Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China
Abstract
1. Introduction
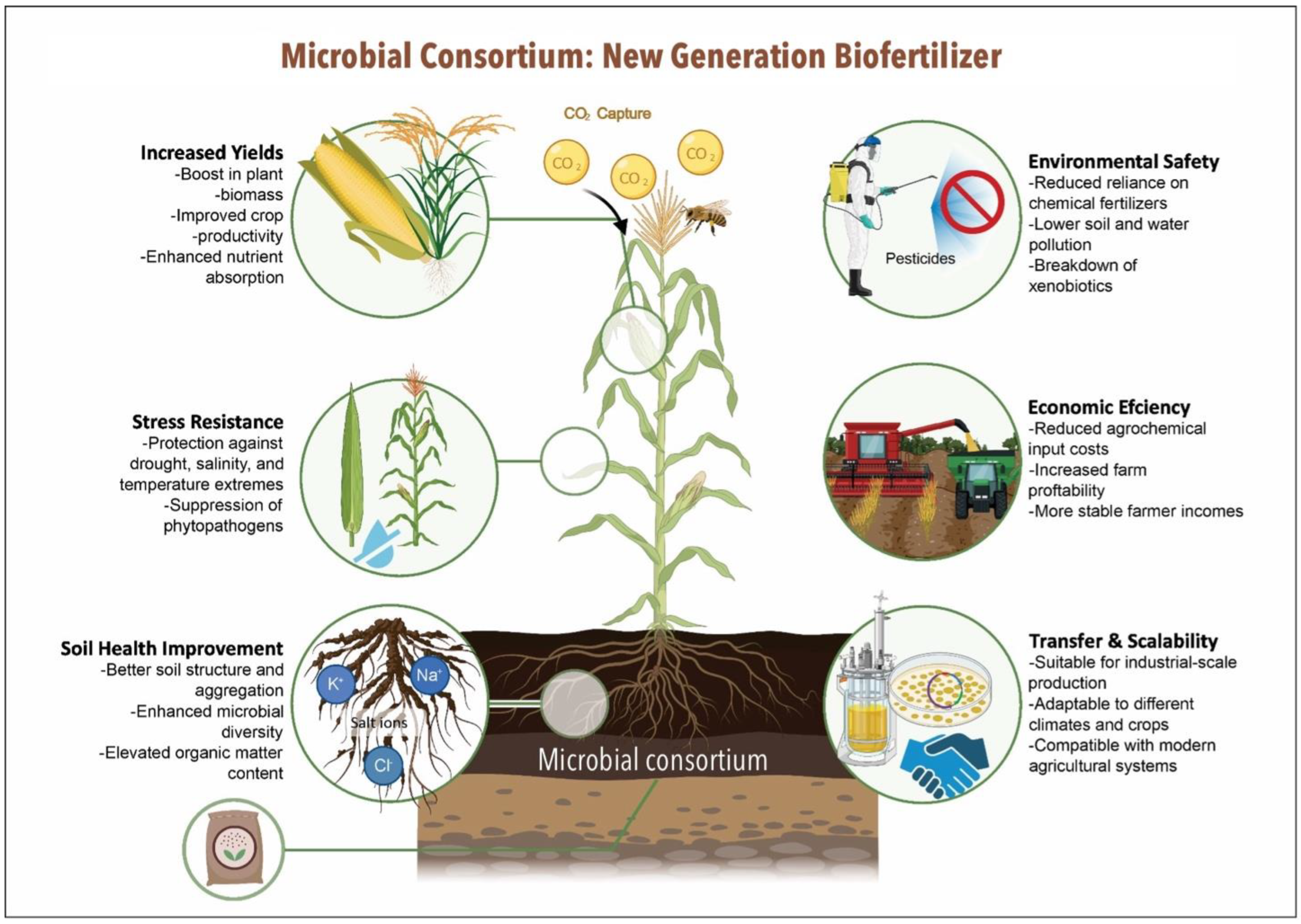
2. Functional Capabilities of Microbial Consortia in the Context of Sustainable Agriculture
3. Scientific Foundations and Applied Research on MCs in China
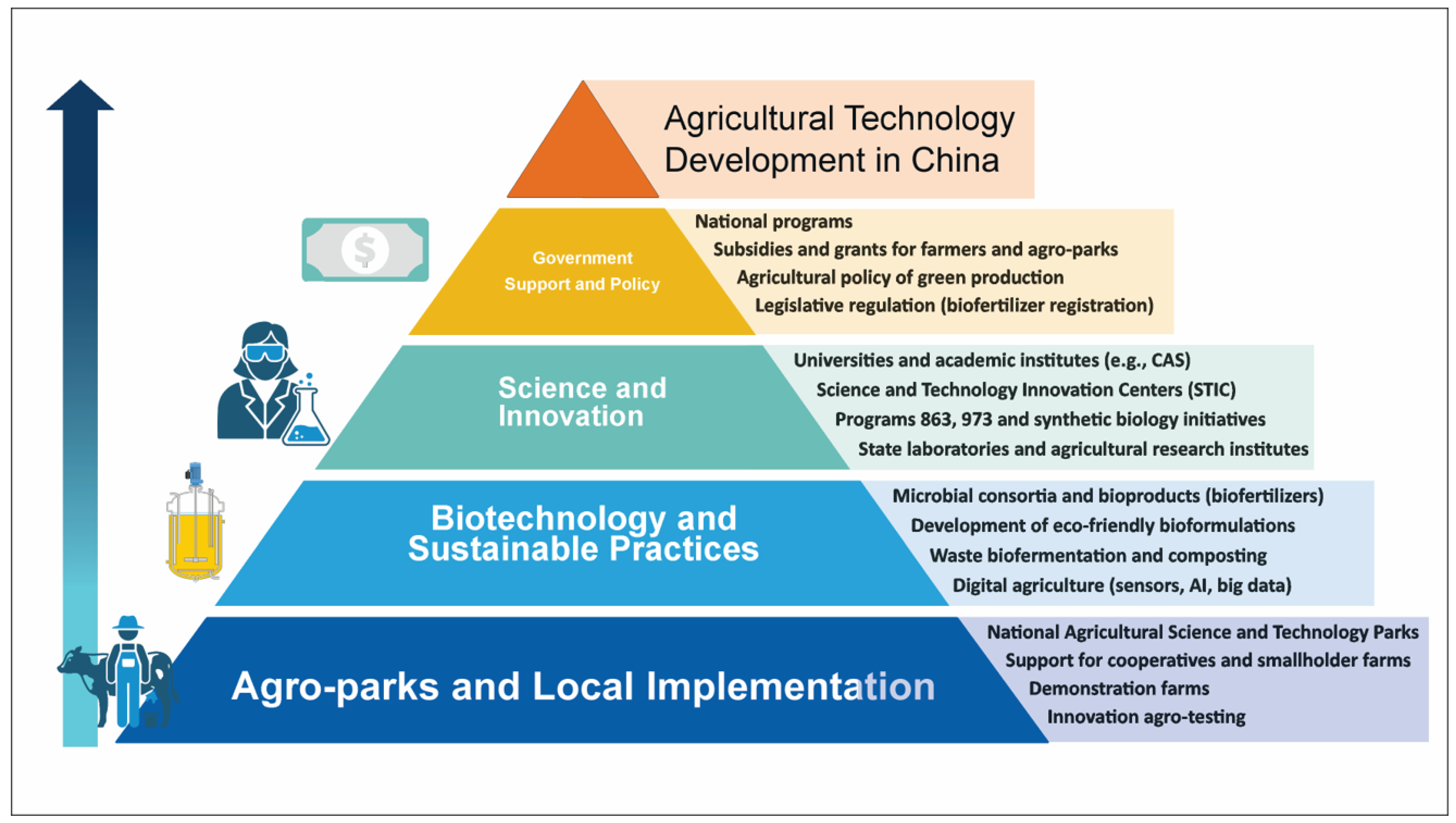
3.1. Microbial Biotechnologies in Chinese Agricultural Modernization
3.2. Scientific Research and Achievements in the Application of MCs in Chinese Agricultural Practice
4. The Development and Application of Microbial Fertilizers in Agricultural Practices in Kazakhstan: A Current State and Scientific Perspectives
5. Technology Transfer in Agricultural Practice: The Cases of China and Kazakhstan
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MC | Microbial Consortium |
| R&D | Research and Development |
| PGPMs | Plant Growth-Promoting Microorganisms |
| HMs | Heavy Metals |
References
- Mousavi, H.; Solberg, S.Ø.; Cottis, T. Nitrogen Enriched Organic Fertilizer (NEO) Elevates Nitrification Rates Shortly after Application but Has No Lasting Effect on Nitrification in Agricultural Soils. Agric. Food Sci. 2023, 32, 179–194. [Google Scholar] [CrossRef]
- Langridge, P.; Alaux, M.; Almeida, N.F.; Ammar, K.; Baum, M.; Bekkaoui, F.; Bentley, A.R.; Beres, B.L.; Berger, B.; Braun, H.-J.; et al. Meeting the Challenges Facing Wheat Production: The Strategic Research Agenda of the Global Wheat Initiative. Agronomy 2022, 12, 2767. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Akpan, U.M. Impacts of Chemical Use in Agricultural Practices: Perspectives of Soil Microorganisms and Vegetation. In One Health Implications of Agrochemicals and their Sustainable Alternatives; Ogwu, M.C., Chibueze Izah, S., Eds.; Sustainable Development and Biodiversity; Springer Nature: Singapore, 2023; Volume 34, pp. 765–792. [Google Scholar] [CrossRef]
- Hossain, M.E.; Shahrukh, S.; Hossain, S.A. Chemical Fertilizers and Pesticides: Impacts on Soil Degradation, Groundwater, and Human Health in Bangladesh. In Environmental Degradation: Challenges and Strategies for Mitigation; Singh, V.P., Yadav, S., Yadav, K.K., Yadava, R.N., Eds.; Water Science and Technology Library; Springer International Publishing: Cham, Switzerland, 2022; Volume 104, pp. 63–92. [Google Scholar] [CrossRef]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-aree, W.; Manzanera, M. Impacts of Agriculture on the Environment and Soil Microbial Biodiversity. Plants 2021, 10, 2325. [Google Scholar] [CrossRef]
- Bhuyan, P.P.; Nayak, R.; Jena, M.; Pradhan, B. Convoluted Role of Cyanobacteria as Biofertilizer: An Insight of Sustainable Agriculture. Vegetos 2022, 36, 309–321. [Google Scholar] [CrossRef]
- Boix-Fayos, C.; De Vente, J. Challenges and Potential Pathways towards Sustainable Agriculture within the European Green Deal. Agric. Syst. 2023, 207, 103634. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated Microbial Consortia Perform Better than Single Strains in Living Soil: A Meta-Analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Yao, Y.; Li, H.; Wang, Q.; Niu, B. Microbial Interactions within Beneficial Consortia Promote Soil Health. Sci. Total Environ. 2023, 900, 165801. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Jan, T.; Kaur, T.; Chowdhury, S.; Kapoor, M.; Singh, S.; Kumar, A.; Rai, A.K.; Rustagi, S.; et al. Microbial Consortia: Promising Tool as Plant Bioinoculants for Agricultural Sustainability. Curr. Microbiol. 2024, 81, 222. [Google Scholar] [CrossRef]
- Sharma, S.; Rathod, Z.R.; Jain, R.; Goswami, D.; Saraf, M. Strategies to Evaluate Microbial Consortia for Mitigating Abiotic Stress in Plants. In Sustainable Agrobiology; Maheshwari, D.K., Dheeman, S., Eds.; Microorganisms for Sustainability; Springer Nature Singapore: Singapore, 2023; Volume 43, pp. 177–203. [Google Scholar] [CrossRef]
- Sunar, K.; Das, K.; Rai, A.K.; Gurung, S.A. Beneficial Microbial Consortia and Their Role in Sustainable Agriculture Under Climate Change Conditions. In Microbial Symbionts and Plant Health: Trends and Applications for Changing Climate; Mathur, P., Kapoor, R., Roy, S., Eds.; Rhizosphere Biology; Springer Nature: Singapore, 2023; pp. 41–73. [Google Scholar] [CrossRef]
- Behera, B.; Das, T.K.; Raj, R.; Ghosh, S.; Raza, M.B.; Sen, S. Microbial Consortia for Sustaining Productivity of Non-Legume Crops: Prospects and Challenges. Agric. Res. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Miller, T.; Kisiel, A.; Cembrowska-Lech, D.; Mikiciuk, M.; Łobodzińska, A.; Bokszczanin, K. Harnessing Beneficial Microbes for Drought Tolerance: A Review of Ecological and Agricultural Innovations. Agriculture 2024, 14, 2228. [Google Scholar] [CrossRef]
- Behera, L.; Datta, D.; Kumar, S.; Kumar, S.; Sravani, B.; Chandra, R. Role of Microbial Consortia in Remediation of Soil, Water and Environmental Pollution Caused by Indiscriminate Use of Chemicals in Agriculture: Opportunities and Challenges. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–418. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Nuti, M. Ecosystem Functions of Microbial Consortia in Sustainable Agriculture. Agronomy 2020, 10, 1902. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H. Microbial Consortia Are Needed to Degrade Soil Pollutants. Microorganisms 2022, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Kushiev, K.; R, K.; Husanov, T.; Rakhimov, B.; Khudayberdiyev, G.; Shavkat, K.; Alhomaidi, E.; Zafar, M.; Majeed, S.; Makhkamov, T.; et al. Pathogen Management in Glycyrrhiza Glabra: Microbial Interactions and Phylogenetic Insights. J. Phytopathol. 2025, 173, e70057. [Google Scholar] [CrossRef]
- Clagnan, E.; Costanzo, M.; Visca, A.; Di Gregorio, L.; Tabacchioni, S.; Colantoni, E.; Sevi, F.; Sbarra, F.; Bindo, A.; Nolfi, L.; et al. Culturomics- and Metagenomics-Based Insights into the Soil Microbiome Preservation and Application for Sustainable Agriculture. Front. Microbiol. 2024, 15, 1473666. [Google Scholar] [CrossRef]
- Khan, S.T. Consortia-Based Microbial Inoculants for Sustaining Agricultural Activities. Appl. Soil Ecol. 2022, 176, 104503. [Google Scholar] [CrossRef]
- Negi, R.; Sharma, B.; Parastesh, F.; Kaur, S.; Khan, S.S.; Kour, D.; Singh, S.; Rai, A.K.; Rustagi, S.; Yadav, N.; et al. Microbial Consortia Mediated Regulation of Plant Defense: A Promising Tool for Sustaining Crops Protection. Physiol. Mol. Plant Pathol. 2024, 134, 102393. [Google Scholar] [CrossRef]
- Kampa, R.; Vittal, R.; Basha, S.A.; Pushpavalli, S.N.C.V.L.; Bokka, V. Plant Growth-Promoting Microbial Consortia for Effective Biocontrol of Groundnut Collar Rot and Chickpea Wilt. Plant Pathol. 2025, 74, 1121–1141. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.N. Microbial Consortium of Mineral Solubilizing and Nitrogen Fixing Bacteria for Plant Growth Promotion of Amaranth (Amaranthus hypochondrius L.). Biocatal. Agric. Biotechnol. 2022, 43, 102404. [Google Scholar] [CrossRef]
- Da Costa Neto, V.P.; De Melo, A.R.P.; Alencar, C.E.S.; De Lima, V.B.C.; Zilli, J.E.; Rodrigues, A.C.; Bonifacio, A. Bacterial Consortia among Bradyrhizobium Species, Azospirillum Baldaniorum and Bacillus Pumilus Promote Plant Growth and Efficient Symbiotic Nitrogen Fixation in Mung Bean. Symbiosis 2024, 93, 255–267. [Google Scholar] [CrossRef]
- Travençoli Rossetim, M.F.; Vargas Motta, A.C.; Rocha Kondo, Y.; Santos Ruthes, B.E.; Hungria, M.; Falcão Salles, J.; Kaschuk, G. Enhancing Soybean Yield Through Inoculation of Multifunctional Microbial Consortia. Int. J. Agron. 2025, 2025, 9491715. [Google Scholar] [CrossRef]
- Karmakar, B.; Thakuria, D.; Begum, R.H.; Joga, R.J. Recent Advances in Experimental Design of Synthetic Microbial Communities for Biocontrol Application. BioControl 2025, 70, 229–244. [Google Scholar] [CrossRef]
- Tariq, A.; Guo, S.; Farhat, F.; Shen, X. Engineering Synthetic Microbial Communities: Diversity and Applications in Soil for Plant Resilience. Agronomy 2025, 15, 513. [Google Scholar] [CrossRef]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.-M.; Širić, I.; Kumar, P.; et al. Application of Synthetic Consortia for Improvement of Soil Fertility, Pollution Remediation, and Agricultural Productivity: A Review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Shayanthan, A.; Ordoñez, P.A.C.; Oresnik, I.J. The Role of Synthetic Microbial Communities (SynCom) in Sustainable Agriculture. Front. Agron. 2022, 4, 896307. [Google Scholar] [CrossRef]
- Li, S.; Xiao, J.; Sun, T.; Yu, F.; Zhang, K.; Feng, Y.; Xu, C.; Wang, B.; Cheng, L. Synthetic Microbial Consortia with Programmable Ecological Interactions. Methods Ecol. Evol. 2022, 13, 1608–1621. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Xiong, C.; Egidi, E.; Singh, B.K. Formulation Challenges Associated with Microbial Biofertilizers in Sustainable Agriculture and Paths Forward. J. Sustain. Agric. Environ. 2024, 3, e70006. [Google Scholar] [CrossRef]
- Arfarita, N.; Higuchi, T.; Prayogo, C. Effects of Seaweed Waste on the Viability of Three Bacterial Isolates in Biological Fertilizer Liquid Formulations to Enhance Soil Aggregation and Fertility. J. Deg. Min. Land. Manag. 2019, 6, 1889–1895. [Google Scholar] [CrossRef][Green Version]
- Beltrán-Rocha, J.C.; Barceló-Quintal, I.D.; García-Martínez, M.; Osornio-Berthet, L.; Saavedra-Villarreal, N.; Villarreal-Chiu, J.; López-Chuken, U.J. Polishing of Municipal Secondary Effluent Using Native Microalgae Consortia. Water Sci. Technol. 2017, 75, 1693–1701. [Google Scholar] [CrossRef]
- Florencio, C.; Bortoletto-Santos, R.; Favaro, C.; Brondi, M.; Velloso, C.; Klaic, R.; Ribeiro, C.; Farinas, C.; Mattoso, L. Avanços na Produção e Formulação de Inoculantes Microbianos Visando uma Agricultura Mais Sustentável. Quím. Nova 2022. [Google Scholar] [CrossRef]
- Ángeles, R.; Arnaiz, E.; Gutiérrez, J.; Sepúlveda-Muñoz, C.A.; Fernández-Ramos, O.; Muñoz, R.; Lebrero, R. Optimization of Photosynthetic Biogas Upgrading in Closed Photobioreactors Combined with Algal Biomass Production. J. Water Process Eng. 2020, 38, 101554. [Google Scholar] [CrossRef]
- Zheng, Y.; Czajka, J.J.; Daiek, C.; Yang, Z.; Sun, L.; Tang, Y.J.; Liu, Y.; Liao, W. An Algal-Bacterial Symbiotic System of Carbon Fixation Using Formate as a Carbon Source. Algal Res. 2023, 72, 103103. [Google Scholar] [CrossRef]
- Fu, W.G.; Yao, T. Effects of the Addition of Medium and Trace Elements and Their Combinations on the Growth Promoting Properties of Microbial Inoculants. Pratacult. Sci. 2024, 41, 2859–2868. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, S.K.; Arya, S.K.; Srivastava, D.; Rajput, V.D.; Husain, R. Multifunctional Growth-Promoting Microbial Consortium-Based Biofertilizers and Their Techno-Commercial Feasibility for Sustainable Agriculture. In Rhizobiome; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–208. [Google Scholar] [CrossRef]
- Praveen, K.; Abinandan, S.; Venkateswarlu, K.; Megharaj, M. Synergy of Eco-Innovation with on-Farm Practices Enhances Circularity beyond Conventional Nutrient Recovery Framework. Resour. Conserv. Recycl. 2024, 208, 107735. [Google Scholar] [CrossRef]
- Rörig, L.R.; Lima, A.O.D.S.; Bonomi-Barufi, J.; Junker, E.; Do Nascimento, M.E.C.; Morillas-España, A.; Abdala-Diaz, R.T.; Vega, J.; Avilés, A.; Acién-Fernandez, F.G.; et al. Beyond the Target Species—Implications of Microalgal Succession and Associated Microbiome in Industrial-Scale Photobioreactors. Algal Res. 2024, 83, 103692. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-Organic Fertilizers Stimulate Indigenous Soil Pseudomonas Populations to Enhance Plant Disease Suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef]
- Bagga, D.; Chauhan, S.; Bhavanam, A.; Nikhil, G.N.; Meena, S.S.; Mohanty, A. Recent Advancements in Fermentation Strategies for Mass Production and Formulation of Biofertilizers: Towards Waste Valorization. J. Soil Sci. Plant Nutr. 2024, 24, 5868–5897. [Google Scholar] [CrossRef]
- History of Agriculture in China: A Journey Through Time and Space. Available online: https://artsandculture.google.com/story/history-of-agriculture-in-china-a-journey-through-time-and-space-world-federation-of-chinese-catering-industry/xwXx9Y9gjbIOeg?hl=en (accessed on 20 June 2025).
- The World Bank. The Greening of China’s Agriculture: A Compendium of Thematic Papers. 2022. Available online: https://documents1.worldbank.org/curated/en/099556012222213645/pdf/P1715180a3bf0d020b09a0e07af942239e.pdf (accessed on 20 June 2025).
- Ma, D.; Peng, K. Agriculture. In The Cambridge Economic History of China; Ma, D., Von Glahn, R., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 87–123. ISBN 978-1-108-34848-5. [Google Scholar]
- Delang, C.O. The Consequences of Soil Degradation in China: A Review. GeoScape 2018, 12, 92–103. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Qi, G.; Tang, L.; Mukwereza, L. Science, Technology, and the Politics of Knowledge: The Case of China’s Agricultural Technology Demonstration Centers in Africa. World Dev. 2016, 81, 82–91. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, W.; Wang, Y. Reflections on the History of Modern Science and Technology in China. Sci. Cult. Rev. 2014, 11, 5–24. Available online: https://core.ac.uk/outputs/48311779/?utm_source=pdf&utm_medium=banner&utm_campaign=pdf-decoration-v1 (accessed on 21 March 2025). (Translated from Chinese).
- Lin, W.-X.; Chen, T.; Zhou, M.-M. New Dimensions in Agroecology. Chin. J. Eco-Agric. 2012, 20, 253–264, (Translated from Chinese). [Google Scholar] [CrossRef]
- Li, W.-H.; Liu, M.-C.; Min, Q.-W. Agricultural Heritage Conservation: New Opportunity for Developing Eco-Agriculture. Chin. J. Eco-Agric. 2012, 20, 663–667. (In Chinese) [Google Scholar] [CrossRef]
- Wang, H.; Gao, H.; Xin, F. Analysis of the Development Trend of Synthetic Biology Industry under the Background of “14th Five-Year Plan”. J. Biol. 2023, 40, 3. (In Chinese) [Google Scholar] [CrossRef]
- Chen, T.; Ren, Y.; Ke, X. Comparative Study on Agricultural Science and Technology Innovation Systems in China and Western Countries. J. Agric. Sci. Technol. 2020, 22, 1. (In Chinese) [Google Scholar] [CrossRef]
- FAO. Scaling up agroecology to achieve the sustainable development goals. In Proceedings of the Second FAO International Symposium, Rome, Italy, 3–5 April 2018; 412p. [Google Scholar]
- Yu, H.; Liu, X. Current Situation and Development Suggestions for the Construction of Agricultural Science and Technology Innovation Centers in China. J. Agric. Sci. Technol. 2021, 23, 10. (In Chinese) [Google Scholar]
- Yu, H.; Liu, X. Current Situation and Development Suggestions for the Construction of National Modern Agricultural Industry Science and Technology Innovation Centers. J. Agric. Sci. Technol. 2023, 25, 1–5. (In Chinese) [Google Scholar]
- Sun, H.; Liu, X.; Ju, Z.; Guo, K.; Dong, B. Development Mode of Cangzhou National Agricultural Science and Technology Park in Hebei Province, China. Chin. J. Eco-Agric. 2016, 24, 1145–1150. [Google Scholar]
- Chen, F.; Ding, C.-J.; Chen, Y.-W.; Zheng, Y.; Deng, Y.; Xu, P.; Yu, J.-R.; Wu, L.-H.; Ma, J.-C.; Zeng, Y.; et al. A Study on the Trend and Prospective of Industrial Biotechnology in China. China Biotechnol. 2016, 36, 1–11. (In Chinese) [Google Scholar]
- Zhou, M. The Realistic Challenge and Countermeasure Analysis of the Development of Biological Pesticide in China. Chin. J. Biol. Control 2021, 37, 184–192. (In Chinese) [Google Scholar]
- Zhuo, F.; Zhang, H.; Liu, W.; Zhang, J. Review and Prospects on Microbiological Pesticides Used on Grain Crops in China. Chin. J. Biol. Control 2023, 39, 747–751. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, M.; Du, J.; Song, Z.; Wu, Q.; Zhu, H. Development of Agricultural Microbial Industry in China. Chin. J. Eng. Sci. 2022, 24, 197. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, D.; Xue, Z.; Gao, Y. Research on the Use of Digital Finance and the Adoption of Green Control Techniques by Family Farms in China. Technol. Soc. 2020, 62, 101323. [Google Scholar] [CrossRef]
- Ding, M.-Z.; Song, H.; Wang, E.-X.; Liu, Y.; Yuan, Y.-J. Design and Construction of Synthetic Microbial Consortia in China. Synth. Syst. Biotechnol. 2016, 1, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, Y.; Peng, J.; Zhao, L. Synthetic Biology Industry in China: Current State and Future Prospects. Synth. Biol. Eng. 2023, 1, 10014. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, X.; Zheng, G.; Meng, G.; Dong, Z.; Baek, J.H.; Jeon, C.O.; Yao, Y.; Xuan, Y.H.; Zhang, J.; et al. Development of Biofertilizers for Sustainable Agriculture over Four Decades (1980–2022). Geogr. Sustain. 2024, 5, 19–28. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, L.; Lu, X.; Cui, F.; Wang, J.; Zhou, C. A Simplified Synthetic Rhizosphere Bacterial Community Steers Plant Oxylipin Pathways for Preventing Foliar Phytopathogens. Plant Physiol. Biochem. 2023, 202, 107941. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Jiang, C.-H.; Si, F.; Song, N.; Yang, W.; Zhu, Y.; Luo, Y.; Guo, J.-H. Long-Term Field Application of a Plant Growth-Promoting Rhizobacterial Consortium Suppressed Root-Knot Disease by Shaping the Rhizosphere Microbiota. Plant Dis. 2024, 108, 94–103. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Uwaremwe, C.; Zhao, X.; Yue, L.; Zhou, Q.; Wang, Y.; Tran, L.-S.P.; Li, W.; Chen, G.; et al. Characterization of Three New Plant Growth-Promoting Microbes and Effects of the Interkingdom Interactions on Plant Growth and Disease Prevention. Plant Cell Rep. 2023, 42, 1757–1776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ahmed, W.; Dai, Z.; Zhou, X.; He, Z.; Wei, L.; Ji, G. Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition. Biology 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Lang, B.; Wang, Y.; Wang, X.; Liu, T.; Chen, J. Designing Synthetic Consortia of Trichoderma Strains That Improve Antagonistic Activities against Pathogens and Cucumber Seedling Growth. Microb. Cell Fact. 2022, 21, 234. [Google Scholar] [CrossRef]
- Lin, L.; Li, L.; Tao, M.; Wu, Q.; Zhou, L.; Wang, B.; Wang, L.; Shao, X.; Zhong, C.; Qian, G. Assembly of an Active Microbial Consortium by Engineering Compatible Combinations Containing Foreign and Native Biocontrol Bacteria of Kiwifruit. Comput. Struct. Biotechnol. J. 2023, 21, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, B.; Han, J.; Kong, N.; Yang, Y.; Zhang, J.; Wang, Y.; Liu, Z. Bacillus-Derived Consortium Enhances Ginkgo biloba’s Health and Resistance to Alternaria tenuissima. Pest Manag. Sci. 2024, 80, 4110–4124. [Google Scholar] [CrossRef]
- Wang, C.-C.; Zhang, Q.-C.; Yan, C.-A.; Tang, G.-Y.; Zhang, M.-Y.; Ma, L.Q.; Gu, R.-H.; Xiang, P. Heavy Metal(Loid)s in Agriculture Soils, Rice, and Wheat across China: Status Assessment and Spatiotemporal Analysis. Sci. Total Environ. 2023, 882, 163361. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium Contamination in Agricultural Soils of China and the Impact on Food Safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Xiang, J.; Li, N.; Feng, J.; Yin, J.; Wang, Y.; Wang, H.; Wang, W.; Yang, Z. Endophytic Consortium Exhibits Varying Effects in Mitigating Cadmium Toxicity in Rice Cultivars with Distinct Cadmium Accumulation Capacities. Environ. Technol. Innov. 2024, 36, 103833. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Hu, K.; Wang, G.; Shi, K. A Coculture of Enterobacter and Comamonas Species Reduces Cadmium Accumulation in Rice. Mol. Plant Microbe Interact. 2023, 36, 95–108. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, H.; Hong, Z.; Wang, X.; Cheng, S.; Xu, J.; Dai, Z. Co-Inoculation Effects of B. licheniformis and P. aeruginosa on Soil Cd and As Availability and Rice Accumulation. J. Environ. Manag. 2024, 351, 119739. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, W.; Beiyuan, J.; Chao, H.; Zhang, Z.; Chen, L.; Cui, Q.; Qiu, T.; Zhang, W.; Huang, M.; et al. Bacterial Consortium Amendment Effectively Reduces Pb/Cd Bioavailability in Soil and Their Accumulation in Wheat. J. Environ. Manag. 2024, 370, 122789. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Wang, J.; Zhou, Y.; Yue, C.; Zhou, X.; Xu, Y.; Tian, S.; Cao, Z.; Wei, X.; Li, S.; et al. The Adsorption and Fixation of Cd and Pb by the Microbial Consortium Weakened the Toxic Effect of Heavy Metal-Contaminated Soil on Rice. Chem. Eng. J. 2024, 497, 154684. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jiao, R.-Q.; Gao, S.-S.; Li, B.L.; Li, Y.-Y.; Han, H.; Chen, Z.-J. Beneficial Microbial Consortia Effectively Alleviated Plant Stress Caused by the Synergistic Toxicity of Microplastics and Cadmium. Ind. Crop. Prod. 2025, 225, 120479. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of Co-Inoculation with Plant-Growth-Promoting Rhizobacteria and Rhizobium on the Biochemical Responses of Alfalfa-Soil System in Copper Contaminated Soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Wang, X.; Cai, D.; Ji, M.; Chen, Z.; Yao, L.; Han, H. Isolation of Heavy Metal-Immobilizing and Plant Growth-Promoting Bacteria and Their Potential in Reducing Cd and Pb Uptake in Water Spinach. Sci. Total Environ. 2022, 819, 153242. [Google Scholar] [CrossRef]
- Mao, Q.; Xie, Z.; Pinzon-Nuñez, D.A.; Issaka, S.; Liu, T.; Zhang, L.; Irshad, S. Leptolyngbya sp. XZMQ and Bacillus XZM Co-Inoculation Reduced Sunflower Arsenic Toxicity by Regulating Rhizosphere Microbial Structure and Enzyme Activity. Environ. Pollut. 2024, 341, 123001. [Google Scholar] [CrossRef]
- Peng, J.; Ma, J.; Wei, X.; Zhang, C.; Jia, N.; Wang, X.; Wang, E.T.; Hu, D.; Wang, Z. Accumulation of Beneficial Bacteria in the Rhizosphere of Maize (Zea mays L.) Grown in a Saline Soil in Responding to a Consortium of Plant Growth Promoting Rhizobacteria. Ann. Microbiol. 2021, 71, 40. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Duan, H.; Dong, H.; Li, J.; Zhang, S.; Zhang, J.; Ding, S.; Xu, T.; Guo, B. Improved Effects of Combined Application of Nitrogen-Fixing Bacteria Azotobacter beijerinckii and Microalgae Chlorella pyrenoidosa on Wheat Growth and Saline-Alkali Soil Quality. Chemosphere 2023, 313, 137409. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Zhou, L.; Han, B.; Huo, S.; El-Sheekh, M.; Dong, H.; Li, X.; Xu, T.; Elshobary, M. Improving Saline-Alkali Soil and Promoting Wheat Growth by Co-Applying Potassium-Solubilizing Bacteria and Cyanobacteria Produced from Brewery Wastewater. Front. Environ. Sci. 2023, 11, 1170734. [Google Scholar] [CrossRef]
- Qi, R.; Lin, W.; Gong, K.; Han, Z.; Ma, H.; Zhang, M.; Zhang, Q.; Gao, Y.; Li, J.; Zhang, X. Bacillus Co-Inoculation Alleviated Salt Stress in Seedlings Cucumber. Agronomy 2021, 11, 966. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Q.; Tang, Q.-Y.; Osman, G.; Gu, M.-Y.; Wang, N.; Zhang, Z.-D. Application of Synthetic Microbial Communities of Kalidium schrenkianum in Enhancing Wheat Salt Stress Tolerance. Int. J. Mol. Sci. 2025, 26, 860. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Barry, G.; Martin, C.S.; Ramsubhag, A.; Eudoxie, G.; Miller, J.R. Multi-Trait Efficiency and Interactivity of Bacterial Consortia Used to Enhance Plant Performance under Water Stress Conditions. Microbiol. Res. 2024, 281, 127610. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, W.; Tang, W.; Zhou, X.; Bu, T.; Tang, Z.; Li, Q. Serendipita indica-Dominated Synthetic Microbial Consortia Enhanced Tartary Buckwheat Growth and Improved Its Tolerance to Drought Stress. Front. Microbiol. 2025, 16, 1562341. [Google Scholar] [CrossRef]
- Hu, J.; Yang, T.; Friman, V.-P.; Kowalchuk, G.A.; Hautier, Y.; Li, M.; Wei, Z.; Xu, Y.; Shen, Q.; Jousset, A. Introduction of Probiotic Bacterial Consortia Promotes Plant Growth via Impacts on the Resident Rhizosphere Microbiome. Proc. R. Soc. B 2021, 288, 20211396. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.; Xu, S.; Zhao, W.; Zhang, X.; Lei, Y.; Zhai, H.; Huang, Z. Co-Inoculation of Antagonistic Bacillus velezensis FH-1 and Brevundimonas diminuta NYM3 Promotes Rice Growth by Regulating the Structure and Nitrification Function of Rhizosphere Microbiome. Front. Microbiol. 2023, 14, 1101773. [Google Scholar] [CrossRef]
- Shang, J.; Liu, B. Application of a Microbial Consortium Improves the Growth of Camellia Sinensis and Influences the Indigenous Rhizosphere Bacterial Communities. J. Appl. Microbiol. 2021, 130, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Zhao, Y.; Zhuang, L.; Yu, Y.; Wang, M.; Liu, J.; Wang, Q. A Microbial Consortium-Based Product Promotes Potato Yield by Recruiting Rhizosphere Bacteria Involved in Nitrogen and Carbon Metabolisms. Microb. Biotechnol. 2021, 14, 1961–1975. [Google Scholar] [CrossRef]
- Dong, M.; Xiao, Y.; Wang, S.; Yang, B.; Zhang, H.; Wu, X. Investigation of Bioremediation Mechanism of Nicosulfuron-Contaminated Soil by Highly Efficient Degrading Bacterial Consortium YM1: Analysis of Degradation Genes and Microbial Community Structure. Appl. Soil Ecol. 2025, 209, 106060. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, W.-J.; Chen, S.-F.; Liu, M.; Ghorab, M.A.; Mishra, S.; Bhatt, P.; Chen, S. Complete Biodegradation of Glyphosate with Microbial Consortium YS622: Structural Analysis, Biochemical Pathways, and Environmental Bioremediation. J. Environ. Chem. Eng. 2024, 12, 114344. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Chen, W.-J.; Chen, S.-F.; Huang, Y.; Lei, Q.; Bhatt, P.; Mishra, S.; Chen, S.; Wang, H. High-Efficiency Degradation of Methomyl by the Novel Bacterial Consortium MF0904: Performance, Structural Analysis, Metabolic Pathways, and Environmental Bioremediation. J. Hazard. Mater. 2023, 452, 131287. [Google Scholar] [CrossRef]
- Lin, Z.; Pang, S.; Zhou, Z.; Wu, X.; Li, J.; Huang, Y.; Zhang, W.; Lei, Q.; Bhatt, P.; Mishra, S.; et al. Novel Pathway of Acephate Degradation by the Microbial Consortium ZQ01 and Its Potential for Environmental Bioremediation. J. Hazard. Mater. 2022, 426, 127841. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Yao, T.; Ma, L.; Zhang, J.; Li, C. Biodegradation Pathway and Detoxification of β-Cyfluthrin by the Bacterial Consortium and Its Bacterial Community Structure. J. Agric. Food Chem. 2022, 70, 7626–7635. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, I.V. Agriculture of Kazakhstan: From the Late 19th to the Early 21st Century; Akimov, A.V., Ed.; Filin: Moscow, Russia, 2021; pp. 1–252. (In Russian) [Google Scholar]
- Tsoy, A.; Nurbatsin, A. Analysis of the Level of Agricultural Development in Kazakhstan: Identifying Agro-Hubs. Eur. J. Econ. Bus. Stud. 2024, 68, 141–154. [Google Scholar] [CrossRef]
- Kulakova, S.V.; Malyarenko, O.I. A Comprehensive Approach to Assessing Innovation Activity in Agriculture in Kazakhstan. Econ. Entrep. 2025, 176, 438–443. (In Russian) [Google Scholar] [CrossRef]
- Kurmanova, G.K.; Sukhanberdina, B.B.; Urazova, B.A. Modernization of Agrarian Economy in the Republic of Kazakhstan. Econ. Strategy Pract. 2021, 16, 35–50. (In Russian) [Google Scholar] [CrossRef]
- Pashkov, S.V.; Imashev, E.Z.; Baubekova, G.K.; Kaimuldinova, K.D.; Tokpanov, Y.A.; Nurgaliyeva, G.Z.; Baimukasheva, G.K.; Kenzhebay, R.N.; Kassenov, S.K.; Ukrainskiy, P.A. Ecological–Economical and Ethno-Cultural Determinants of the Development of Organic Farming in Kazakhstan. Sustainability 2024, 16, 4065. [Google Scholar] [CrossRef]
- Esimkhan, G.; Sartanova, N.; Zhanibekova, G.; Baisholanova, K.; Kuzenbayeva, E. Financing the Agricultural Sector of Kazakhstan in Modern Conditions. Econ. Entrep. 2024, 95, 176–189. (In Russian) [Google Scholar] [CrossRef]
- Nurgaliyeva, A.; Zhumagalieva, B.; Asrepov, G.; Bekniyazova, D.; Kussainov, K. Development of Innovative Processes in the Field of Agriculture of the Republic of Kazakhstan. Sci. Horiz. 2024, 27, 141–151. [Google Scholar] [CrossRef]
- Taubayev, A.; Rakhmetova, A.; Kalkabayeva, G.; Saifullina, Y.; Zhukenov, B. Problems of Research Funding in the Agro-Industrial Complex of Kazakhstan. J. Asian Afr. Stud. 2023, 58, 1656–1664. [Google Scholar] [CrossRef]
- Zhambakin, K.; Zhapar, K. Current Status and Prospects of Plant Biotechnology in Kazakhstan. Plant Biotechnol. Rep. 2020, 14, 177–184. [Google Scholar] [CrossRef]
- Bissenova, G.; Tekebayeva, Z.; Tynybayeva, I.; Temirkhanov, A.; Sarmurzina, Z. Screening of Microorganisms with High Biological Activity to Create Consortia as a Growth Stimulator for Wheat Seeds. Int. J. Des. Nat. Ecodyn. 2023, 18, 819–829. [Google Scholar] [CrossRef]
- Nauanova, A.; Shaikhin, S.; Ospanova, S.; Makenova, M.; Shumenova, N.; Bostubayeva, M. Enhancing Spring Barley Grain Yield with Local Biofertilizers in the Semi-Arid Steppe Zone of Northern Kazakhstan. Int. J. Des. Nat. Ecodyn. 2024, 19, 371–378. [Google Scholar] [CrossRef]
- Smirnova, I.; Sadanov, A.; Baimakhanova, G.; Faizulina, E.; Tatarkina, L. Use of a Consortium of Agronomically Important Microorganisms for Growing Soybean (Glycine max (L.) Merr.). Open Agric. Sci. J. 2023, 17, e187433152302140. [Google Scholar] [CrossRef]
- Smirnova, I.; Sadanov, A.; Gul, B.; Elmira, F.; Larisa, T. Salt Tolerant Rhizobacteria Mitigating the Effects of Salinity Stress on Growth of Soybean (Glycine max (L.) Merr.) on Salinity Soil. Agric. Food 2023, 11, 268–279. [Google Scholar] [CrossRef]
- Zayadan, B.K.; Matorin, D.N.; Baimakhanova, G.B.; Bolathan, K.; Oraz, G.D.; Sadanov, A.K. Promising Microbial Consortia for Producing Biofertilizers for Rice Fields. Microbiology 2014, 83, 391–397. [Google Scholar] [CrossRef]
- Jiang, H. Review of Chinese Agricultural Policy. Transnatl. Corp. Rev. 2009, 1, 42–58. [Google Scholar] [CrossRef]
- The Astana Times. China’s Belt and Road Initiative: Kazakhstan and Geopolitics. Available online: https://astanatimes.com/2020/06/chinas-belt-and-road-initiative-kazakhstan-and-geopolitics/ (accessed on 28 March 2025).
- Press Service of Al-Farabi Kazakh National University. Available online: https://farabi.university/news/82111?lang=ru (accessed on 30 March 2025).
- Dinis Sousa, R.; Boranbayeva, A.; Satpayeva, Z.; Gassanova, A. Management of Successful Technology Transfer in Agriculture: The Case of Kazakhstan. Probl. Perspect. Manag. 2021, 19, 488–501. [Google Scholar] [CrossRef]
- Ibyzhanova, A.; Rustenova, E.; Akhmetzhanova, N.; Talapbayeva, G.; Yerniyazova, Z. Opportunities for Kazakhstan’s Agricultural Exports to the Chinese Market. J. East. Eur. Cent. Asian Res. 2024, 11, 871–886. [Google Scholar] [CrossRef]

| № | Consortium Composition | Host Crop | Key Effect | Reference |
|---|---|---|---|---|
| 1 | Asticcacaulis sp. + Arachidicoccus sp. + Phenylobacterium sp. | Tomato | Reliable protection against Botrytis cinerea via induced immunity and biofilm formation | [69] |
| 2 | Bacillus cereus AR156 + B. subtilis SM21 + Serratia sp. XY21 | Cucumber | Nematode-induced root galls reduced by 56–72%; improved yield and rhizosphere health | [70] |
| 3 | Rhodotorula graminis JJ10.1 + Pseudomonas psychrotolerans YY7 + P. chlororaphis T8 + Bacillus amyloliquefaciens FZB42 | Arabidopsis thaliana and tomato | Cross-kingdom consortium prevented bacterial–fungal infections and promoted growth via synergistic biofilm | [71] |
| 4 | Bacillus cereus BT-23 + Lysobacter antibioticus 13-6 + L. capsici ZST1-2 | Chinese cabbage | Kiel’s disease caused by Plasmodiophora brassicae is reduced reduced by ~66%; increased yield and reduced soil acidity | [72] |
| 5 | Trichoderma asperellum GDSF1009 + T. asperelloides Z4-1 + T. harzianum 10569 + T. asperellum 10264 | Cucumber | Suppressed Fusarium wilt; enhanced seedling growth and amino acid accumulation compared to monocultures | [73] |
| 6 | Lysobacter enzymogenes OH11W (ΔWAP-8294A2) + Bacillus safensis ZK-1 | Kiwi | Controlled bacterial canker and associated fungal infections | [74] |
| 7 | Bacillus subtilis 503 + B. safensis 537 + B. amyloliquefaciens 337 + B. sonorensis 544 | Ginkgo | Field control of leaf blight up to 100%; increased biomass and antioxidant activity, and improved soil microbiota | [75] |
| № | Consortium Composition | Host Crop | Key Effect | Reference |
| Heavy metals | ||||
| 1 | Bacillus cereus + B. thuringiensis + Herbaspirillum huttiense | Wheat | Reduced available Pb/Cd in soil and shoot translocation; enhanced root development | [81] |
| 2 | Cellulomonas iranensis ZJW-6 + Pseudomonas brassicacearum wj1 | Rice | Removed 94% Cd and 74% Pb in 7 days; improved soil structure and adsorption | [82] |
| 3 | Bacillus subtilis SQ4 + Enterobacter hormaechei VY5 + B. velezensis SQ6 | Sorghum | Alleviated combined PVC + Cd stress; increased dry biomass and nutrient availability | [83] |
| 4 | Paenibacillusmucilaginosus ACCC10013 + Sinorhizobium meliloti CCNWSX0020 | Alfalfa | Mitigated Cu toxicity by reducing ROS and lipid peroxidation | [84] |
| 5 | Enterobacter bugandensis XY1 + Serratia marcescens X43 | Water spinach | Reduced Cd and Pb in aboveground biomass by 51–80% via polyamine-mediated immobilization | [85] |
| 6 | Leptolyngbya sp. XZMQ + Bacillus XZM | Sunflower | Reduced As in roots/stems/leaves by 38–70%; enhanced soil enzymatic activity | [86] |
| Salinity | ||||
| 7 | Pseudomonas sp. P8 + Peribacillus sp. P10 + Streptomyces sp. X52 | Maize | Improved growth and enriched nitrogen fixers in rhizosphere under salinity stress | [87] |
| 8 | Azotobacter beijerinckii B3 + Chlorella pyrenoidosa | Wheat | Increased biomass by 67% under alkaline stress; reduced pH, enhanced fertility | [88] |
| 9 | Paenibacillus sabinae + Leptolyngbya sp. RBD05 | Wheat | Co-inoculation increased dry weight by 85% and K:Na ratio by 41%; improved salt tolerance | [89] |
| 10 | Bacillus licheniformis (NX-3/59) + B. subtilis (NX-4/48/62) | Cucumber (seedling) | Enhanced stem diameter and fresh weight under salt stress by activating substrate nutrients | [90] |
| 11 | 11-strain-SMC from Kalidium schrenkianum | Wheat | Stimulated germination, antioxidant enzymes, and chlorophyll; reduced oxidative stress | [91] |
| Drought | ||||
| 12 | Burkholderia sp. UWIGT-83 + Burkholderia sp. UWIGT-120 | Red hot pepper | Improved germination and growth under simulated drought via ACC deaminase and biofilm | [92] |
| 13 | Bacillus cereus JQB1 + Rhodococcus sphaeroides JQB3 + Serendipita indica JQF1 + Mortierella alpina JQF2 + Ceriporia lacerata JQF3 + Fusarium equiseti JQF4 | Tartary buckwheat | Increased biomass, photosynthesis, and reduced H2O2/MDA; improved drought tolerance | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nygymetova, A.M.; Sadvakasova, A.K.; Zaletova, D.E.; Kossalbayev, B.D.; Bauenova, M.O.; Wang, J.; Huang, Z.; Sarsekeyeva, F.K.; Kirbayeva, D.K.; Allakhverdiev, S.I. Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China. Plants 2025, 14, 2208. https://doi.org/10.3390/plants14142208
Nygymetova AM, Sadvakasova AK, Zaletova DE, Kossalbayev BD, Bauenova MO, Wang J, Huang Z, Sarsekeyeva FK, Kirbayeva DK, Allakhverdiev SI. Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China. Plants. 2025; 14(14):2208. https://doi.org/10.3390/plants14142208
Chicago/Turabian StyleNygymetova, Aimeken M., Assemgul K. Sadvakasova, Dilnaz E. Zaletova, Bekzhan D. Kossalbayev, Meruyert O. Bauenova, Jingjing Wang, Zhiyong Huang, Fariza K. Sarsekeyeva, Dariga K. Kirbayeva, and Suleyman I. Allakhverdiev. 2025. "Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China" Plants 14, no. 14: 2208. https://doi.org/10.3390/plants14142208
APA StyleNygymetova, A. M., Sadvakasova, A. K., Zaletova, D. E., Kossalbayev, B. D., Bauenova, M. O., Wang, J., Huang, Z., Sarsekeyeva, F. K., Kirbayeva, D. K., & Allakhverdiev, S. I. (2025). Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China. Plants, 14(14), 2208. https://doi.org/10.3390/plants14142208









