Phenolic Exudation Control and Indirect Somatic Embryogenesis of Garlic-Fruit Tree (Malania oleifera Chun & S.K. Lee)—An Endangered Woody Tree Species of Southeastern Yunnan Province, China
Abstract
1. Introduction
2. Results
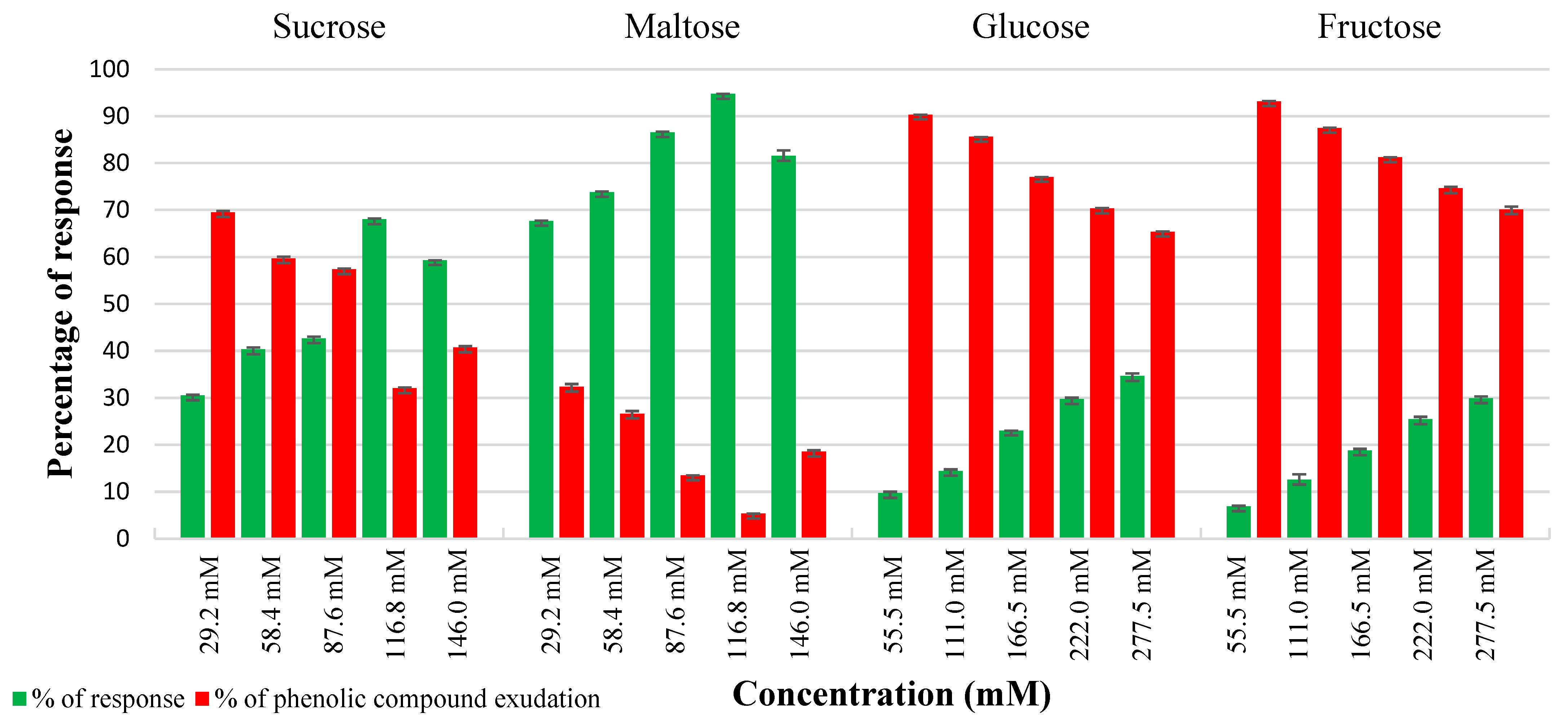
2.1. Effect of Carbon Sources in Controlling Phenolic Exudation from Explants and Callus Culture
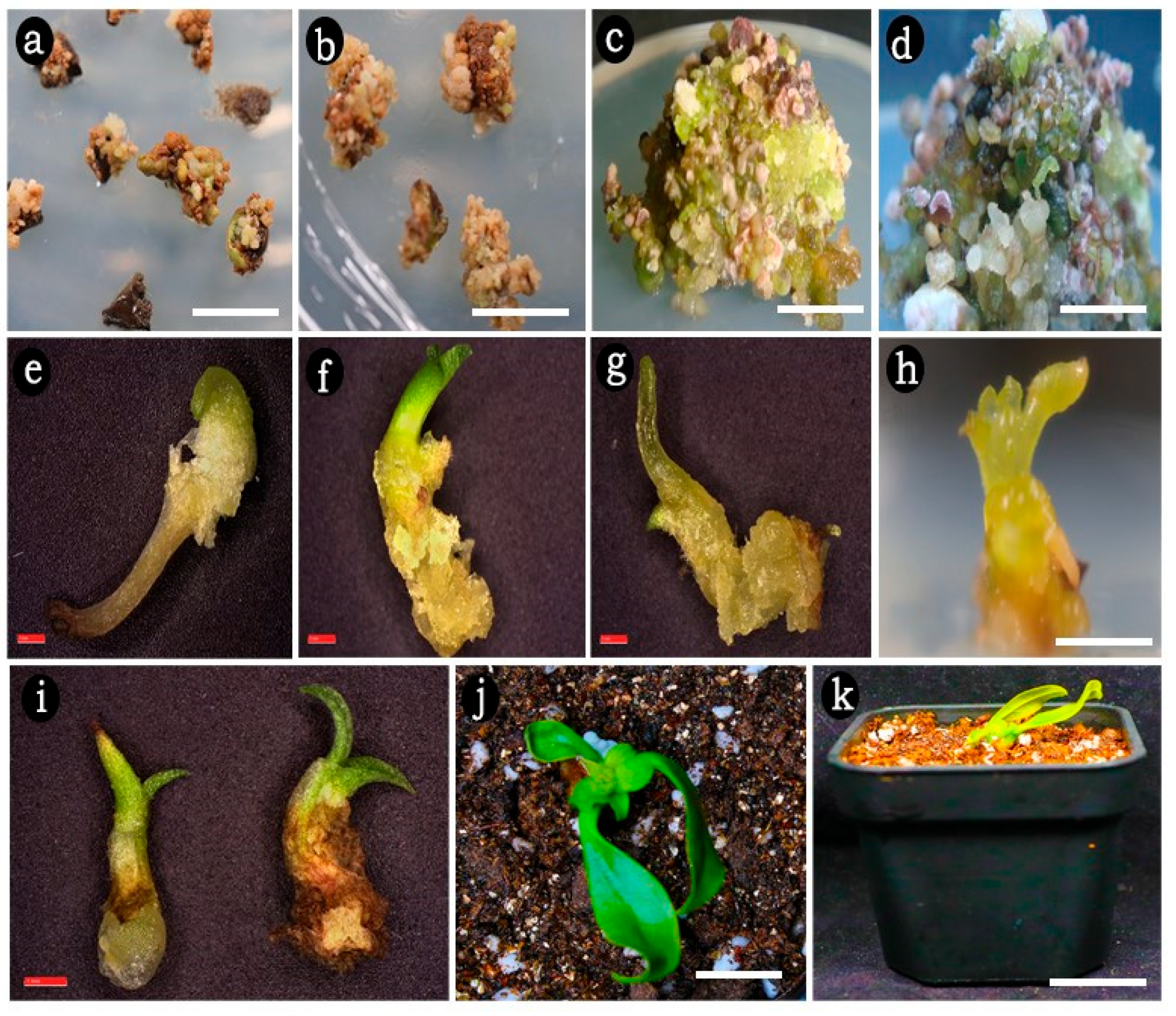
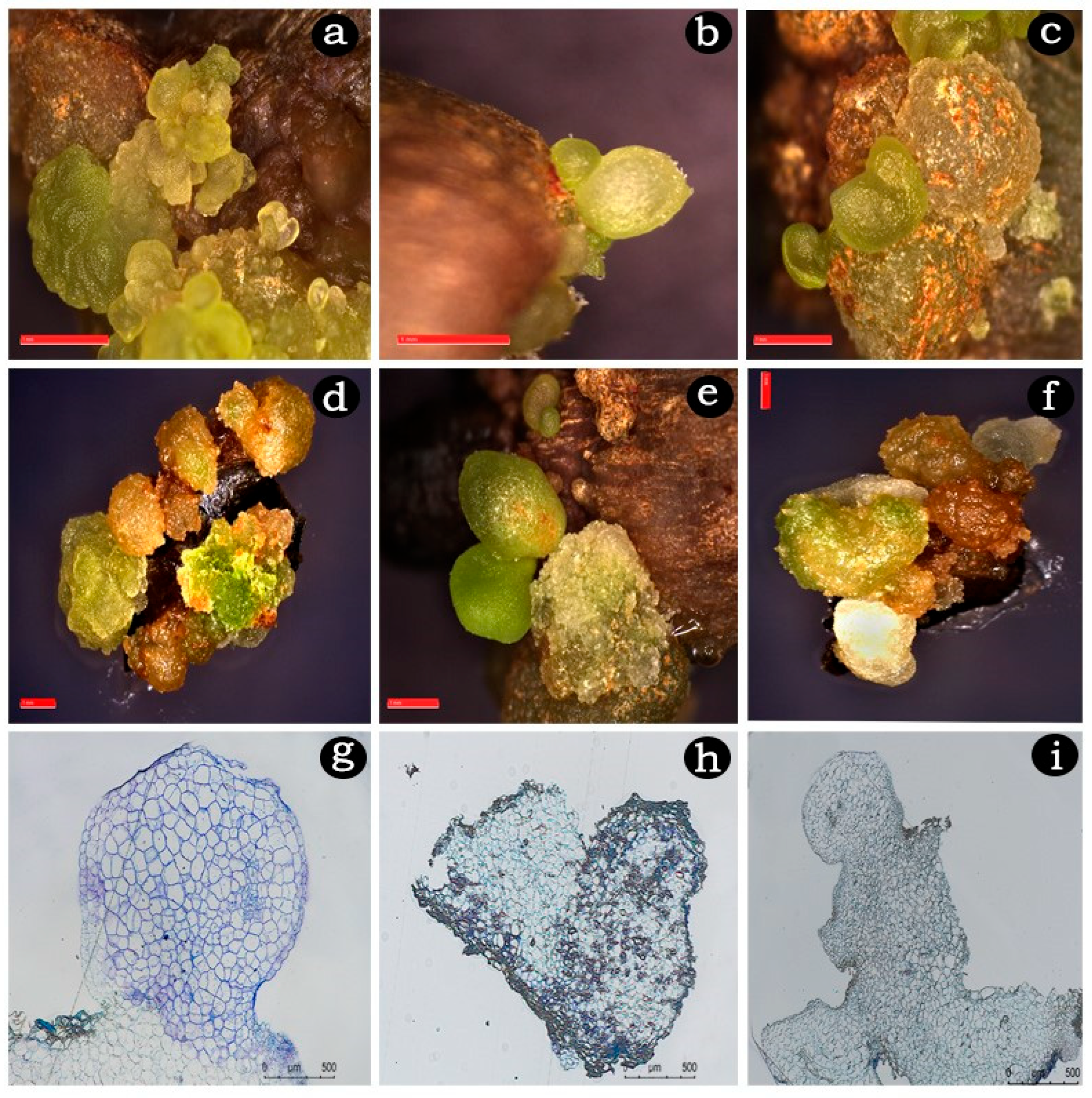
2.2. Embryogenic Callus Induction
2.3. Somatic Embryo Maturation
2.4. Effect of GA3 on Germination of Somatic Embryos
2.5. Histological Analysis
3. Discussion
4. Materials and Methods
4.1. Explant Source
4.2. Explant Surface Sterilization and Preparation
4.3. Basal Media and Culture Conditions
4.4. Influence of Carbon Source in Controlling Phenolic Exudation from Explants
4.5. Induction of Embryogenic Callus
4.6. Effect of PGRs on Somatic Embryo Induction and Maturation
4.7. Somatic Embryo Germination
4.8. Resin Sections and Histological Observation for Light Microscopy
4.9. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, T.; Yu, Q.; Xu, W.; Li, D.Z.; Chen, F.; Liu, A. Transcriptome analysis reveals crucial genes involved in the biosynthesis of nervonic acid in woody Malania oleifera oilseeds. BMC Plant Biol. 2018, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, R.; Tian, Z.; Zhou, B.; Duan, R.; He, R.; Dong, L. Population variation in fatty acid composition and response to climatic factors in Malania oleifera Chun et S.K Lee. BMC Plant Biol. 2025, 25, 73. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liang, S.; Zhao, D.; Xu, A.; Zhang, K. Study on plants containing nervonic acid. Acta Bot. Boreali-Occident. Sin. 2004, 24, 2362–2365. [Google Scholar]
- Tang, T.F.; Liu, X.M.; Ling, M.; Lai, F.; Zhang, L.; Zhou, Y.H.; Sun, R.R. Constituents of the essential oil and fatty acid from Malania oleifera. Ind. Crops Prod. 2013, 43, 1–5. [Google Scholar] [CrossRef]
- Cook, C.; Barnett, J.; Coupland, K.; Sargent, J. Effects of feeding Lunaria oil rich in nervonic and erucic acids on the fatty acid compositions of sphingomyelins from erythrocytes, liver, and brain of the quaking mouse mutant. Lipids 1998, 33, 993–1000. [Google Scholar] [CrossRef]
- Evans, D.A.; Bennett, D.A.; Wilson, R.S.; Bienias, J.L.; Morris, M.C.; Scherr, P.A.; Schneider, J. Incidence of Alzheimer disease in a biracial urban community: Relation to apolipoprotein E allele status. Arch. Neurol. 2003, 60, 185–189. [Google Scholar] [CrossRef]
- Chen, J.R.; Hsu, S.F.; Hsu, C.D.; Hwang, L.H.; Yang, S.C. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J. Nutr. Biochem. 2004, 15, 467–472. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimizu, T.; Ohtsuka, Y.; Yamashiro, Y.; Oshida, K. Early dietary treatments with Lorenzo’s oil and docosahexaenoic acid for neurological development in a case with Zellweger syndrome. Brain Dev. Jpn. 2007, 29, 586–589. [Google Scholar] [CrossRef]
- Yuan, Y.; Dai, X.; Wang, D.; Zeng, X. Purification, characterization and cytotoxicity of malanin, a novel plant toxin from the seeds of Malania oleifera. Toxicon 2009, 54, 121–127. [Google Scholar] [CrossRef]
- Qiu, H.X.; Gilbert, M.G. Olacaceae. Flora China 2003, 5, 200–204. [Google Scholar]
- Liang, Y.; Wu, S.; Li, X. Study on the endangered causes for Malania oleifera. Guangxi Zhiwu 2003, 23, 404–407. [Google Scholar]
- Sun, W.B.; Ma, Y.P.; Blackmore, S. How a new conservation action concept has accelerated plant conservation in China. Trends Plant Sci. 2020, 24, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, G.; Edward Grumbine, R.; Dao, Z.; Sun, W.; Guo, H. Conserving plant species with extremely small populations (PSESP) in China. Biodivers. Conserv. 2013, 22, 803–809. [Google Scholar] [CrossRef]
- Xu, C.Q.; Liu, H.; Zhou, S.S.; Zhang, D.X.; Zhao, W.; Wang, S.; Mao, J.F. Genome sequence of Malania oleifera, a tree with great value for nervonic acid production. GigaScience 2019, 82, giy164. [Google Scholar] [CrossRef]
- Shen, Y.; Tao, L.; Zhang, R.; Yao, G.; Zhou, M.; Sun, W.; Ma, Y. Genomic insights into endangerment and conservation of the garlic-fruit tree (Malania oleifera), a plant species with extremely small populations. GigaScience 2024, 13, giae070. [Google Scholar] [CrossRef]
- Li, A.R.; Mao, P.; Li, Y.J. Root hemiparasitism in Malania oleifera (Olacaceae), a neglected aspect in research of the highly valued tree species. Plant Divers. 2019, 415, 347–351. [Google Scholar] [CrossRef]
- Xu, S.S.; Kan, W.; Kong, B.H.; Ma, J.; Yang, G.Z. First report of Fusarium oxysporum and Fusarium solani causing root rot on Malania oleifera in China. Plant Dis. 2020, 104, 584. [Google Scholar] [CrossRef]
- Su, X.; Wang, X.; Cui, N.; Xu, H.; Tian, T.; Wei, B. Enhancing germination and growth in Malania oleifera Chun & SK Lee seeds through gibberellic acid priming. J. Appl. Res. Med. Aromat. Plants 2025, 45, 100629. [Google Scholar] [CrossRef]
- Isah, T. Induction of somatic embryogenesis in woody plants. Acta Physiol. Plant. 2016, 38, 118. [Google Scholar] [CrossRef]
- Pei, L.; Zhao, Y.; Shi, X.; Chen, R.; Yan, J.; Li, X.; Shi, S. The role of γ-aminobutyric acid (GABA) in the occurrence of adventitious roots and somatic embryos in woody plants. Plants 2022, 11, 3512. [Google Scholar] [CrossRef]
- Mihai, R.A.; Melo Heras, E.J.; Pinto Valdiviezo, E.A.; Espinoza Caiza, I.A.; Cubi Insuaste, N.S.; Mejía, J.P.; Florescu, L.I. Somatic embryogenesis of representative medicinal trees in South America—Current status. Forests 2023, 14, 2066. [Google Scholar] [CrossRef]
- Liu, X.; Wei, L.; Miao, C.; Zhang, Q.; Yan, J.; Li, S.; Qin, W. Application of exogenous phenolic compounds in improving postharvest fruits quality: Classification, potential biochemical mechanisms and synergistic treatment. Food Rev. Int. 2024, 40, 1776–1795. [Google Scholar] [CrossRef]
- Gibson, S. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Mexudhan, J.Z.; Singh, L.; Lal, J.; Tirkey, J. Problem and its remedy of micropropagation in woody trees: A review. Environ. Ecol. 2023, 41, 633–639. [Google Scholar]
- Liu, R.; Fang, Y.; Peng, S.; Benani, N.; Wu, X.; Chen, Y.; Yang, P. Study on factors influencing carbon dioxide emissions and carbon peak heterogenous pathways in Chinese provinces. J. Environ. Manag. 2024, 365, 121667. [Google Scholar] [CrossRef]
- Sae-leaw, T.; Benjakul, S.; Simpson, B.K. Effect of catechin and its derivatives on inhibition of polyphenoloxidase and melanosis of Pacific white shrimp. J. Food Sci. Technol. 2017, 54, 1098–1107. [Google Scholar] [CrossRef]
- Titov, S.; Bhowmik, S.K.; Mandal, A.; Alam, M.S.; Uddin, S.N. Control of phenolic compound secretion and effect of growth regulators for organ formation from Musa spp. cv. Kanthali floral bud explants. Am. J. Biochem. Biotechnol. 2006, 2, 97–104. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid—A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Swarnkar, P.L.; Bohra, S.P.; Chandra, N. Biochemical changes during growth and differentiation of the callus of Solanum surattense. J. Plant Physiol. 1986, 126, 75–81. [Google Scholar] [CrossRef]
- Mozafar, A.; Oertli, J.J. Vitamin C (ascorbic acid): Uptake and metabolism by soybean. J. Plant Physiol. 1993, 141, 316–321. [Google Scholar] [CrossRef]
- Zhong, J. Preliminary Study of Tissue Culture System of Sapindus mukorossi Gaertn. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Thorpe, T.; Stasolla, C.; Yeung, E.C.; de Klerk, G.-J.; Roberts, A.; George, E.F. The components of plant tissue culture media II: Organic additions, Osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., De Klerk, G.-J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Petersen, K.K.; Hansen, J.; Krogstrup, P. Significance of different carbon sources and sterilization methods on callus induction and plant regeneration of Miscanthus x ogiformis Honda ‘Giganteus’. Plant Cell Tissue Organ Cult. 1999, 58, 189–197. [Google Scholar] [CrossRef]
- Dantas, L.A.; Faria, P.S.A.; Dário, B.M.M.; Arantes, A.L.M.; Silva, F.G.; Avila, R.G.; Pereira, P.S.; Neto, A.R. The impact of carbon source on cell growth and the production of bioactive compounds in cell suspensions of Hancornia speciosa Gomes. Sci. Rep. 2021, 11, 24315. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhu, Z.; Hong, Y.; Gong, Z.; Zhang, H. The effect of carbon taxes and subsidies on forest carbon sequestration in China. For. Policy Econ. 2022, 169, 103316. [Google Scholar] [CrossRef]
- Khan, T.; Reddy, V.S.; Leelavathi, S. High-frequency regeneration via somatic embryogenesis of an elite recalcitrant cotton genotype (Gossypium hirsutum L.) and efficient Agrobacterium-mediated transformation. Plant Cell Tissue Organ Cult. 2010, 101, 323–330. [Google Scholar] [CrossRef]
- Kumria, R.; Sunnichan, V.G.; Das, D.K.; Gupta, S.K.; Reddy, V.S.; Bhatnagar, R.K.; Leelavathi, S. High-frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) through metabolic stress. Plant Cell Rep. 2003, 21, 635–639. [Google Scholar] [CrossRef]
- Kumar, G.P.P.; Subiramani, S.; Govindarajan, S.; Sadasivam, V.; Manickam, V.; Mogilicherla, K.; Narayanasamy, J. Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Plant Biotechnol. Rep. 2015, 7, 72–80. [Google Scholar] [CrossRef]
- Pereira, A.V.C.; Midori Takamori, L.; Yaguinuma, D.H.; de Oliveira, A.M.; Ribas, A.F. Maltose in culture media improves the in vitro regeneration of Urochloa brizantha cv. ‘Marandu’ plants. Biotecnol. Veg. 2019, 19, 205–213. [Google Scholar]
- Julkunen-Tiitto, R. Defensive efforts of Salix myrsinifolia plantlets in photomixotrophic culture conditions: The effect of sucrose, nitrogen and pH on the phytomass and secondary phenolic accumulation. Écoscience 1996, 3, 297–303. [Google Scholar] [CrossRef]
- Curtis, O.F.; Shetty, K. Growth medium effects on vitrification, total phenolics, chlorophyll, and water content of in vitro propagated oregano clones. Int. Symp. Med. Aromat. Plants 1995, 426, 489–504. [Google Scholar] [CrossRef]
- Khosroushahi, A.Y.; Naderi-Manesh, H.; Simonsen, H.T. Effect of antioxidants and carbohydrates in callus cultures of Taxus brevifolia: Evaluation of browning, callus growth, total phenolics and paclitaxel production. Bioimpacts 2011, 1, 37–45. [Google Scholar] [CrossRef]
- Cloutier, M.; Chen, J.; De Dobbeleer, C.; Perrier, M.; Jolicoeur, M. A systems approach to plant bioprocess optimization. Plant Biotechnol. 2009, 7, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Hsiao, K.C. Sugar degradation during autoclaving: Effects of duration and solution volume on breakdown of glucose. Physiol. Plant. 1995, 94, 415–418. [Google Scholar] [CrossRef]
- Leitzen, S.; Vogel, M.; Steffens, M.; Zapf, T.; Müller, C.E.; Brandl, M. Quantification of degradation products formed during heat sterilization of glucose solutions by LC-MS/MS: Impact of autoclaving temperature and duration on degradation. Pharmaceuticals 2021, 14, 1121. [Google Scholar] [CrossRef]
- Kjellstrand, P.; Erixon, M.; Wieslander, A.; Lindén, T.; Martinson, E. Temperature the Single Most Important Factor for Degradation of Glucose Fluids during Storage. Perit. Dial. Int. 2004, 24, 385–391. [Google Scholar] [CrossRef]
- Qian, X.; Nimlos, M.R.; Johnson, D.K.; Himmel, M.E. Acidic sugar degradation pathways. Appl. Biochem. Biotechnol. 2005, 124, 989–997. [Google Scholar] [CrossRef]
- Che, P.; Anand, A.; Wu, E.; Sander, J.D.; Simon, M.K.; Zhu, W.; Jones, T.J. Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application. Plant Biotechnol. J. 2018, 16, 1388–1395. [Google Scholar] [CrossRef]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Davies, M.E. Polyphenol synthesis in cell suspension cultures of Paul’s Scarlet rose. Planta 1972, 104, 50–65. [Google Scholar] [CrossRef]
- Nunes, S.; Marum, L.; Farinha, N.; Pereira, V.T.; Almeida, T.; Sousa, D.; Santos, C. Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Organ Cult. 2018, 132, 71–84. [Google Scholar] [CrossRef]
- Kim, Y.W. Initiation of embryogenic callus from mature zygotic embryos in Japanese larch (Larix kaempferi). J. Plant Biotechnol. 2015, 42, 223–227. [Google Scholar] [CrossRef]
- Xu, K.D.; Wang, W.; Yu, D.S.; Li, X.L.; Chen, J.M.; Feng, B.J.; Li, C.W. NAA at a high concentration promotes efficient plant regeneration via direct somatic embryogenesis and SE-mediated transformation system in Ranunculus sceleratus. Sci. Rep. 2019, 9, 18321. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, M.; Susanthi, B.; Murali Mohan, N.; Mandal, P.K. In vitro somatic embryogenesis and plantlet regeneration from immature male inflorescence of adult dura and tenera palms of Elaeis guineensis (Jacq.). SpringerPlus 2015, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, E.; Ballester, A.; Ibarra, M.; Vieitez, A.M. Induction of somatic embryogenesis in explants of shoot cultures established from adult Eucalyptus globulus and E. saligna × E. maidenii trees. Tree Physiol. 2015, 35, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhong, Y.; Hong, W.; Luo, H.; Li, D.; Zhao, L.; Wang, J. Genetic diversity evaluation and core collection construction of pomegranate (Punica granatum L.) using genomic SSR markers. Sci. Hortic. 2023, 319, 112192. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Y.; Jeong, B.R. Callus induction and browning suppression in tree peony Paeonia ostii ‘Fengdan’. Hortic. Environ. Biotechnol. 2020, 61, 591–600. [Google Scholar] [CrossRef]
- Raji, M.R.; Lotfi, M.; Tohidfar, M.; Zahedi, B.; Carra, A.; Abbate, L.; Carimi, F. Somatic embryogenesis of muskmelon (Cucumis melo L.) and genetic stability assessment of regenerants using flow cytometry and ISSR markers. Protoplasma 2018, 255, 873–883. [Google Scholar] [CrossRef]
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Induction of somatic embryogenesis in leaf and root explants of Digitalis lanata Ehrh.: Direct and indirect method. S. Afr. J. Bot. 2020, 130, 356–365. [Google Scholar] [CrossRef]
- Thaniarasu, R.; Kumar, T.S.; Rao, M.V. Indirect somatic embryogenesis and genetic homogeneity assessment in Plectranthus bourneae Gamble an endemic plant to Western Ghats of Tamil Nadu, India. J. Appl. Hortic. 2021, 23, 224–230. [Google Scholar] [CrossRef]
- Woo, H.A.; Ku, S.S.; Jie, E.Y.; Kim, H.; Kim, H.S.; Cho, H.S.; Kim, S.W. Efficient plant regeneration from embryogenic cell suspension cultures of Euonymus alatus. Sci. Rep. 2021, 11, 15120. [Google Scholar] [CrossRef]
- Daniel, M.A.; David, R.H.A.; Caesar, S.A.; Ramakrishnan, M.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Effect of L-glutamine and casein hydrolysate in the development of somatic embryos from cotyledonary leaf explants in okra (Abelmoschus esculentus L. monech). S. Afr. J. Bot. 2018, 114, 223–231. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhu, X.M.; Yin, H.; Li, B.; Chen, X.J.; Fan, X.L.; Han, J.J. Symbiosis between Dendrobium catenatum protocorms and Serendipita indica involves the plant hypoxia response pathway. Plant Physiolol. 2023, 192, 2554–2568. [Google Scholar] [CrossRef]

| Auxins | Percentage of Embryogenic Callus (%) | Number of Somatic Embryos | ||
|---|---|---|---|---|
| Internode | Leaf | Internode | Leaf | |
| NAA | ||||
| 1.0 | 26.54 ± 0.12 i | 33.04 ± 0.38 i | 4.61 ± 0.17 h | 8.47 ± 0.06 i |
| 1.5 | 33.83 ± 0.24 g | 37.66 ± 0.16 g | 9.06 ± 0.36 f | 14.84 ± 0.30 g |
| 2.0 | 53.77 ± 0.19 a | 45.66 ± 0.10 f | 14.06 ± 0.40 e | 19.59 ± 0.90 e |
| 2.5 | 49.43 ± 0.10 b | 57.43 ± 0.6 a | 21.03 ± 0.30 c | 31.88 ± 0.72 b |
| 3.0 | 45.69 ± 0.06 d | 51.07 ± 0.39 c | 27.65 ± 0.11 a | 36.66 ± 0.12 a |
| 2,4-D | ||||
| 1.0 | 23.65 ± 0.34 j | 30.69 ± 0.41 j | 6.65 ± 0.06 g | 10.44 ± 0.04 g |
| 1.5 | 27.62 ± 0.13 h | 34.66 ± 0.24 h | 9.54 ± 0.07 f | 15.63 ± 0.12 f |
| 2.0 | 39.16 ± 0.31 f | 54.61 ± 0.05 b | 15.65 ± 0.22 d | 20.58 ± 0.13 d |
| 2.5 | 46.69 ± 0.09 c | 48.61 ± 0.07 d | 21.65 ± 0.06 c | 27.79 ± 0.13 c |
| 3.0 | 43.29 ± 0.09 e | 46.76 ± 0.11 e | 26.76 ± 0.11 b | 32.51 ± 0.13 b |
| Cytokinins | Percentage of Response (SE) | Average Number of Somatic Embryos | ||||||
|---|---|---|---|---|---|---|---|---|
| Internode | Leaf | Internode | Leaf | |||||
| Globular | Heart | Torpedo | Globular | Heart | Torpedo | |||
| TDZ | ||||||||
| 0.5 | 50.73 ± 0.20 j | 58.21 ± 0.56 i | 44.51 ± 0.33 k | 0.00 ± 0.00 g | 0.00 ± 0.00 e | 56.22 ± 0.33 j | 0.00 ± 0.00 j | 0.00 ± 0.00 e |
| 1.0 | 59.57 ± 0.30 g | 67.65 ± 0.16 g | 47.62 ± 0.08 j | 0.00 ± 0.00 g | 0.00 ± 0.00 e | 67.36 ± 0.30 i | 7.44 ± 0.19 g | 0.00 ± 0.00 e |
| 1.5 | 69.55 ± 0.16 d | 76.04 ± 0.19 d | 54.41 ± 0.09 i | 2.51 ± 0.26 c | 0.00 ± 0.00 e | 82.17 ± 0.81 g | 9.75 ± 0.18 f | 0.00 ± 0.00 e |
| 2.0 | 63.48 ± 0.22 e | 74.69 ± 0.07 e | 60.62 ± 0.70 g | 0.00 ± 0.00 g | 0.00 ± 0.00 e | 86.33 ± 0.44 f | 11.48 ± 0.09 e | 0.00 ± 0.00 e |
| BA | ||||||||
| 0.5 | 61.70 ± 0.25 f | 73.04 ± 0.39 f | 78.69 ± 0.10 c | 2.33 ± 0.01 d | 1.25 ± 0.31 d | 98.56 ± 0.12 d | 25.66 ± 0.05 d | 4.29 ± 0.03 d |
| 1.0 | 85.22 ± 0.36 a | 93.80 ± 0.31 a | 85.77 ± 0.16 b | 4.29 ± 0.05 a | 1.94 ± 0.02 c | 116.25 ± 3.64 a | 37.58 ± 0.04 a | 9.55 ± 0.57 a |
| 1.5 | 79.66 ± 0.11 b | 86.78 ± 0.06 b | 95.66 ± 0.57 a | 3.44 ± 0.21 b | 2.33 ± 0.06 b | 104.85 ± 0.70 c | 31.22 ± 0.38 c | 7.71 ± 0.08 b |
| 2.0 | 72.71 ± 0.22 c | 80.55 ± 0.77 c | 79.35 ± 0.35 c | 2.57 ± 0.06 c | 2.88 ± 0.12 a | 109.18 ± 0.36 b | 34.83 ± 0.06 b | 6.55 ± 0.06 c |
| Kin | ||||||||
| 0.5 | 49.59 ± 0.04 k | 57.47 ± 0.12 i | 58.62 ± 0.03 h | 0.00 ± 0.00 g | 0.00 ± 0.00 e | 78.65 ± 0.10 h | 00 ± 0.00 j | 00 ± 0.00 e |
| 1.0 | 55.85 ± 0.16 i | 61.74 ± 0.26 h | 67.72 ± 0.07 e | 1.28 ± 0.04 f | 0.00 ± 0.00 e | 86.51 ± 0.60 f | 00 ± 0.00 j | 00 ± 0.00 e |
| 1.5 | 63.52 ± 0.22 e | 67.77 ± 0.11 g | 77.77 ± 0.06 d | 1.95 ± 0.06 e | 0.00 ± 0.00 e | 94.33 ± 0.48 e | 2.51 ± 0.03 i | 00 ± 0.00 e |
| 2.0 | 58.62 ± 0.21 h | 61.11 ± 0.11 h | 66.84 ± 0.03 f | 2.28 ± 0.9 d | 0.00 ± 0.00 e | 87.84 ± 0.34 f | 5.65 ± 0.06 h | 00 ± 0.00 e |
| GA3 (mg/L) | SEs Germination (%) | Mean Number of Somatic Plantlets Recovered | ||
|---|---|---|---|---|
| Internode | Leaf | Internode | Leaf | |
| 1.0 | 9.43 ± 0.05 e | 11.51 ± 0.12 d | 2.65 ± 0.10 e | 3.47 ± 0.13 e |
| 1.5 | 11.50 ± 0.10 d | 13.54 ± 0.12 c | 3.65 ± 0.11 d | 4.72 ± 0.10 d |
| 2.0 | 16.54 ± 0.06 a | 17.47 ± 0.03 a | 5.87 ± 0.02 a | 7.62 ± 0.13 a |
| 2.5 | 14.40 ± 0.15 b | 15.54 ± 0.06 b | 5.44 ± 0.23 b | 7.09 ± 0.01 b |
| 3.0 | 12.51 ± 0.07 c | 13.54 ± 0.12 c | 4.88 ± 0.05 c | 5.69 ± 0.13 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anbazhakan, R.; Zhu, X.-M.; Li, N.-Q.; Poudel, B.; Gao, J.-Y. Phenolic Exudation Control and Indirect Somatic Embryogenesis of Garlic-Fruit Tree (Malania oleifera Chun & S.K. Lee)—An Endangered Woody Tree Species of Southeastern Yunnan Province, China. Plants 2025, 14, 2186. https://doi.org/10.3390/plants14142186
Anbazhakan R, Zhu X-M, Li N-Q, Poudel B, Gao J-Y. Phenolic Exudation Control and Indirect Somatic Embryogenesis of Garlic-Fruit Tree (Malania oleifera Chun & S.K. Lee)—An Endangered Woody Tree Species of Southeastern Yunnan Province, China. Plants. 2025; 14(14):2186. https://doi.org/10.3390/plants14142186
Chicago/Turabian StyleAnbazhakan, Rengasamy, Xin-Meng Zhu, Neng-Qi Li, Brihaspati Poudel, and Jiang-Yun Gao. 2025. "Phenolic Exudation Control and Indirect Somatic Embryogenesis of Garlic-Fruit Tree (Malania oleifera Chun & S.K. Lee)—An Endangered Woody Tree Species of Southeastern Yunnan Province, China" Plants 14, no. 14: 2186. https://doi.org/10.3390/plants14142186
APA StyleAnbazhakan, R., Zhu, X.-M., Li, N.-Q., Poudel, B., & Gao, J.-Y. (2025). Phenolic Exudation Control and Indirect Somatic Embryogenesis of Garlic-Fruit Tree (Malania oleifera Chun & S.K. Lee)—An Endangered Woody Tree Species of Southeastern Yunnan Province, China. Plants, 14(14), 2186. https://doi.org/10.3390/plants14142186






