Desiccation-Tolerant Vascular Plants: A Group of Species Largely Neglected in Conservation
Abstract
1. Introduction
2. Results
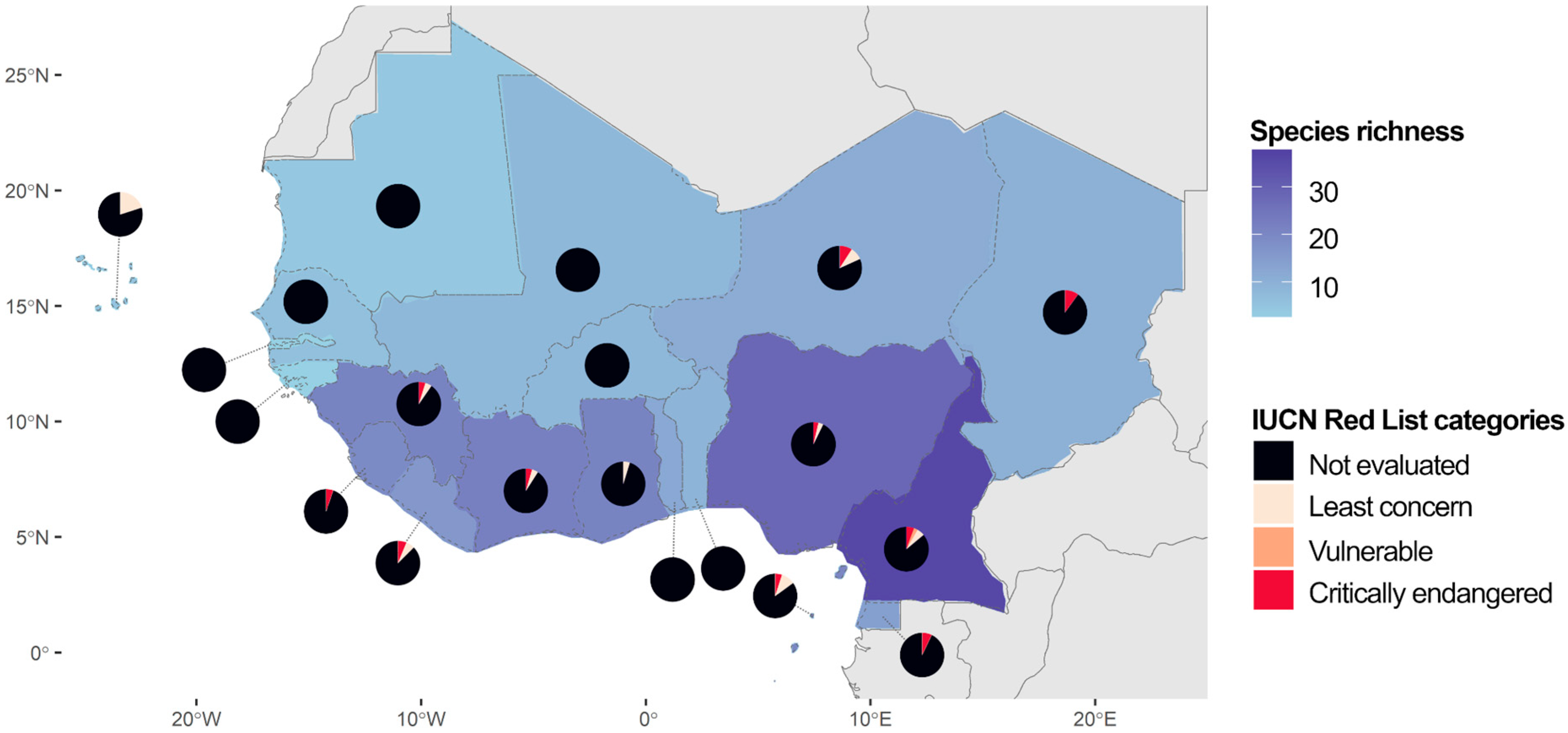
2.1. Diversity, Distribution, and Conservation of DT Plants in West Africa
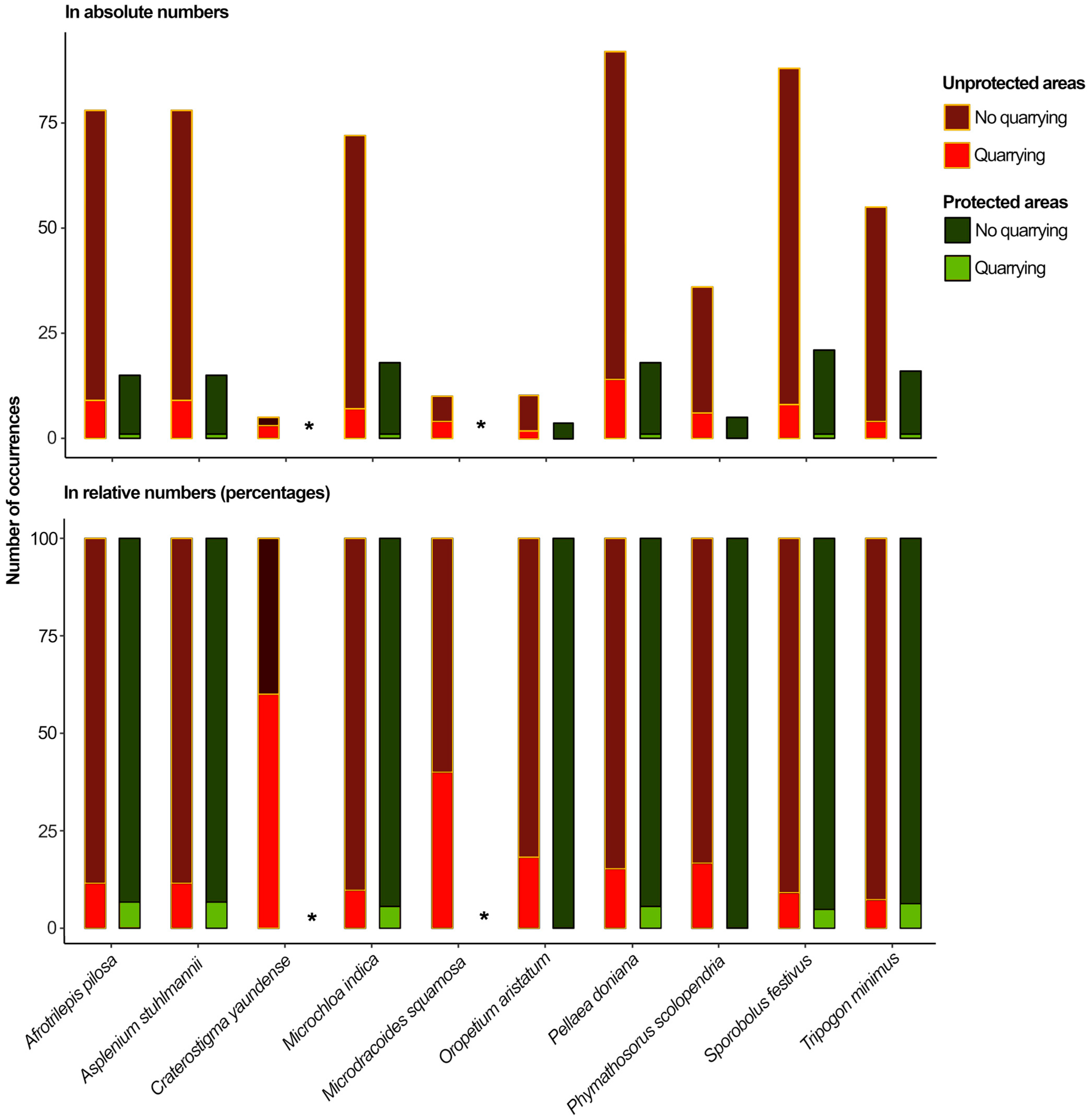
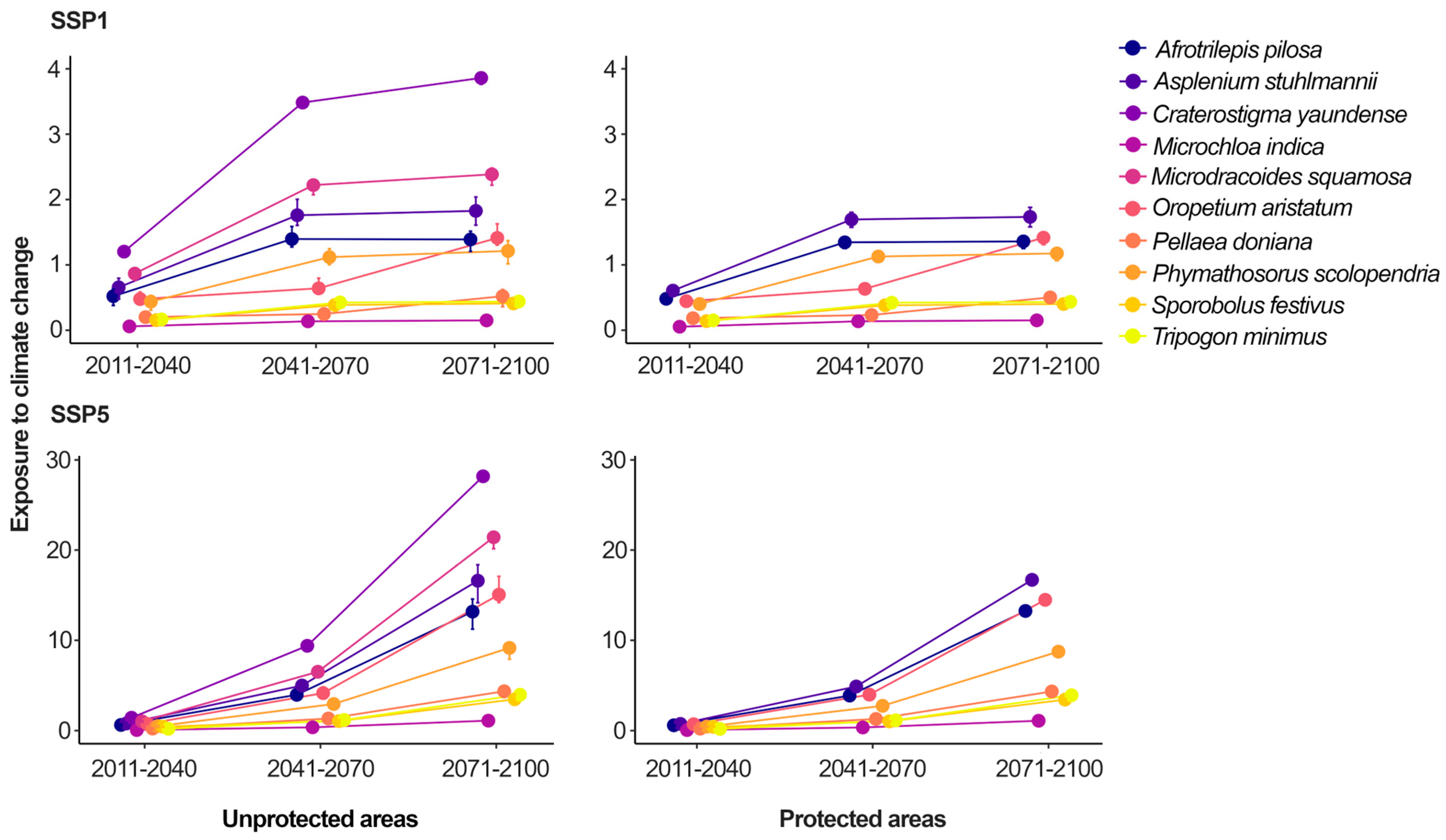
2.2. The Exposure of DT Plants to Anthropogenic Drivers of Biodiversity Loss and Their Protection on West African Inselbergs
3. Discussion
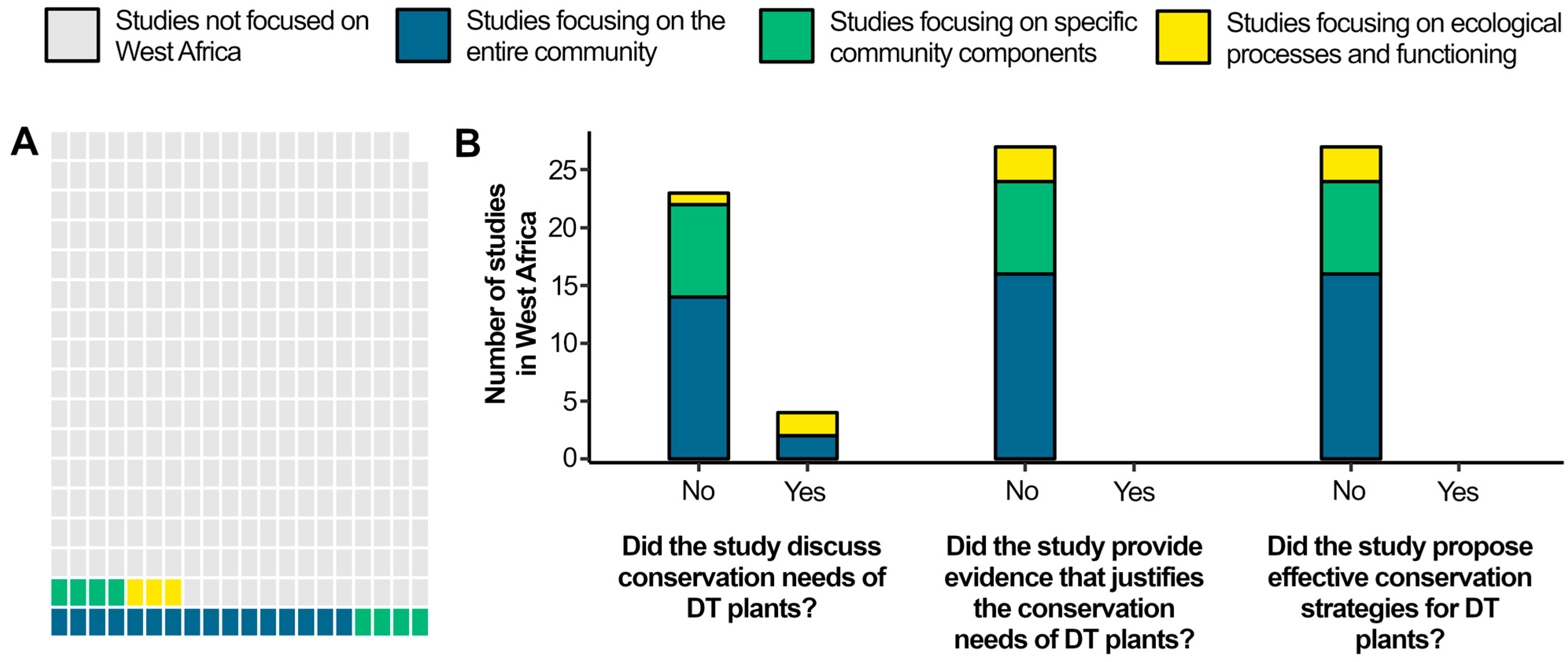
3.1. The Need of Studies Specifically Designed to Assess the Conservation of DT Plants
3.2. Protected Areas Are Not Similarly Effective in Face of Different Conservation Challenges
4. Materials and Methods
4.1. Study Area
4.2. Desiccation-Tolerant Vascular Plants in West Africa
4.3. Study Case to Evaluated the Exposure of DT Plants to Land Use Change and Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DT | Desiccation-tolerant |
| IUCN | International Union for Conservation of Nature |
| SSP | shared socioeconomic pathway |
References
- Sinclair, A.R.E. The loss of biodiversity: The sixth great extinction. In Conserving Nature’s Diversity; Ashgate: Burlington, VT, USA, 2000; pp. 9–15. [Google Scholar]
- Bergès, L.; Roche, P.; Avon, C. Corridors écologiques et conservation de la biodiversité. Sci. Eaux Territ. 2010, 3, 34–39. [Google Scholar]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Purvis, A. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef]
- Bellard, C.; Marino, C.; Courchamp, F. Ranking threats to biodiversity and why it doesn’t matter. Nat. Commun. 2022, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Chaplin-Kramer, R.; Sharp, R.P.; Weil, C.; Bennett, E.M.; Pascual, U.; Arkema, K.K.; Daily, G.C. Global modeling of nature’s contributions to people. Science 2019, 366, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Sixteen years of change in the global terrestrial human footprint. Nat. Commun. 2016, 7, 12558. [Google Scholar] [CrossRef]
- World Meteorological Organization. State of the Climate in Africa 2024; WMO: Geneva, Switzerland, 2025. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Marks, R.A.; Farrant, J.M.; McLetchie, D.N.; VanBuren, R. Unexplored dimensions of variability in vegetative desiccation tolerance. Am. J. Bot. 2021, 108, 346–358. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Costa, M.C.D.; Farrant, J.M.; Oliver, M.J.; Ligterink, W.; Buitink, J.; Hilhorst, H.M. Key genes involved in desiccation tolerance and dormancy across life forms. Plant Sci. 2016, 251, 162–168. [Google Scholar] [CrossRef]
- Hilhorst, H.W.; Farrant, J.M. Plant desiccation tolerance: A survival strategy with exceptional prospects for climate-smart agriculture. Annu. Plant Rev. Online 2018, 1, 327–354. [Google Scholar]
- Gaff, D.F. Protoplasmic tolerance of extreme water stress. In Encyclopedia of Plant Physiology; Springer: Berlin, Germany, 1981. [Google Scholar]
- Porembski, S.; Silveira, F.A.; Fiedler, P.L.; Watve, A.; Rabarimanarivo, M.; Kouame, F.; Hopper, S.D. Worldwide destruction of inselbergs and related rock outcrops threatens a unique ecosystem. Biodivers. Conserv. 2016, 25, 2827–2830. [Google Scholar] [CrossRef]
- Porembski, S. Tropical inselbergs: Habitat types, adaptive strategies and diversity patterns. Braz. J. Bot. 2007, 30, 579–586. [Google Scholar] [CrossRef]
- Porembski, S. The invasibility of tropical granite outcrops (‘inselbergs’) by exotic weeds. J. R. Soc. West. Aust. 2000, 83, 131–137. [Google Scholar]
- Porembski, S.; Barthlott, W.; Dörrstock, S.; Biedinger, N. Vegetation of rock outcrops in Guinea: Granite inselbergs, sandstone table mountains and ferricretes. Flora 1994, 189, 315–326. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J.; Tuba, Z.; Mishler, B.D. The evolution of vegetative desiccation tolerance in land plants. Plant Ecol. 2000, 151, 85–100. [Google Scholar] [CrossRef]
- Gould, S.J. Dollo on Dollo’s law: Irreversibility and the status of evolutionary laws. J. Hist. Biol. 1970, 3, 189–212. [Google Scholar] [CrossRef]
- Cullen, R. Biodiversity protection prioritisation: A 25-year review. Wildl. Res. 2013, 40, 108–116. [Google Scholar] [CrossRef]
- IUCN Species Survival Commission (SSC) West Africa Plant Red List Authority. Advancing Plant Conservation in West Africa through Red List Assessments. 2025. Available online: https://www.iucn.org (accessed on 11 April 2025).
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland, 2012. [Google Scholar]
- Zizka, A.; Andermann, T.; Silvestro, D. IUCNN—Deep learning approaches to approximate species’ extinction risk. Divers. Distrib. 2022, 28, 227–241. [Google Scholar] [CrossRef]
- Bachman, S.P.; Brown, M.J.; Leão, T.C.; Nic Lughadha, E.; Walker, B.E. Extinction risk predictions for the world’s flowering plants to support their conservation. New Phytol. 2024, 242, 797–808. [Google Scholar] [CrossRef]
- Cazalis, V.; Di Marco, M.; Zizka, A.; Butchart, S.H.M.; González-Suárez, M.; Böhm, M.; Bachman, S.P.; Hoffmann, M.; Rosati, I.; De Leo, F.; et al. Accelerating and standardising IUCN Red List assessments with sRedList. Biol. Conserv. 2024, 298, 110761. [Google Scholar] [CrossRef]
- Tecco, P.A.; Díaz, S.; Cabido, M.; Urcelay, C. Functional traits of alien plants across contrasting climatic and land-use regimes. J. Ecol. 2010, 98, 17–27. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Davis, M.; Chew, M.; Hobbs, R.; Lugo, A.E.; Ewel, J.J.; Vermeij, G.J.; Brown, J.H.; Rosenzweig, M.L.; Gardener, M.R.; Carroll, S.P.; et al. Don’t judge species on their origins. Nature 2011, 474, 153–154. [Google Scholar] [CrossRef]
- Moro, M.F.; Souza, V.C.; Oliveira-Filho, A.T.; Queiroz, L.P.; Fraga, C.N.; Rodal, M.J.N.; Araújo, F.S.; Martins, F.R. Alienígenas na sala: O que fazer com espécies exóticas em trabalhos de taxonomia, florística e fitossociologia? Acta Bot. Bras. 2012, 26, 991–999. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- de Paula, L.F.A.; Negreiros, D.; Azevedo, L.O.; Fernandes, R.L.; Stehmann, J.R.; Silveira, F.A.O. Functional ecology as a missing link for conservation of a resource-limited flora in the Atlantic forest. Biodivers. Conserv. 2015, 24, 2239–2253. [Google Scholar] [CrossRef]
- Kumschick, S.; Gaertner, M.; Vila, M.; Essl, F.; Jeschke, J.M.; Pyšek, P.; Ricciardi, A.; Bacher, S.; Blackburn, T.M.; Dick, J.T.A.; et al. Ecological impacts of alien species: Quantification, scope, caveats, and recommendations. BioScience 2015, 65, 55–63. [Google Scholar] [CrossRef]
- Dehling, D.M.; Stouffer, D.B. Bringing the Eltonian niche into functional diversity. Oikos 2018, 127, 1711–1723. [Google Scholar] [CrossRef]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Johnson, C.N. Species extinction and the relationship between distribution and abundance. Nature 1998, 394, 272–274. [Google Scholar] [CrossRef]
- Violle, C.; Thuiller, W.; Mouquet, N.; Munoz, F.; Kraft, N.J.B.; Cadotte, M.W.; Livingstone, S.W.; Mouillot, D. Functional rarity: The ecology of outliers. Trends Ecol. Evol. 2017, 32, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Zokov, E. First Insights into Alternative Functional Designs of Desiccation-Tolerant Plants’ Desiccation. Master’s Thesis, University of Rostock, Rostock, Germany, 2024. [Google Scholar]
- Porembski, S.; Barthlott, W. Plant species diversity of West African inselbergs. In The Biodiversity of African Plants; Springer: Dordrecht, The Netherlands, 1996; pp. 180–187. [Google Scholar]
- Smith, R.J.; Veríssimo, D.; Isaac, N.J.; Jones, K.E. Identifying Cinderella species: Uncovering mammals with conservation flagship appeal. Conserv. Lett. 2012, 5, 205–212. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single-species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Moynes, E.; Bhathe, V.; Brennan, C.; Ellis, S.; Bennett, J.; Landsman, S. Flagship species: Do they help or hurt conservation? Front. Young Minds 2021, 9, 576035. [Google Scholar] [CrossRef]
- Williams, P.; Burgess, N.; Rahbek, C. Flagship species, ecological complementarity and conserving the diversity of mammals and birds in sub-Saharan Africa. Anim. Conserv. 2000, 3, 249–260. [Google Scholar]
- Mazel, F.; Mooers, A.Ø.; Riva, G.V.D.; Pennell, M.W. Prioritizing phylogenetic diversity captures functional diversity unreliably. Nat. Commun. 2018, 9, 2888. [Google Scholar] [CrossRef] [PubMed]
- Balding, M.; Williams, K.J.H. Plant blindness and the implications for plant conservation. Conserv. Biol. 2016, 30, 1192–1199. [Google Scholar] [CrossRef]
- Caro, T.M.; O’Doherty, G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Murphy, D.D.; Weiland, P.S. The use of surrogates in implementation of the federal Endangered Species Act—Proposed fixes to a proposed rule. J. Environ. Stud. Sci. 2014, 4, 156–162. [Google Scholar] [CrossRef]
- Lawton, J.H. Range, population abundance and conservation. Trends Ecol. Evol. 1993, 8, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Flather, C.H.; Sieg, C.H. Species rarity: Definition, causes and classification. In Conservation of Rare or Little-Known Species; Island Press: Washington, DC, USA, 2007; pp. 40–66. [Google Scholar]
- CBD. COP 10 Decisions: Strategic Plan for Biodiversity 2011–2020. 2025. Available online: https://www.cbd.int/decision/cop?id=12268 (accessed on 11 April 2025).
- UNEP-WCMC; IUCN. Protected Planet Report 2024; UNEP-WCMC: Cambridge, UK, 2024. [Google Scholar]
- Daru, B.H. Predicting undetected native vascular plant diversity at a global scale. Proc. Natl. Acad. Sci. USA 2024, 121, e2319989121. [Google Scholar] [CrossRef]
- Torres, A.; zu Ermgassen, S.O.; Ferri-Yanez, F.; Navarro, L.M.; Rosa, I.M.; Teixeira, F.Z.; Liu, J. Unearthing the global impact of mining construction minerals on biodiversity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Timpte, M.; Marquard, E.; Paulsch, C. Analysis of the Strategic Plan 2011–2020 of the Convention on Biological Diversity (CBD); IBN: Regensburg, Germany, 2018. [Google Scholar]
- McIntyre, N.E.; Wiens, J.A. Interactions between habitat abundance and configuration: Experimental validation of some predictions from percolation theory. Oikos 1999, 86, 129–137. [Google Scholar] [CrossRef]
- Desmet, P.G. Using landscape fragmentation thresholds to determine ecological process targets in systematic conservation plans. Biol. Conserv. 2018, 221, 257–260. [Google Scholar] [CrossRef]
- Keitt, T.H.; Urban, D.L.; Milne, B.T. Detecting critical scales in fragmented landscapes. Conserv. Ecol. 1997, 1, 4. [Google Scholar] [CrossRef]
- Dickinson, M.G.; Orme, C.D.L.; Suttle, K.B.; Mace, G.M. Separating sensitivity from exposure in assessing extinction risk from climate change. Sci. Rep. 2014, 4, 6898. [Google Scholar] [CrossRef]
- Carriquí, M.; Fortesa, J.; Brodribb, T.J. A loss of stomata exposes a critical vulnerability to variable atmospheric humidity in ferns. Curr. Biol. 2025, 35, 1539–1548. [Google Scholar] [CrossRef]
- Fage, J.D.; McCaskie, T. Western Africa. In Encyclopedia Britannica; Encyclopaedia Britannica (UK) Ltd.: London, UK, 2025; Available online: https://www.britannica.com/place/western-Africa (accessed on 10 April 2025).
- Sinsin, B.; Kampmann, D.; Thiombiano, A.; Konaté, S. Atlas de la Biodiversité de l’Afrique de l’Ouest; Tome I: Cotonou, Benin; Frankfurt/Main, Germany, 2010. [Google Scholar]
- Mallon, D.P.; Hoffmann, M.; Grainger, M.J.; Hibert, F.; van Vliet, N.; McGowan, P.J.K. An IUCN Situation Analysis of Terrestrial and Freshwater Fauna in West and Central Africa; IUCN Occasional Paper; IUCN: Gland, Switzerland, 2015; p. 54. [Google Scholar]
- Tindano, E.; Kadéba, A.; Traoré, I.C.E.; Thiombiano, A. Effects of abiotic factors on the flora and vegetation of inselbergs in Burkina Faso. Environ. Adv. 2023, 12, 100378. [Google Scholar] [CrossRef]
- Tindano, E.; Lankoandé, B.; Porembski, S.; Thiombiano, A. Inselbergs: Potential conservation areas for plant diversity in the face of anthropization. J. Phytol. 2023, 15, 70–79. [Google Scholar] [CrossRef]
- Tindano, E.; Kaboré, G.E.; Porembski, S.; Thiombiano, A. Plant communities on inselbergs in Burkina Faso. Heliyon 2024, 10, e23653. [Google Scholar] [CrossRef]
- Hutchinson, J.; Dalziel, J.M. Flora of West Tropical Africa; Crown Agents: London, UK, 1958; Volume 1. [Google Scholar]
- Adjanohoun, E. Végétation des savanes et des rochers découverts en Côte d’Ivoire Centrale. Mém. ORSTOM 1964, 7, 1–178. [Google Scholar]
- POWO. Plants of the World Online. 2025. Available online: https://powo.science.kew.org/ (accessed on 12 April 2025).
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. 2025. Available online: https://www.iucnredlist.org (accessed on 12 April 2025).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Rinnan, D.S.; Lawler, J. Climate-niche factor analysis: A spatial approach to quantifying species vulnerability to climate change. Ecography 2019, 42, 1494–1503. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Home Page. 2024. Available online: https://www.gbif.org (accessed on 23 February 2024).
- Phillips, N.; Lawrence, T.B.; Hardy, C. Discourse and institutions. Acad. Manag. Rev. 2004, 29, 635–652. [Google Scholar] [CrossRef]
- Karger, D.N.; Lange, S.; Hari, C.; Reyer, C.P.O.; Conrad, O.; Zimmermann, N.E.; Frieler, K. CHELSA-W5E5: Daily 1km meteorological forcing data for climate impact studies. Earth Syst. Sci. Data 2023, 15, 2445–2464. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2025. [Google Scholar]


| Distribution in West Africa | IUCN Red List Categories | |||
|---|---|---|---|---|
| Lycophytes | ||||
| Selaginellaceae | ||||
| Selaginella njamnjamensis Hieron. | BEN, CMR, MLI, NGA | Not evaluated | ||
| Pteridophytes | ||||
| Aspleniaceae | ||||
| Asplenium aethiopicum (Burm.f.) Becherer | CIV, CMR, GGN, GIN, GNQ, LBR, NER, NGA, SLE, TCD | Vulnerable | ||
| Asplenium friesiorum C.Chr. | CMR, GGN, NGA | Not evaluated | ||
| Asplenium megalura Hieron. | CIV, CMR, GHA, GGN, GIN, LBR, SLE, TGO | Not evaluated | ||
| Asplenium monanthes L. | CMR, GGN | Least Concern | ||
| Asplenium sandersonii Hook. | CMR, GGN, GNQ, NGA | Not evaluated | ||
| Asplenium stuhlmannii Hieron. | CIV, CMR, GIN, NGA, SLE | Not evaluated | ||
| Dryopteridaceae | ||||
| Elaphoglossum acrostichoides (Hook. & Grev.) Schelpe | CIV, CMR, GGN, GHA, GIN, LBR | Least Concern | ||
| Hymenophyllaceae | ||||
| Crepidomanes chevalieri (Christ) Ebihara & Dubuisson | CIV, CMR, GGN, GHA, GIN, LBR, NGA, SLE | Not evaluated | ||
| Crepidomanes melanotrichum (Schltdl.) J.P.Roux | CIV, CMR, GGN, GHA, GIN, LBR, NGA, SLE | Not evaluated | ||
| Didymoglossum erosum (Willd.) Beentje | CIV, CMR, GHA, GIN, LBR, NGA, SLE | Not evaluated | ||
| Hymenophyllum capillare Desv. | CMR, GGN, GHA | Not evaluated | ||
| Hymenophyllum hirsutum (L.) Sw. | CMR, GGN, GHA, GIN, GNQ, CIV, LBR | Not evaluated | ||
| Hymenophyllum kuhnii C.Chr. | CMR, GGN, GHA, GIN, GNQ, LBR, NGA, SLE | Not evaluated | ||
| Hymenophyllum splendidum Bosch | CMR, GNQ | Not evaluated | ||
| Polyphlebium borbonicum (Bosch) Ebihara & Dubuisson | CIV, CMR, GGN, GNQ, GHA, GIN, LBR | Not evaluated | ||
| Polypodiaceae | ||||
| Loxogramme abyssinica (Baker) M.G.Price | CIV, CMR, GGN, GHA, GIN, GNQ, LBR, NGA, SLE, TGO | Not evaluated | ||
| Melpomene flabelliformis (Poir.) A.R.Sm. & R.C.Moran | CMR, GGN | Not evaluated | ||
| Phymatosorus scolopendria (Burm.f.) Pic.Serm. | BEN, CIV, CMR, GGN, GNQ, GHA, GIN, LBR, NGA, SLE, TGO | Not evaluated | ||
| Platycerium stemaria (P.Beauv.) Desv. | BEN, CIV, CMR, GGN, GNQ, GHA, GIN, LBR, NGA, SEN, SLE | Not evaluated | ||
| Pleopeltis macrocarpa (Willd.) Kaulf. | CIV, CMR, GGN, GIN, LBR, NGA, SLE | Not evaluated | ||
| Pteridaceae | ||||
| Actiniopteris radiata (Sw.) Link | CMR, CPV, MLI, NGA, TCD, TGO | Not evaluated | ||
| Actiniopteris semiflabellata Pic.Serm. | MRT | Not evaluated | ||
| Adiantum incisum Forssk. | CIV, CMR, CPV, GHA, NGA, TGO | Not evaluated | ||
| Cheilanthes coriacea Decne. | NER, TCD | Not evaluated | ||
| Cheilanthes inaequalis Mett. | CMR, GIN, NGA | Not evaluated | ||
| Cosentinia vellea (Aiton) Tod. | CPV | Least Concern | ||
| Hemionitis farinosa (Forssk.) Christenh. | CMR, GGN, NGA, SLE | Not evaluated | ||
| Pellaea doniana Hook. | GNQ | Not evaluated | ||
| Vittaria guineensis Desv. | CIV, CMR, GGN, GHA, GIN, GNQ, LBR, NGA, SLE, TGO | Not evaluated | ||
| Tectariaceae | ||||
| Arthropteris orientalis (J.F.Gmel.) Posth. | CIV, CMR, GGN, GHA, GIN, LBR, NGA, SLE | Not evaluated | ||
| Angiosperms | ||||
| Cyperaceae | ||||
| Afrotrilepis jaegeri J.Raynal | SLE * | Not evaluated | ||
| Afrotrilepis pilosa (Boeckeler) J.Raynal | BEN, BFA, CIV, CMR, GHA, GIN, GNQ, LBR, MLI, NGA, SEN, SLE, TGO | Not evaluated | ||
| Coleochloa abyssinica (Hochst. ex A.Rich.) Gilly | CMR, NGA | Not evaluated | ||
| Coleochloa domensis Muasya & D.A.Simpson | CMR * | Critically endangered | ||
| Microdracoides squamosa Hua | CMR, GIN, NGA, SLE * | Not evaluated | ||
| Linderniaceae | ||||
| Craterostigma plantagineum Hochst. | BFA, NER, TCD | Not evaluated | ||
| Craterostigma yaundense (S.Moore) Eb.Fisch., Schäferh. & Kai Müll. | CMR * | Vulnerable | ||
| Poaceae | ||||
| Microchloa indica (L.f.) P.Beauv. | BEN, BFA, CIV, CMR, GHA, GIN, GNB, MLI, NER, NGA, SEN, SLE, TCD, TGO | Not evaluated | ||
| Microchloa kunthii Desv. | BEN, BFA, CIV, CMR, GHA, NGA, TGO | Not evaluated | ||
| Oropetium aristatum (Stapf) Pilg. | BEN, BFA, CIV, GHA, GMB, GNB, MLI, NER, SEN, TGO * | Not evaluated | ||
| Oropetium capense Stapf | MLI, MRT, NER, TCD | Not evaluated | ||
| Sporobolus festivus Hochst. ex A.Rich. | BEN, BFA, CIV, CMR, GHA, GIN, GMB, GNQ, MLI, MRT, NER, NGA, SEN, TCD, TGO | Not evaluated | ||
| Sporobolus pellucidus Hochst. | BFA, NER, TCD | Not evaluated | ||
| Sporobolus stapfianus Gand. | NER, NGA | Least Concern | ||
| Tripogon major Hook.f. | SLE | Not evaluated | ||
| Tripogon multiflorus Miré & H.Gillet | CPV, NER, TCD | Not evaluated | ||
| Tripogonella minima (A.Rich.) P.M.Peterson & Romasch. | BEN, BFA, CIV, CMR, CPV, GHA, MLI, MRT, NER, NGA, SEN, TCD, TGO | Not evaluated | ||
| Velloziaceae | ||||
| Xerophyta schnitzleinia (Hochst.) Baker | NGA, GNQ | Not evaluated | ||
| W-Value/t-Value | p-Value | |||
|---|---|---|---|---|
| Quarrying | 80 | 0.0004 | ||
| Climate change | ||||
| SSP1 | ||||
| 2011–2040 | 1.17 | 0.7809 | ||
| 2041–2070 | 1.04 | 0.9456 | ||
| 2071–2100 | 1.05 | 0.9297 | ||
| SSP5 | ||||
| 2011–2040 | 1.1 | 0.8592 | ||
| 2041–2070 | 1.03 | 0.9519 | ||
| 2071–2100 | 0.93 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidou, W.I.; Bondi, L.; Porembski, S.; Bomisso, E.L. Desiccation-Tolerant Vascular Plants: A Group of Species Largely Neglected in Conservation. Plants 2025, 14, 2184. https://doi.org/10.3390/plants14142184
Seidou WI, Bondi L, Porembski S, Bomisso EL. Desiccation-Tolerant Vascular Plants: A Group of Species Largely Neglected in Conservation. Plants. 2025; 14(14):2184. https://doi.org/10.3390/plants14142184
Chicago/Turabian StyleSeidou, Wassila Ibrahim, Luiz Bondi, Stefan Porembski, and Edson Lezin Bomisso. 2025. "Desiccation-Tolerant Vascular Plants: A Group of Species Largely Neglected in Conservation" Plants 14, no. 14: 2184. https://doi.org/10.3390/plants14142184
APA StyleSeidou, W. I., Bondi, L., Porembski, S., & Bomisso, E. L. (2025). Desiccation-Tolerant Vascular Plants: A Group of Species Largely Neglected in Conservation. Plants, 14(14), 2184. https://doi.org/10.3390/plants14142184






