Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat
Abstract
1. Introduction
2. Results
2.1. Biomass Accumulation
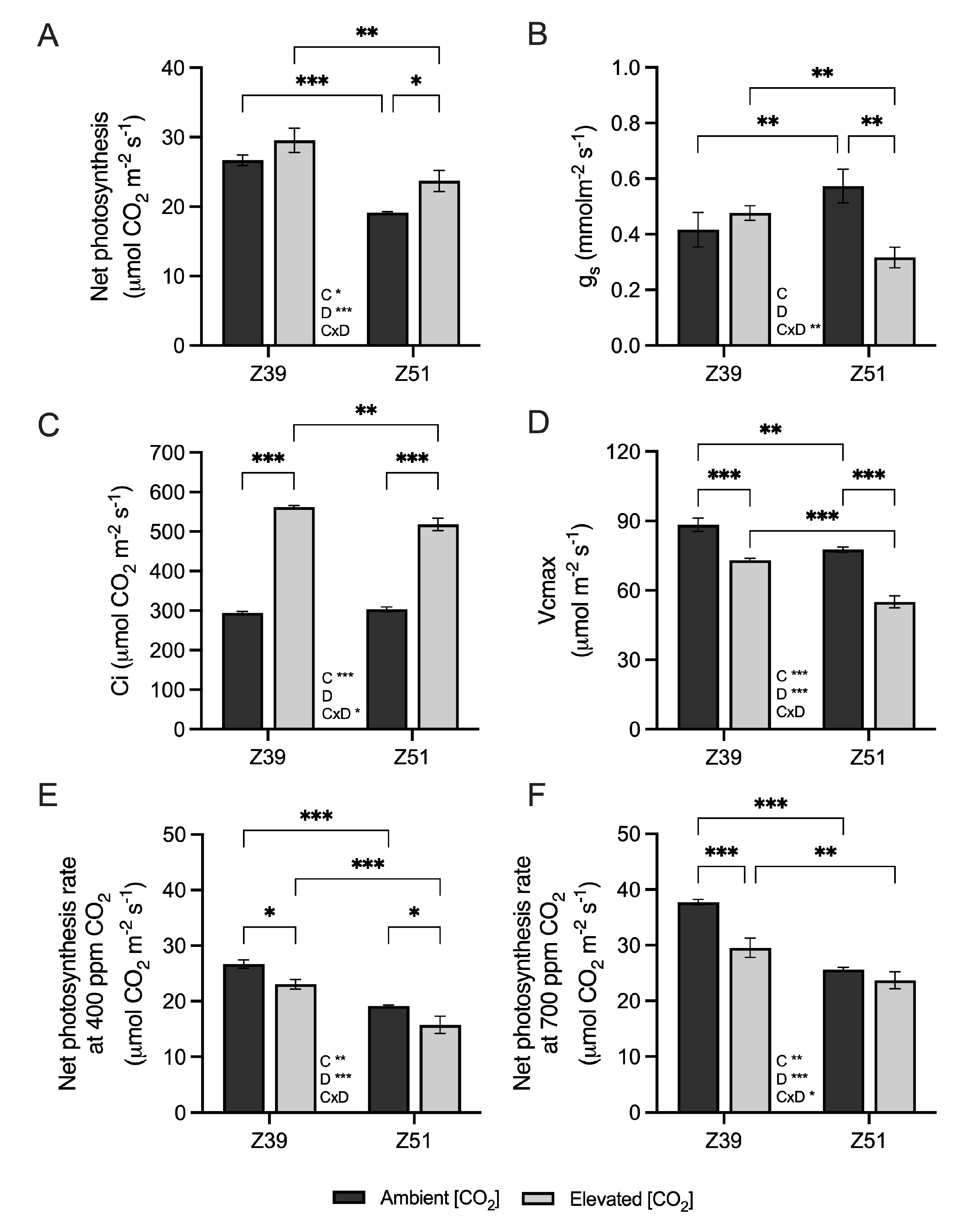
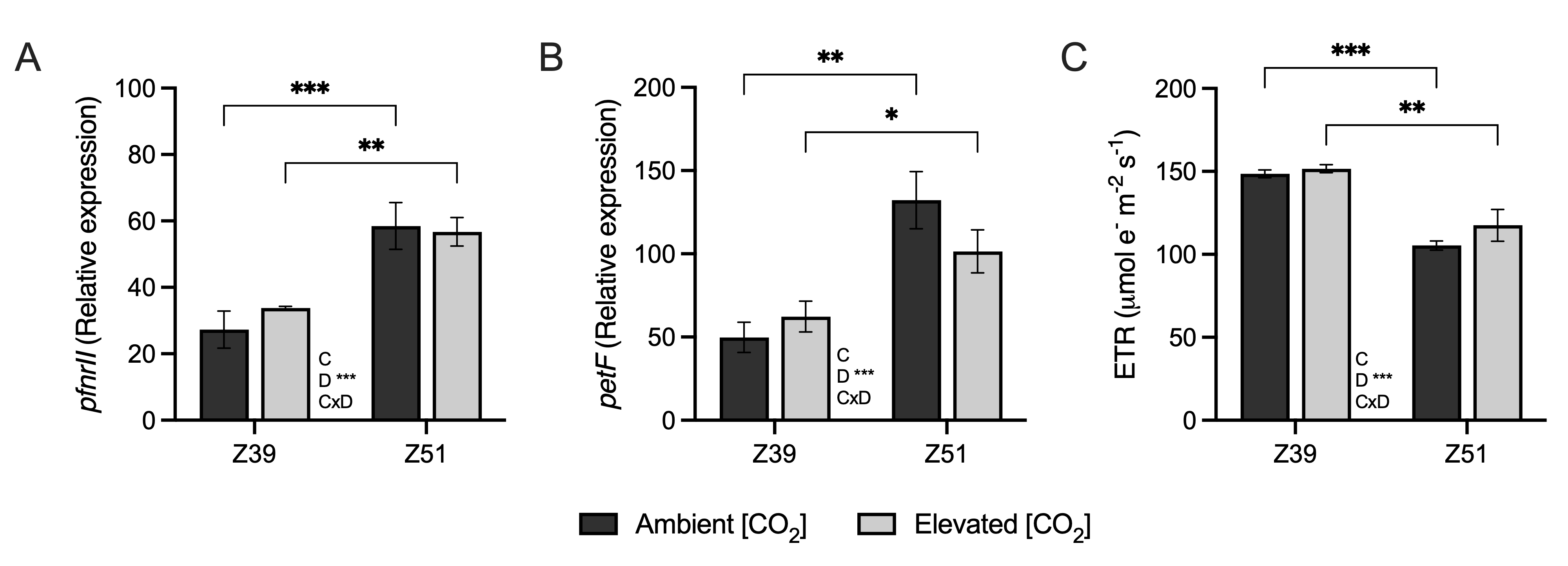
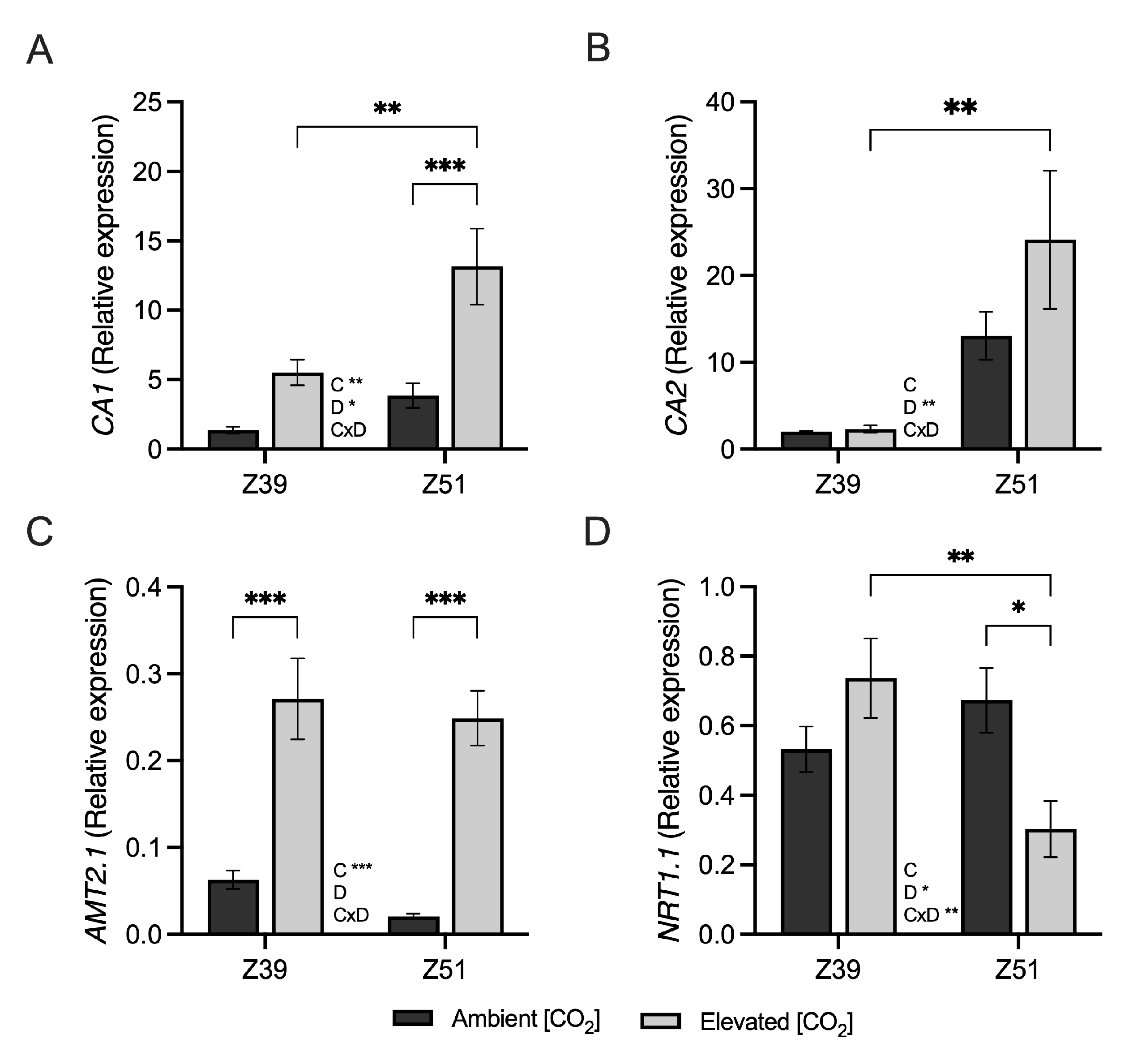
2.2. Leaf Carbon Assimilation and Related Genes
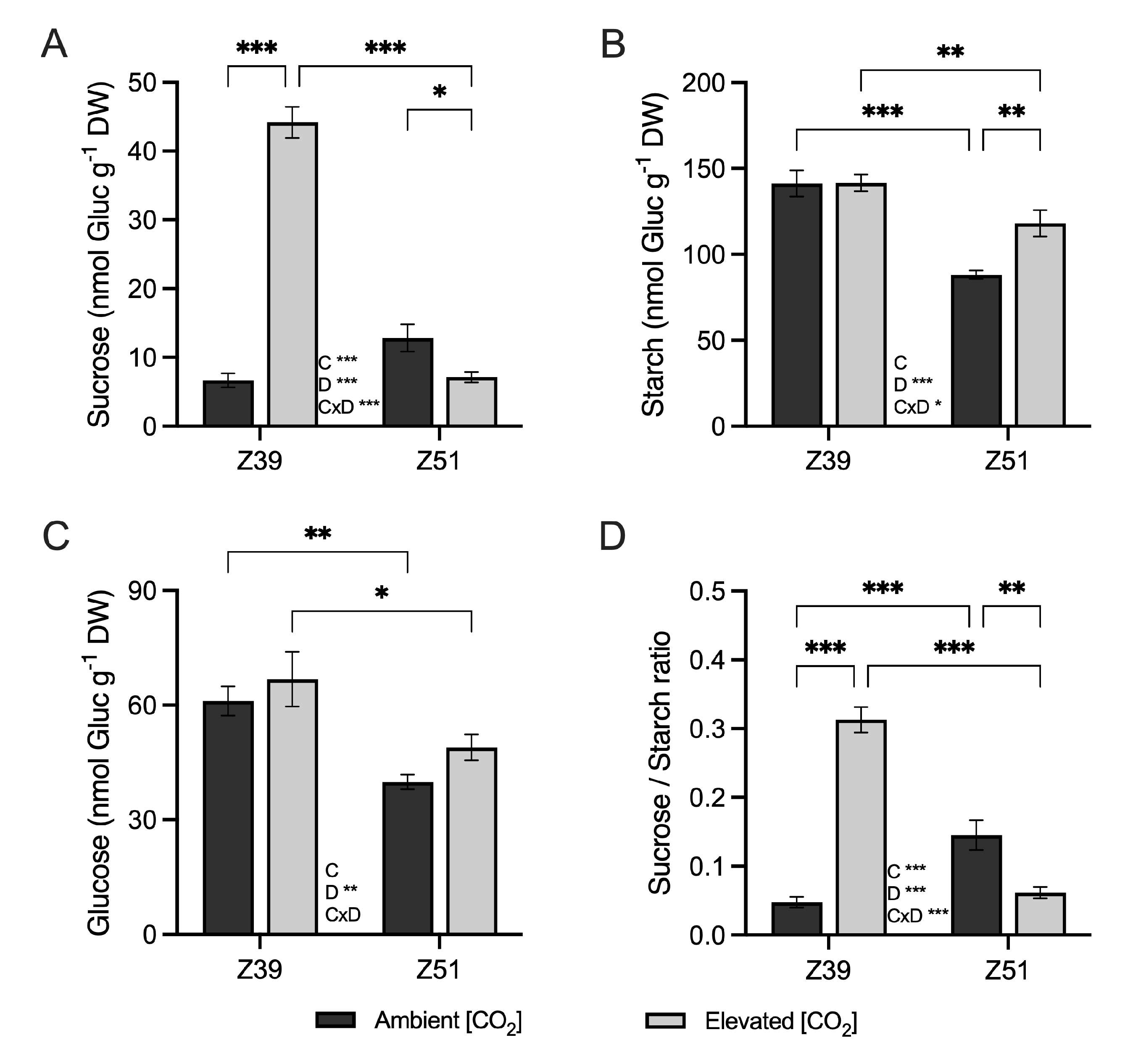
2.3. Soluble Carbohydrate Accumulation
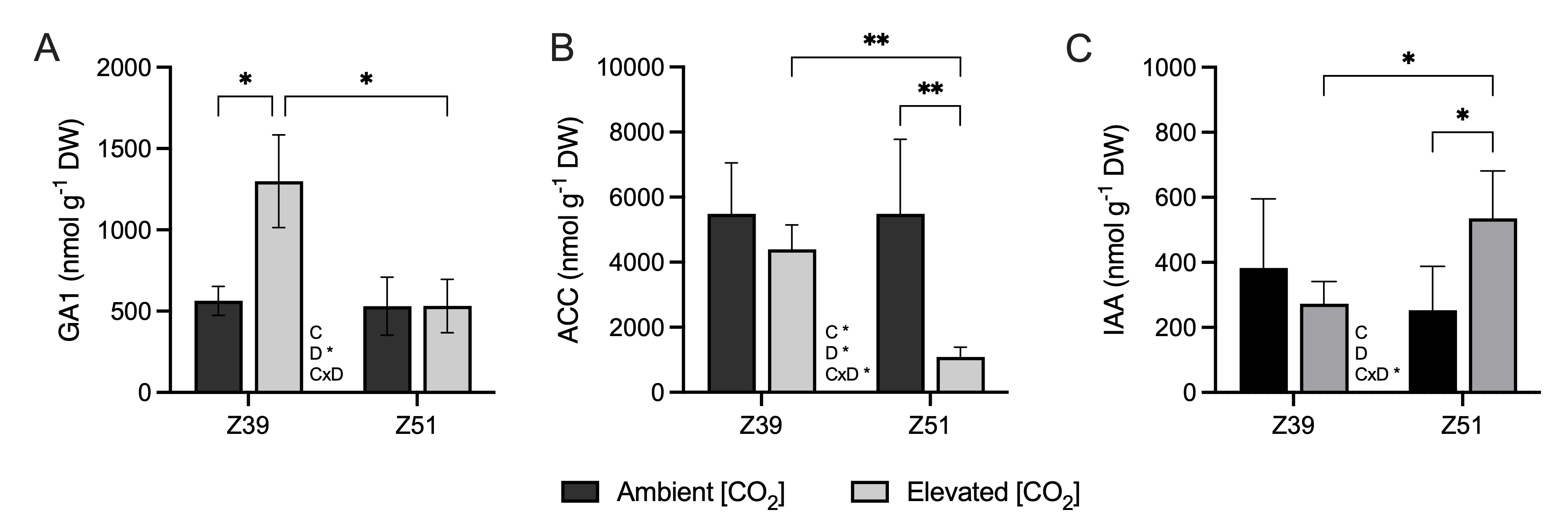
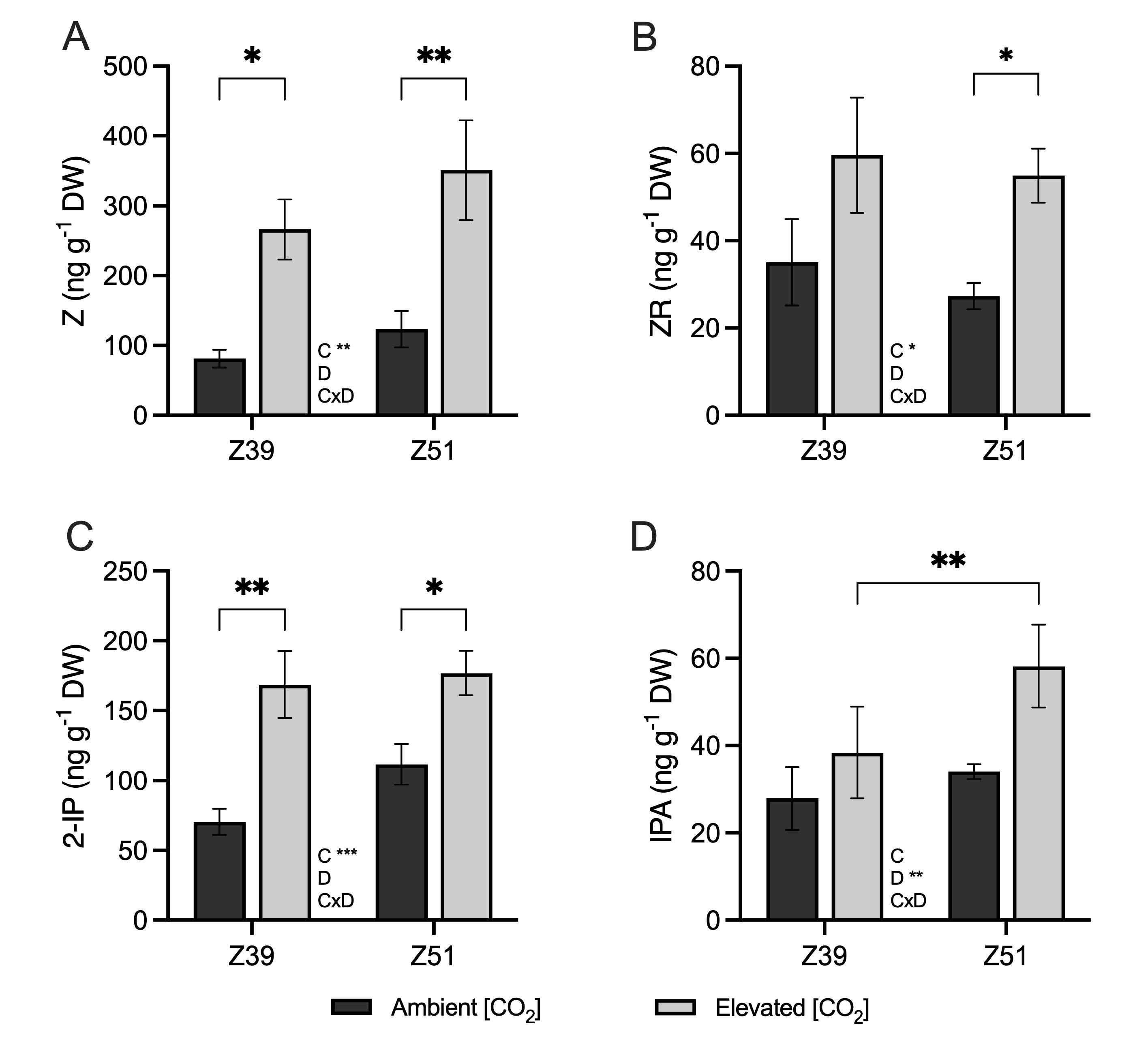
2.4. Phytohormone Contents
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Plant Material
4.2. Leaf Gas Exchange and Chlorophyll Fluorescence Determinations
4.3. Determination of Non-Structural Carbohydrates
4.4. Determination of Phytohormones
4.5. Extraction of Leaf RNA and Relative Gene Expression
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; 3056p. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 Years of Free-Air Carbon Dioxide Enrichment (FACE): What Have We Learned about Future Crop Productivity and Its Potential for Adaptation? Glob. Change Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.G.; Gonzàlez-Meler, M.A. More Efficient Plants: A Consequence of Rising Atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “Source-Sink” Hypothesis of down-Regulation of Photosynthesis in Elevated [CO2] in the Field with Single Gene Substitutions in Glycine Max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-CO2 Response of Stomata and Its Dependence on Environmental Factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Erice, G.; Nogués, S.; Moran, J.F.; Irigoyen, J.J.; Sánchez-Díaz, M. The Mechanism(s) Involved in the Photoprotection of PSII at Elevated CO2 in Nodulated Alfalfa Plants. Environ. Exp. Bot. 2008, 64, 295–306. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M.; Nogués, S. Carbon Partitioning in N2 Fixing Medicago Sativa Plants Exposed to Different CO2 and Temperature Conditions. Funct. Plant Biol. 2008, 35, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an Integrated View of Nitrogen Metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising Atmospheric Carbon Dioxide: Plants FACE the Future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The Response of Photosynthesis and Stomatal Conductance to Rising [CO2]: Mechanisms and Environmental Interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.D.; Cheng, S.; Sims, D.; Seemann, J.R. The Biochemical and Molecular Basis for Photosynthetic Acclimation to Elevated Atmospheric CO2. Plant Soil. Environ. 1999, 22, 567–582. [Google Scholar] [CrossRef]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and Primary Photosynthetic Metabolism—More than the Icing on the Cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Cabrerizo, P.M.; Arrese-Igor, C.; Aparicio-Tejo, P.M. Pea Plant Responsiveness under Elevated [CO2] Is Conditioned by the N Source (N2 Fixation versus NO3- Fertilization). Environ. Exp. Bot. 2013, 95, 34–40. [Google Scholar] [CrossRef]
- Erice, G.; Sanz-Sáez, Á.; Urdiain, A.; Araus, J.L.; Irigoyen, J.J.; Aranjuelo, I. Harvest Index Combined with Impaired N Availability Constrains the Responsiveness of Durum Wheat to Elevated CO2 Concentration and Terminal Water Stress. Funct. Plant Biol. 2014, 41, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen Assimilation and Growth of Wheat under Elevated Carbon Dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Morcuende, R.; Del Pozo, A.; Martínez-Carrasco, R.; Pérez, P. Involvement of Nitrogen and Cytokinins in Photosynthetic Acclimation to Elevated CO2 of Spring Wheat. J. Plant Physiol. 2013, 170, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Lacuesta, M.; Saiz-Fernández, I.; Podlešáková, K.; Miranda-Apodaca, J.; Novák, O.; Doležal, K.; De Diego, N. The Trans and Cis Zeatin Isomers Play Different Roles in Regulating Growth Inhibition Induced by High Nitrate Concentrations in Maize. Plant Growth Regul. 2018, 85, 199–209. [Google Scholar] [CrossRef]
- Soba, D.; Zhou, B.; Arrese-Igor, C.; Munné-Bosch, S.; Aranjuelo, I. Physiological, Hormonal and Metabolic Responses of Two Alfalfa Cultivars with Contrasting Responses to Drought. Int. J. Mol. Sci. 2019, 20, 5099. [Google Scholar] [CrossRef] [PubMed]
- Yaronskaya, E.; Vershilovskaya, I.; Poers, Y.; Alawady, A.E.; Averina, N.; Grimm, B. Cytokinin Effects on Tetrapyrrole Biosynthesis and Photosynthetic Activity in Barley Seedlings. Planta 2006, 224, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The Diverse Roles of Cytokinins in Regulating Leaf Development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Zhu, C.; Gu, J.; Zhang, W.; Liu, G.; Zhu, J. Elevated CO2-Induced Changes in Cytokinin and Nitrogen Metabolism Are Associated with Different Responses in the Panicle Architecture of Two Contrasting Rice Genotypes. Plant Growth Regul. 2019, 89, 119–129. [Google Scholar] [CrossRef]
- Shaheen, A.; Li, Z.; Yang, Y.; Xie, J.; Zhu, L.; Li, C.; Nie, F.; Wang, M.; Wang, Y.; Rasheed, A.; et al. Genetic Regulation of Wheat Plant Architecture and Future Prospects for Its Improvement. New Crops 2025, 2, 100048. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and Biochemical Impacts of Environmental Factors on Wheat Grain Development and Protein Synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Vicente, R.; Molero, G.; Serret, M.D.; Maydup, M.L.; Araus, J.L. New Avenues for Increasing Yield and Stability in C3 Cereals: Exploring Ear Photosynthesis. Curr. Opin. Plant Biol. 2020, 56, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Gebbing, T.; Schnyder, H. Pre-Anthesis Reserve Utilization for Protein and Carbohydrate Synthesis in Grains of Wheat. Plant Physiol. 1999, 121, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Gámez, A.L.; Vicente, R.; Sanchez-Bragado, R.; Jauregui, I.; Morcuende, R.; Goicoechea, N.; Aranjuelo, I. Differential Flag Leaf and Ear Photosynthetic Performance Under Elevated (CO2) Conditions During Grain Filling Period in Durum Wheat. Front. Plant Sci. 2020, 11, 587958. [Google Scholar] [CrossRef] [PubMed]
- Gámez, A.L.; Han, X.; Aranjuelo, I. Differential Effect of Free-Air CO2 Enrichment (FACE) in Different Organs and Growth Stages of Two Cultivars of Durum Wheat. Plants 2023, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Erice, G.; Sanz-Sáez, A.; Abadie, C.; Gilard, F.; Gil-Quintana, E.; Avice, J.C.; Staudinger, C.; Wienkoop, S.; Araus, J.L.; et al. Differential CO2 Effect on Primary Carbon Metabolism of Flag Leaves in Durum Wheat (Triticum durum Desf.). Plant Cell Environ. 2015, 38, 2780–2794. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed. Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Ancín, M.; Gámez, A.L.; Jauregui, I.; Galmes, J.; Sharwood, R.E.; Erice, G.; Ainsworth, E.A.; Tissue, D.T.; Sanz-Sáez, A.; Aranjuelo, I. Does the Response of Rubisco and Photosynthesis to Elevated [CO2] Change with Unfavourable Environmental Conditions? J. Exp. Bot. 2024, 75, 7351–7364. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Aranjuelo, I.; Medrano, H.; Flexas, J. Variation in Rubisco Content and Activity under Variable Climatic Factors. Photosynth. Res. 2013, 117, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT–PCR Platform to Measure Transcript Levels of C and N Metabolism-Related Genes in Durum Wheat: Transcript Profiles in Elevated [CO2] and High Temperature at Different Levels of N Supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Ineson, P.; Scoot, A. Elevated CO2 Reduces the Nitrogen Concentration of Plant. Glob. Change Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Del Pozo, A.; Pérez, P.; Gutiérrez, D.; Alonso, A.; Morcuende, R.; Martínez-Carrasco, R. Gas Exchange Acclimation to Elevated CO2 in Upper-Sunlit and Lower-Shaded Canopy Leaves in Relation to Nitrogen Acquisition and Partitioning in Wheat Grown in Field Chambers. Environ. Exp. Bot. 2007, 59, 371–380. [Google Scholar] [CrossRef]
- De la Mata, L.; De la Haba, P.; Alamillo, J.M.; Pineda, M.; Agüera, E. Elevated CO2 Concentrations Alter Nitrogen Metabolism and Accelerate Senescence in Sunflower (Helianthus annuus L.) Plants. Plant Soil Environ. 2013, 59, 303–308. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, J.P., Jr. Nitrate Assimilation Is Inhibited by Elevated CO2 in Field-Grown Wheat. Nat. Clim. Change 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The Interaction between Elevated Carbon Dioxide and Nitrogen Nutrition: The Physiological and Molecular Background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Rubio Asensio, J.S.; Cousins, A.B. Carbon Dioxide Enrichment Inhibits Nitrate Assimilation in Wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Torralbo, F.; González-Moro, M.B.; Baroja-Fernández, E.; Aranjuelo, I.; González-Murua, C. Differential Regulation of Stomatal Conductance as a Strategy to Cope with Ammonium Fertilizer under Ambient versus Elevated CO2. Front. Plant Sci. 2019, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does Ear C Sink Strength Contribute to Overcoming Photosynthetic Acclimation of Wheat Plants Exposed to Elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef] [PubMed]
- Erice, G.; Sanz-Sáez, A.; Aroca, R.; Ruíz-Lozano, J.M.; Avice, J.C.; Irigoyen, J.J.; Sanchez-Diaz, M.; Aranjuelo, I. Photosynthetic Down-Regulation in N2-Fixing Alfalfa under Elevated CO2 Alters Rubisco Content and Decreases Nodule Metabolism via Nitrogenase and Tricarboxylic Acid Cycle. Acta. Physiol. Plant 2014, 36, 2607–2617. [Google Scholar] [CrossRef]
- Hu, H.; Boisson-Dernier, A.; Israelsson-Nordström, M.; Böhmer, M.; Xue, S.; Ries, A.; Godoski, J.; Kuhn, J.M.; Schroeder, J.I. Carbonic Anhydrases Are Upstream Regulators of CO2-Controlled Stomatal Movements in Guard Cells. Nat. Cell Biol. 2010, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Dier, M.; Sickora, J.; Erbs, M.; Weigel, H.J.; Zörb, C.; Manderscheid, R. Positive Effects of Free Air CO2 Enrichment on N Remobilization and Post-Anthesis N Uptake in Winter Wheat. Field Crops Res. 2019, 234, 107–118. [Google Scholar] [CrossRef]
- Dier, M.; Meinen, R.; Erbs, M.; Kollhorst, L.; Baillie, C.-K.; Kaufholdt, D.; Kücke, M.; Weigel, H.-J.; Zörb, C.; Hänsch, R.; et al. Effects of Free Air Carbon Dioxide Enrichment (FACE) on Nitrogen Assimilation and Growth of Winter Wheat under Nitrate and Ammonium Fertilization. Glob. Change Biol. 2017, 24, e40–e54. [Google Scholar] [CrossRef] [PubMed]
- John, I.; Drake, R.; Farrell, A.; Cooper, W.; Lee, P.; Horton, P.; Grierson, D. Delayed Leaf Senescence in Ethylene-deficient ACC-oxidase Antisense Tomato Plants: Molecular and Physiological Analysis. Plant J. 1995, 7, 483–490. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Serret, M.D.; Elazab, A.; Bort Pie, J.; Araus, J.L.; Aranjuelo, I.; Sanz-Sáez, Á. Wheat Ear Carbon Assimilation and Nitrogen Remobilization Contribute Significantly to Grain Yield. J. Integr. Plant Biol. 2016, 58, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.V.; Caputo, C.; Roberts, I.N.; Castro, M.A.; Barneix, A.J. Cytokinin-Induced Changes of Nitrogen Remobilization and Chloroplast Ultrastructure in Wheat (Triticum aestivum). J. Plant Physiol. 2009, 166, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta-Mancera, H.A.; Thomas, B.J.; Thomas, H.; Scott, I.M. Regreening of Senescent Nicotiana Leaves. II. Redifferentiation of Plastids. J. Exp. Bot. 1999, 50, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-Dependent Photorespiration and the Protection of Photosynthesis during Water Deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.W.H.; Wong, S.C.; Letham, D.S.; Hocart, C.H.; Farquhar, G.D. Effects of Elevated [CO2] and Nitrogen Nutrition on Cytokinins in the Xylem Sap and Leaves of Cotton. Plant Physiol. 2000, 124, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 Induces Physiological, Biochemical and Structural Changes in Leaves of Arabidopsis Thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Amasino, R.M. Cytokinins in Plant Senescence: From Spray and Pray to Clone and Play. BioEssays 1996, 18, 557–565. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Peleg, Z.; Abdel-Tawab, Y.M.; Tumimbang, E.B.; Delatorre, C.A.; Blumwald, E. Stress-Induced Cytokinin Synthesis Increases Drought Tolerance through the Coordinated Regulation of Carbon and Nitrogen Assimilation in Rice. Plant Physiol. 2013, 163, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Koprna, R.; Humplík, J.F.; Špíšek, Z.; Bryksová, M.; Zatloukal, M.; Mik, V.; Novák, O.; Nisler, J.; Doležal, K. Improvement of Tillering and Grain Yield by Application of Cytokinin Derivatives in Wheat and Barley. Agronomy 2021, 11, 67. [Google Scholar] [CrossRef]
- Robles-Zazueta, C.A.; Crespo-Herrera, L.A.; Piñera-Chavez, F.J.; Rivera-Amado, C.; Aradottir, G.I. Climate Change Impacts on Crop Breeding: Targeting Interacting Biotic and Abiotic Stresses for Wheat Improvement. Plant Genome 2024, 17, e20365. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I.; Hoagland, D.R. A Comparison of Water Culture and Soil as Media for Crop Production. Science 1939, 89, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 Leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Mendizábal, T.; Setién, I.; Estavillo, J.M.; González-Moro, M.B. Late Nitrogen Fertilization Affects Carbohydrate Mobilization in Wheat. J. Plant Nutr. Soil Sci. 2010, 173, 907–919. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Rapid and Sensitive Hormonal Profiling of Complex Plant Samples by Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Plant Methods 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Pistón, F.; Atienza, S.G. Identification of Suitable Reference Genes for Normalization of QPCR Data in Comparative Transcriptomics Analyses in the Triticeae. Planta 2011, 233, 163–173. [Google Scholar] [CrossRef] [PubMed]
| Zadoks | [CO2] | Shoot Dry Biomass (g) | N Content (%) | C Content (%) | C/N Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| Z39 | 400 | 5.28 ± 0.11 | b | 2.4 ± 0.26 | ab | 44 ± 0.37 | a | 18.97 ± 2.08 | ab |
| 700 | 7.67 ± 0.51 | b | 1.85 ± 0.04 | b | 43.53 ± 0.47 | a | 23.62 ± 0.52 | a | |
| Z51 | 400 | 21.77 ± 2.73 | a | 2.69 ± 0.25 | a | 43.45 ± 0.47 | a | 16.68 ± 1.83 | b |
| 700 | 26.26 ± 1.1 | a | 2.18 ± 0.17 | ab | 43.5 ± 0.49 | a | 20.28 ± 1.59 | a | |
| [CO2]: * Shoot Dry Biomass (g), * N content (%), * C/N ratio | |||||||||
| Dev.: *** Shoot Dry Biomass (g), * N content (%), * C/N ratio | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torralbo, F.; Munné-Bosch, S.; González-Murua, C.; Aranjuelo, I. Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat. Plants 2025, 14, 2224. https://doi.org/10.3390/plants14142224
Torralbo F, Munné-Bosch S, González-Murua C, Aranjuelo I. Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat. Plants. 2025; 14(14):2224. https://doi.org/10.3390/plants14142224
Chicago/Turabian StyleTorralbo, Fernando, Sergi Munné-Bosch, Carmen González-Murua, and Iker Aranjuelo. 2025. "Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat" Plants 14, no. 14: 2224. https://doi.org/10.3390/plants14142224
APA StyleTorralbo, F., Munné-Bosch, S., González-Murua, C., & Aranjuelo, I. (2025). Impact of Plant Developmental Stage on Photosynthetic Acclimation to Elevated [CO2] in Durum Wheat. Plants, 14(14), 2224. https://doi.org/10.3390/plants14142224







