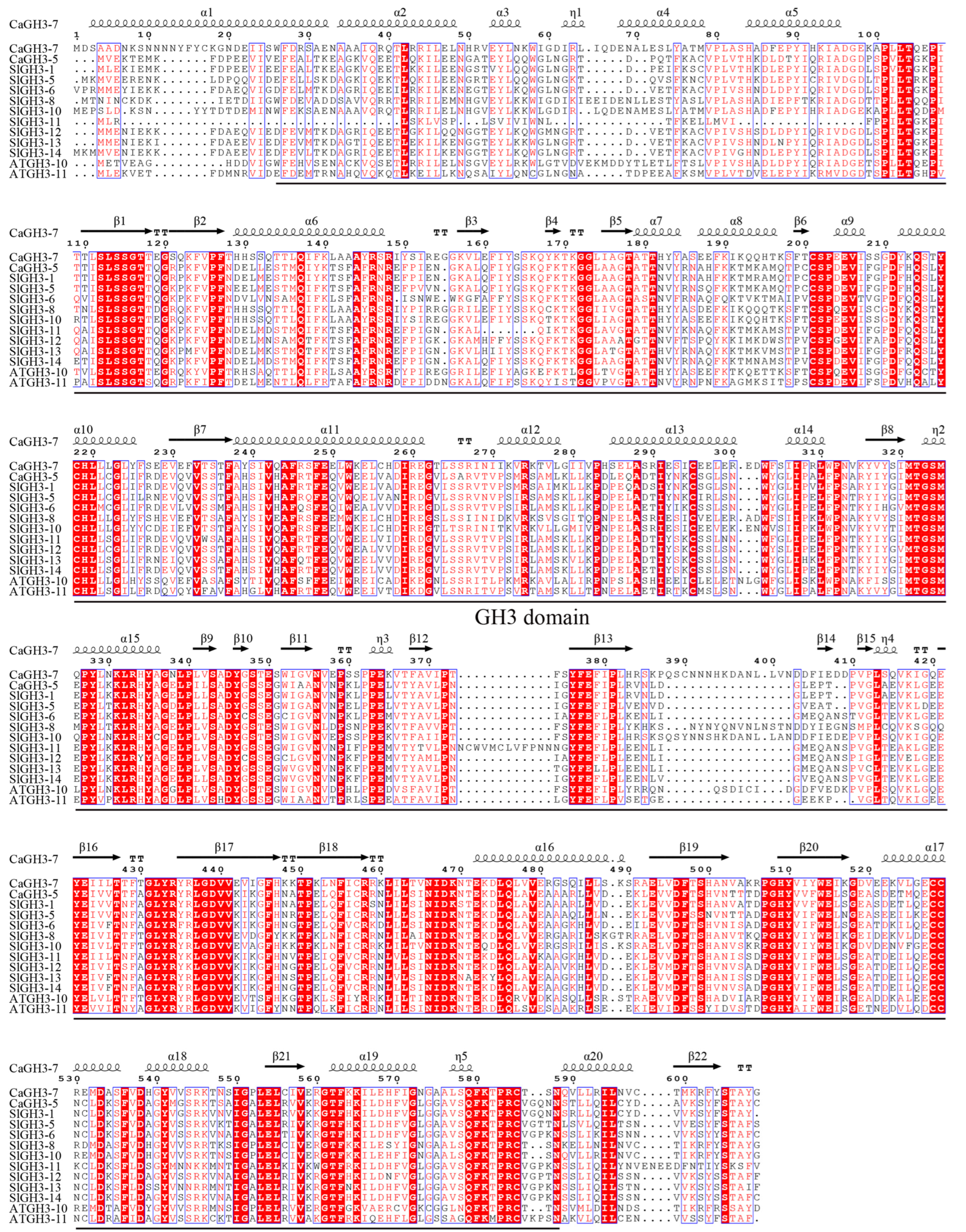
2.4. Phylogenetic, Conserved Motifs, Domains, and Gene Structures of CaGH3 Family Genes
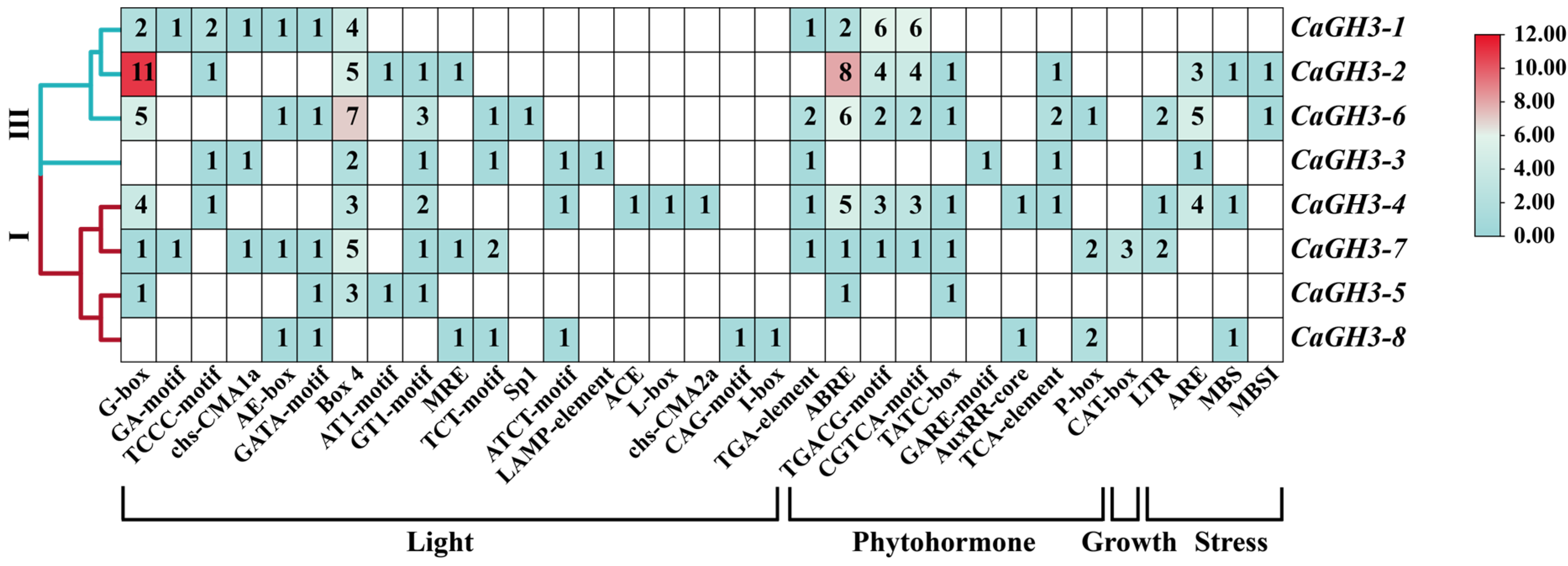
2.5. Analysis of Cis-Acting Elements of CaGH3 Promoters
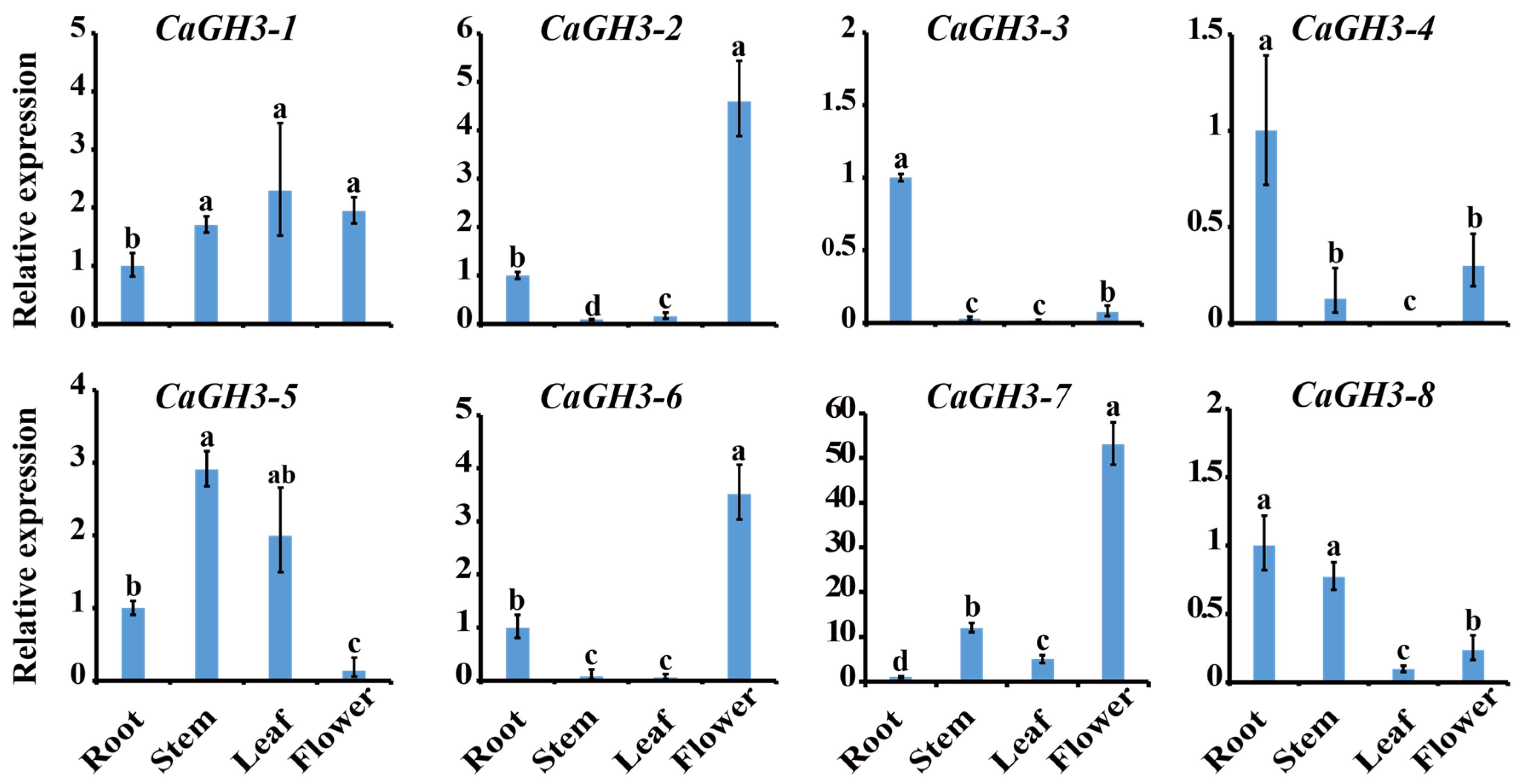
2.6. Expression Analysis of CaGH3 Genes in Different Tissues
2.7. Expression Patterns of CaGH3 Genes in Response to Different Hormone Treatments
2.8. Expression Patterns of CaGH3 Genes in Response to Different Stresses
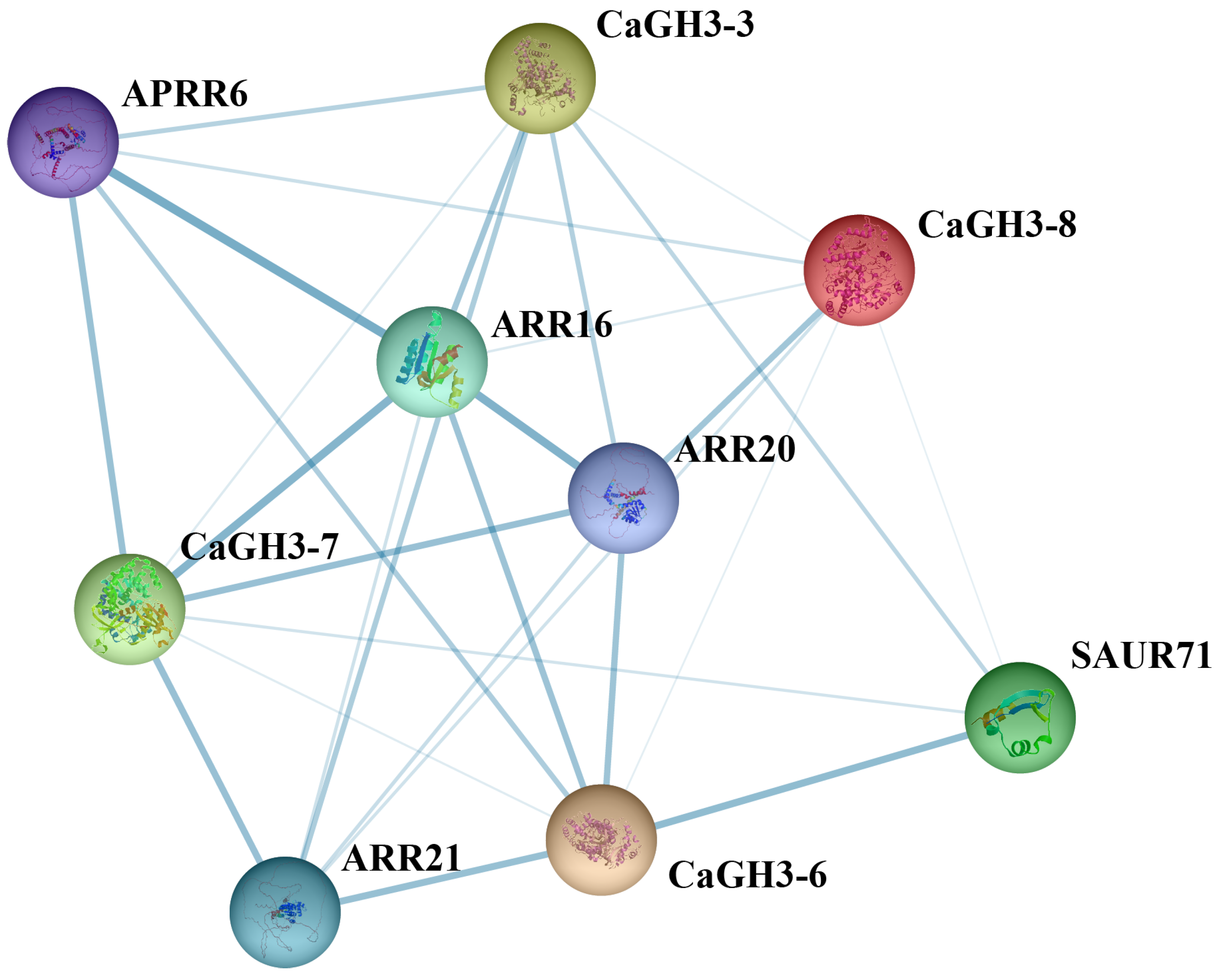
2.9. Prediction of Interacting Proteins Among CaGH3 Family Genes
2.10. Cloning and Sequence Analysis of CaGH3-5 and CaGH3-7
2.11. CaGH3-5 and CaGH3-7 Are Membrane and Chloroplast Localization Proteins
2.12. Transcriptome Analysis of Tomato with Overexpression of CaGH3-7
3. Discussion
4. Materials and Methods
4.1. Identification of the CaGH3 Gene Family
4.2. Sequence Features and Structures of CaGH3 Gene Family
4.3. Chromosomal Location and Collinearity Analysis of the CaGH3 Gene Family
4.4. Phylogenetic Analysis of the CaGH3 Gene Family
4.5. Conserved Motifs, Domains, and Gene Structures of the CaGH3 Gene Family
4.6. Cis-Acting Regulatory Element Analysis of CaGH3 Gene Promoters
4.7. Plant Materials, Growth Conditions, and Hormone and Stress Treatments
4.8. RNA Extraction, qRT PCR, and Statistical Analysis
4.9. Protein Interaction Prediction Analysis of the CaGH3 Gene Family
4.10. Gene Cloning and Sequence Analysis of CaGH3-5 and CaGH3-7
4.11. Subcellular Localization of CaGH3-5 and CaGH3-7
4.12. Vector Construction, Plant Transformation, and Transcriptome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GH3 | Gretchen Hagen 3 |
| Aux | Auxin |
| IAA | Indole-3-acetic acid |
| SAUR | Small auxin up RNA |
| SA | Salicylic acid |
| JA | Jasmonic acid |
| ABA | Abscisic acid |
| HMM | Hidden Markov Model |
| MeJA | Methyl jasmonate |
| PEG-6000 | Polyethylene glycol-6000 |
| MW | Molecular weight |
| pI | Isoelectric point |
| ARR | Arabidopsis response regulator |
| APRR | Arabidopsis putative response regulator-like |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | Mitogen-activated protein kinase |
References
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Smolko, A.; Bauer, N.; Pavlović, I.; Pěnčík, A.; Novák, O.; Salopek-Sondi, B. Altered Root Growth, Auxin Metabolism and Distribution in Arabidopsis thaliana Exposed to Salt and Osmotic Stress. Int. J. Mol. Sci. 2021, 22, 7993. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Hu, Y.; Aoi, Y.; Hira, H.; Ge, C.; Dai, X.; Kasahara, H.; Zhao, Y. Local conjugation of auxin by the GH3 amido synthetases is required for normal development of roots and flowers in Arabidopsis. Biochem. Biophys. Res. Commun. 2022, 589, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Liao, D.; Ye, H.; Li, C.; Luo, Z.; Yan, A.; Zhao, Q.; Xie, K.; Li, Y. Auxin-mediated regulation of arbuscular mycorrhizal symbiosis: A role of SlGH3.4 in tomato. Plant Cell Environ. 2022, 45, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Lin, P.; Zhao, Z.; Wang, D.; Qin, L.; Xu, F.; Su, Y.; Wu, Q.; Que, Y. Genome-Wide Identification of Auxin-Responsive GH3 Gene Family in Saccharum and the Expression of ScGH3-1 in Stress Response. Int. J. Mol. Sci. 2022, 23, 12750. [Google Scholar] [CrossRef] [PubMed]
- Takase, T.; Nakazawa, M.; Ishikawa, A.; Kawashima, M.; Ichikawa, T.; Takahashi, N.; Shimada, H.; Manabe, K.; Matsui, M. Ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J. 2004, 37, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kang, B.C.; Jiang, H.; Moore, S.L.; Li, H.; Watkins, C.B.; Setter, T.L.; Jahn, M.M. A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol. Biol. 2005, 58, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Stone, J. Arabidopsis thaliana GH3.9 in Auxin and Jasmonate Cross Talk. Plant Signal. Behav. 2007, 2, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; De-Gernier, H.; Duan, X.; Xie, Y.; Geelen, D.; Hayashi, K.I.; Xuan, W.; Geisler, M.; Ten-Tusscher, K.; Beeckman, T.; et al. GH3-Mediated Auxin Inactivation Attenuates Multiple Stages of Lateral Root Development. New Phytol. 2023, 240, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Sherp, A.M.; Westfall, C.S.; Alvarez, S.; Jez, J.M. Arabidopsis thaliana GH3.15 acyl acid amido synthetase has a highly specific substrate preference for the auxin precursor indole-3-butyric acid. J. Biol. Chem. 2018, 293, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M. Connecting primary and specialized metabolism: Amino acid conjugation of phytohormones by GRETCHEN HAGEN 3 (GH3) acyl acid amido synthetases. Curr. Opin. Plant Biol. 2022, 66, 102194. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the Indole-3-Acetic Acid–Amido Synthetase GH3-8 Suppresses Expansin Expression and Promotes Salicylate- and Jasmonate-Independent Basal Immunity in Rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhang, Y.; Deng, X.; Li, S.; Zhang, C.; Li, Y. Comparative Genomic and Transcriptomic Analysis Suggests the Evolutionary Dynamic of GH3 Genes in Gramineae Crops. Front. Plant Sci. 2019, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Li, C.H.; Cao, J.; Zhang, Y.C.; Zhang, S.Q.; Xia, Y.F.; Sun, D.Y.; Sun, Y. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol. 2009, 151, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef] [PubMed]
- Kirungu, J.N.; Magwanga, R.O.; Lu, P.; Cai, X.; Zhou, Z.; Wang, X.; Peng, R.; Wang, K.; Liu, F. Functional characterization of Gh_A08G1120 (GH3.5) gene reveal their significant role in enhancing drought and salt stress tolerance in cotton. BMC Genet. 2019, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Delfin, J.C.; Kanno, Y.; Seo, M.; Kitaoka, N.; Matsuura, H.; Tohge, T.; Shimizu, T. AtGH3.10 is another jasmonic acid-amido synthetase in Arabidopsis thaliana. Plant J. 2022, 110, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Yeom, S.I.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jeong, H.B.; Yu, N.; Park, B.M.; Chae, W.B.; Lee, O.J.; Lee, H.E.; Kim, S. Comparative heat stress responses of three hot pepper (Capsicum annuum L.) genotypes differing temperature sensitivity. Sci. Rep. 2023, 13, 14203. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, Q.; Nie, K.; Wu, H.; Chen, G.; Wang, J.; Ma, Z. Exogenous phthalanilic acid induces resistance to drought stress in pepper seedlings (Capsicum annuum L.). Front. Plant Sci. 2023, 14, 1156276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, S.; Chen, C.; Li, M.; Du, Q.; Wang, J.; Yin, Y.; Xiao, H. CaCP15 Gene Negatively Regulates Salt and Osmotic Stress Responses in Capsicum annuum L. Genes 2023, 14, 1409. Genes 2023, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Yu, T.; Li, J.; Qiu, X.; Zhu, C.; Liu, J.; Dang, F.; Yang, Y. Characterization of the B-BOX gene family in pepper and the role of CaBBX14 in defense response against Phytophthora capsici infection. Int. J. Biol. Macromol. 2023, 237, 124071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Ma, S.; Wang, Y.; Yang, H.; Liu, J.; Li, M.; Cui, X.; Liang, S.; Cheng, Q.; et al. Characterization of the Molecular Events Underlying the Establishment of Axillary Meristem Region in Pepper. Int. J. Mol. Sci. 2023, 24, 12718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Hu, Z.; Li, B.; Zheng, X.; Qiu, R.; Chen, Y.; Li, J.; Dong, J.; Zhang, Z. Tomato zonate spot virus induced hypersensitive resistance via an auxin-related pathway in pepper. Gene 2022, 823, 146320. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Zhang, C.; Qin, T.; Liu, Y.; Liu, Z.; Xie, X.; Bai, J.; Sun, C.; Bi, Z. Comprehensive Analysis of GH3 Gene Family in Potato and Functional Characterization of StGH3.3 under Drought Stress. Int. J. Mol. Sci. 2023, 24, 15122. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Li, T.T.; Shi, W.M.; Ma, Q.; Di, D.W. The Roles of GRETCHEN HAGEN3 (GH3)-Dependent Auxin Conjugation in the Regulation of Plant Development and Stress Adaptation. Plants 2023, 12, 4111. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Agarwal, P.; Tyagi, A.K.; Sharma, A.K. Genome-wide investigation and expression analysis suggest diverse roles of auxin-responsive GH3 genes during development and response to different stimuli in tomato (Solanum lycopersicum). Mol. Genet. Genomics 2012, 287, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Cai, X.; Liang, J.; Freeling, M.; Wang, X. Gene retention, fractionation and subgenome differences in polyploid plants. Nat. Plants 2018, 4, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Hao, M.; Liu, H.; Xiao, J.; Li, X.; Yuan, M.; Wang, S. The group I GH3 family genes encoding JA-Ile synthetase act as positive regulator in the resistance of rice to Xanthomonas oryzae pv. oryzae. Biochem. Biophys. Res. Commun. 2019, 508, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Klein, M.; Hossbach, B.; Feussner, K.; Hornung, E.; Herrfurth, C.; Hamberg, M.; Feussner, I. Arabidopsis GH3.10 conjugates jasmonates. Plant Biol. 2025, 27, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Nobuta, K.; Okrent, R.A.; Stoutemyer, M.; Rodibaugh, N.; Kempema, L.; Wildermuth, M.C.; Innes, R.W. The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 2007, 144, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yin, J.; Zhang, H.; He, Y.; Shuai, S.; Chen, S.; Cao, S.; Li, W.; Ma, D.; Chen, H. Genome-wide identification, characterization analysis and expression profiling of auxin-responsive GH3 family genes in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2020, 47, 3885–3907. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Luo, D.X.; Khan, A.; Haq, S.U.; Gai, W.X.; Zhang, H.X.; Cheng, G.X.; Muhammad, I.; Gong, Z.H. Classification and Genome-Wide Analysis of Chitin-Binding Proteins Gene Family in Pepper (Capsicum annuum L.) and Transcriptional Regulation to Phytophthora capsici, Abiotic Stresses and Hormonal Applications. Int. J. Mol. Sci. 2018, 19, 2216. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, C.E.; Duan, J.; Wu, K. Research progresses on GH3s, one family of primary auxin-responsive genes. Plant Growth Regul. 2008, 56, 225–232. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Z.; Zhao, K.; Lei, H.; Shen, X.; Liu, Y.; Liao, X.; Li, T. Genome-wide analysis of the GH3 family in apple (Malus × domestica). BMC Genomics 2013, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Min, X.; Luo, K.; Hamidou Abdoulaye, A.; Zhang, X.; Huang, W.; Zhang, R.; Chen, Y. Molecular characterization of the GH3 family in alfalfa under abiotic stress. Gene 2023, 851, 146982. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yue, R.; Tao, S.; Yang, Y.; Zhang, L.; Xu, M.; Wang, H.; Shen, C. Genome-wide identification, expression analysis of auxin-responsive GH3 family genes in maize (Zea mays L.) under abiotic stresses. J. Integr. Plant Biol. 2015, 57, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Kaur, N.; Tyagi, A.K.; Khurana, J.P. The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct. Integr. Genomics 2006, 6, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Wang, S.K.; Xu, Y.X.; Yu, C.J.; Shen, C.J.; Qian, Q.; Geisler, M.; Jiang, D.A.; Qi, Y.H. The auxin response factor, OsARF 19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.; Krzeszowiec, W.; Waligórski, P.; Gabryś, H. Auxin and chloroplast movements. Physiol. Plant 2016, 156, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.Y.; Cho, C.; Kim, J. Inducible expression of Arabidopsis response regulator 22 (ARR22), a type-C ARR, in transgenic Arabidopsis enhances drought and freezing tolerance. PLoS ONE 2013, 8, 79248. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, Q.; Li, Z.; Liu, F.; Su, W.; Zhang, C.; Ling, H.; Luo, J.; Su, Y.; Que, Y. A PIP-mediated osmotic stress signaling cascade plays a positive role in the salt tolerance of sugarcane. BMC Plant Biol. 2021, 21, 589. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Geng, J.; Zhang, Z.H.; Wang, W.; Ma, T.; Ni, P.; Zhang, Z.; Li, X.; Xing, J.; Sun, Q.; et al. The sucrose transporter TaSWEET11 is critical for grain filling and yield potential in wheat (Triticum aestivum L.). Plant J. 2025, 122, e70133. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, J.; Yue, M.; Xu, C.; Jian, W.; Liu, Y.; Song, B.; Gao, Y.; Cheng, Y.; Li, Z. Tomato transcriptional repressor MYB70 directly regulates ethylene-dependent fruit ripening. Plant J. 2020, 104, 1568–1581. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Gene ID | Protein Length (aa) | Molecular Weights (KDa) | Isoelectric Point (pI) | Instability Index | Hydrophilic Coefficient | Chromosome | Subcellular Localization | α-Helix (%) | β-Turn (%) | Extended Strand (%) | Random Coil (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaGH3-1 | Capana02g000676 | 602 | 68.04 | 6.07 | 40.99 | −0.24 | 2 | Chloroplast | 42.86 | 5.15 | 14.45 | 37.54 |
| CaGH3-2 | Capana02g003021 | 599 | 67.71 | 5.48 | 43.18 | −0.23 | 2 | Chloroplast | 41.74 | 5.01 | 13.69 | 39.57 |
| CaGH3-3 | Capana07g001573 | 605 | 68.58 | 5.39 | 40.33 | −0.27 | 7 | Chloroplast | 41.65 | 4.46 | 13.06 | 40.83 |
| CaGH3-4 | Capana07g001662 | 599 | 68.34 | 6.21 | 40.53 | −0.34 | 7 | Chloroplast | 43.57 | 4.51 | 14.69 | 37.23 |
| CaGH3-5 | Capana08g001036 | 575 | 64.28 | 5.67 | 31.81 | −0.14 | 8 | Chloroplast | 44.35 | 4.70 | 14.43 | 36.52 |
| CaGH3-6 | Capana08g002278 | 595 | 67.47 | 6.27 | 43.75 | −0.28 | 8 | Chloroplast | 41.85 | 4.20 | 13.95 | 40.00 |
| CaGH3-7 | Capana10g000405 | 609 | 69.42 | 7.26 | 47.34 | −0.29 | 10 | Chloroplast | 43.19 | 4.93 | 13.30 | 38.59 |
| CaGH3-8 | Capana10g000854 | 579 | 64.73 | 5.54 | 35.22 | −0.11 | 10 | Chloroplast | 41.62 | 4.49 | 15.54 | 38.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Q.-L.; Wang, M.; Liu, L.; Zheng, X.-M.; Cheng, Y. Genome-Wide Identification and Expression Analysis of Auxin-Responsive GH3 Gene Family in Pepper (Capsicum annuum L.). Plants 2025, 14, 2231. https://doi.org/10.3390/plants14142231
Zang Q-L, Wang M, Liu L, Zheng X-M, Cheng Y. Genome-Wide Identification and Expression Analysis of Auxin-Responsive GH3 Gene Family in Pepper (Capsicum annuum L.). Plants. 2025; 14(14):2231. https://doi.org/10.3390/plants14142231
Chicago/Turabian StyleZang, Qiao-Lu, Meng Wang, Lu Liu, Xiao-Mei Zheng, and Yan Cheng. 2025. "Genome-Wide Identification and Expression Analysis of Auxin-Responsive GH3 Gene Family in Pepper (Capsicum annuum L.)" Plants 14, no. 14: 2231. https://doi.org/10.3390/plants14142231
APA StyleZang, Q.-L., Wang, M., Liu, L., Zheng, X.-M., & Cheng, Y. (2025). Genome-Wide Identification and Expression Analysis of Auxin-Responsive GH3 Gene Family in Pepper (Capsicum annuum L.). Plants, 14(14), 2231. https://doi.org/10.3390/plants14142231






