Yield, Phytonutritional and Essential Mineral Element Profiles of Selected Aromatic Herbs: A Comparative Study of Hydroponics, Soilless and In-Soil Production Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytonutrional Profiling of Selected Herbs Grown Under Different Environmental Conditions
2.1.1. Vitamin C Content
2.1.2. β-Carotene Content
2.1.3. Total Phenolic Content
2.1.4. Total Flavonoid Content
2.1.5. Condensed Tannins
2.1.6. DPPH Antioxidant Capacity
2.1.7. β-Carotene Linoleic Acid Content
2.2. Leaf Mineral Content and Yield
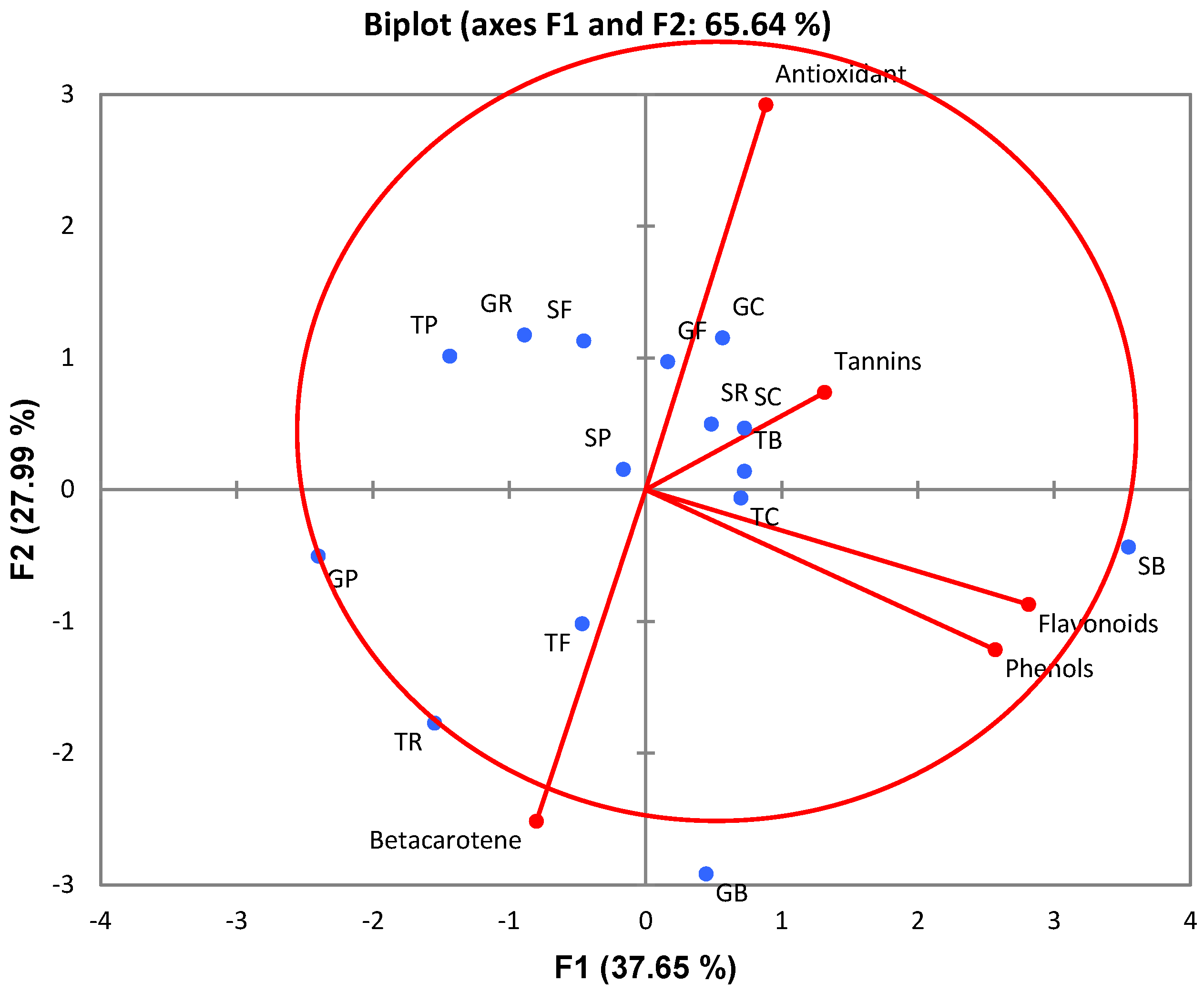
2.3. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Aromatic Herbs
3.1.1. Gravel-Film Technique (Closed Hydroponic System)
3.1.2. In-Soil Cultivation Under Shade-Net Structure
3.1.3. Soilless Cultivation Under a Non-Temperature-Controlled Plastic Tunnel (NTC)
3.2. Fresh Leaf Mass
3.3. The Phytonutritional Analysis at Harvest
3.3.1. Quantification of Vitamin C Content
3.3.2. Determination of β-Carotene Content
3.3.3. Determination of β-Carotene Linoleic Acid Content
3.3.4. Determination of Condensed Tannins
3.3.5. Quantification of the Total Phenolic Content
3.3.6. The Total Flavonoid Content
3.3.7. Determination of the Antioxidant Scavenging Activity
3.3.8. Determination of Mineral Nutrients
3.3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, R.S.; Pal, Y.; Saraswat, N.; Pranay, W. A review on the recent flavoring herbal medicines of today. Open Med. J. 2020, 7, 1874–2203. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (poly)phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Okpoghono, J. Preservative approaches, culinary and medicinal prospective mechanisms of herbs and spices: A compiled update. In Culinary and Medicinal Prospective Mechanisms of Herbs and Spices: A Compiled Update; Elsevier BV: Amsterdam, The Netherlands, 2024; 29p. [Google Scholar]
- Barbaś, P.; Pszczółkowski, P.; Krochmal-Marczak, B.; Hameed, T.S.; Sawicka, B. Soil-specific effects of the bio-growth regulator supporter on seed potato yield and quality across varieties: Unlocking Sustain Potential in Diverse Environments. Land 2025, 14, 595. [Google Scholar]
- Alén, R. Chemistry for Biomass Utilization; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2023. [Google Scholar]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-lanS, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Abd Elgadir, M.; Chigurupati, S.; Mariod, A.A. Selected potential pharmaceutical and medical benefits of phenolic compounds: Recent advances. Funct. Food Sci.-Online ISSN 2021, 3, 108–128. [Google Scholar] [CrossRef]
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Sharma, P.; Nandave, M.; Nandave, D.; Yadav, S.; Vargas-De-La-Cruz, C.; Singh, S.; Tandon, R.; Ramniwas, S.; Behl, T. Reactive oxygen species (ROS)-mediated oxidative stress in chronic liver diseases and its mitigation by medicinal plants. Am. J. Transl. Res. 2023, 15, 6321–6341. [Google Scholar]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Sawicka, B. Post-harvest losses of agricultural produce. In Zero Hunger; Springer International Publishing: Cham, Switzerland, 2020; pp. 654–669. [Google Scholar]
- Jayasekara, C.; Mendis, E.; Kim, S.K. Seafood in the human diet for better nutrition and health. In Encyclopedia of Marine Biotechnology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 2939–2959. [Google Scholar]
- Kumar, S.; Sharma, S.; Kumar, V.; Sharma, R.; Minhas, A.; Boddu, R. Cruciferous vegetables: A mine of phytonutrients for functional and nutraceutical enrichment. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Academic Press: Cambridge, MA, USA, 2022; pp. 401–426. [Google Scholar]
- Hodges, D.M.; Lester, G.E. Cucurbits. In Health-Promoting Properties of Fruits and Vegetables; CABI: Walingford, UK, 2011; pp. 118–134. [Google Scholar]
- Zhitkovich, A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Agwu, E.; Ezihe, C.; Kaigama, G. Antioxidant roles/functions of ascorbic acid (vitamin C). In Ascorbic Acid: Biochemistry and Functions; IntechOpen: London, UK, 2023; p. 42. [Google Scholar]
- Basrowi, R.W.; Dilantika, C. Optimizing iron adequacy and absorption to prevent iron deficiency anemia: The role of combination of fortified iron and vitamin C. World Nutr. J. 2021, 5, 33–39. [Google Scholar] [CrossRef]
- Kükürt, A.; Gelen, V. (Eds.) Ascorbic Acid: Biochemistry and Functions; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, R.I.S.; Maboko, M.M.; Mudau, F.N. Growth, yield and mineral content of basil and cultivated rocket due to plant density and nitrogen level. Int. J. Veg. Sci. 2020, 26, 558–572. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic acid—The little-known antioxidant in woody plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M. Historical and introductory aspects of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–42. [Google Scholar]
- Rao, A.V.; Rao, L.; Brzozowski, T. Dietary Carotenoids: Sources, Properties, and Role in Human Health; IntechOpen: London, UK, 2024. [Google Scholar]
- Maurya, V.K.; Shakya, A.; Aggarwal, M.; Gothandam, K.M.; Bohn, T.; Pareek, S. Fate of β-carotene within loaded delivery systems in food: State of knowledge. Antioxidants 2021, 10, 426. [Google Scholar] [CrossRef]
- Yisak, H.; Elmneh, R.; Taklual, W.; Ewunetei, A.; Kefale, B. Prevalence and associated factors of clinical vitamin A deficiency among pre-school children 1–5 years of age in rural Kebeles in Farta District, South Gondar Zone, Ethiopia: A mixed methods study. J. Multidiscip. Healthc. 2020, 13, 1191–1201. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Poledica, M.; Starace, G.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Pearl grey shading net boosts the accumulation of total carotenoids and phenolic compounds that accentuate the antioxidant activity of processing tomato. Antioxidants 2021, 10, 1999. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Yue, C.; Wang, Z.; Yang, P. The effect of light on the key pigment compounds of photosensitive etiolated tea plant. Bot. Stud. 2021, 62, 21. [Google Scholar] [CrossRef]
- De Souza, G.S.; De Castro, E.M.; Soares, Â.M.; Pinto, J.E.B.P. Característicasbiométricas e fisiológicas de plantas jovens de Mikania glomerataSprengel e Mikania laevigata Schultz Bip. ex Baker cultivadas sobmalhas coloridas. Rev. Bras. Biociênc. 2010, 8, 330–335. [Google Scholar]
- Lone, R.; Bhat, A.; Nazim, N.; Malla, N.A.; Rohella, G.K.; Mohamed, H.I. Role of Phenolics in Plant–Microbe Interaction: A Review. In Plant Phenolics in Biotic Stress Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–33. [Google Scholar]
- Choudhary, G.K.; Nirala, R.K.; Kumar, N.; Choudhary, P.K.; Kumar, R.; Singh, S.P. Reactive oxygen nitrogen species and antioxidant. J. Vet. Pharmacol. Toxicol. 2023, 22, 1–7. [Google Scholar]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Eskandarzade, P.; Mehrjerdi, M.Z.; Gruda, N.S.; Aliniaeifard, S. Phytochemical compositions and antioxidant activity of green and purple basils altered by light intensity and harvesting time. Heliyon 2024, 10, e30931. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants-a comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of salinity stress on color parameters, leaf pigmentation, polyphenol and flavonoid contents, and antioxidant activity of Amaranthus lividus leafy vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Iqbal, N.; Poór, P. Plant protection by tannins depends on defence-related phytohormones. J. Plant Growth Regul. 2025, 44, 22–39. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar]
- Cunha de Melo, K.; Silva de Oliveira, I.; Helena de Oliveira Pires, L.; Santos do Nascimento, L.A.; Roberto Zamian, J.; Narciso da Rocha Filho, G.; Fonseca Passos, M.; Santos Lopes, A.; Converti, A.; Costa, C.E.F.D. Study of the antioxidant power of the waste oil from palm oil bleaching clay. Energies 2020, 13, 804. [Google Scholar] [CrossRef]
- Kruk, J.; Szymańska, R. Singlet oxygen oxidation products of carotenoids, fatty acids and phenolic prenyllipids. J. Photochem. Photobiol. B Biol. 2021, 216, 112148. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular mechanisms of plant responses to copper: From deficiency to excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef]
- Kumar, D.; Punetha, A.; Verma, P.P.; Padalia, R.C. Micronutrient based approach to increase yield and quality of essential oil in aromatic crops. J. Appl. Res. Med. Aromat. Plants 2020, 26, 100361. [Google Scholar] [CrossRef]
- Vogt, A.C.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On iron metabolism and its regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Maboko, M.; Du Plooy, I. High-density of tomato cultivar’s with early decapitation of growing point increased yield in a closed hydroponic system. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2013, 63, 676–682. [Google Scholar] [CrossRef]
- Shiva, S.; Enninful, R.; Roth, M.R.; Tamura, P.; Jagadish, K.; Welti, R. An efficient modified method for plant leaf lipid extraction results in improved recovery of phosphatidic acid. Plant Methods 2018, 14, 14. [Google Scholar] [CrossRef]
- Xu, J.; Lin, J.; Peng, S.; Zhao, H.; Wang, Y.; Rao, L.; Liao, X.; Zhao, L. Development of an HPLC-PDA method for the determination of capsanthin, zeaxanthin, lutein, β-cryptoxanthin and β-carotene simultaneously in chili peppers and products. Molecules 2023, 28, 2362. [Google Scholar] [CrossRef]
- Chelghoum, M.; Guenane, H.; Harrat, M.; Yousfi, M. Total tocopherols, carotenoids, and fatty acids content variation of Pistacia atlantica from different organs’ crude oils and their antioxidant activity during development stages. Chem. Biodivers. 2020, 17, e2000117. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Zeller, W.E. Direct versus sequential analysis of procyanidin-and prodelphinidin-based condensed tannins by the HCl–butanol–acetone–iron assay. J. Agric. Food Chem. 2019, 68, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Mampholo, M.B.; Sivakumar, D.; Van Rensburg, J. Variation in bioactive compounds and quality parameters in different modified atmosphere packaging during postharvest storage of traditional leafy vegetables (Amaranthus cruentus L. and Solanum retroflexum). J. Food Qual. 2015, 38, 1–12. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of digestion methods using atomic absorption spectrometry for the determination of metal levels in plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
| Herbs | Phytonutritional Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Vitamin C (mg/100 g) | Β-Carotene (mg/100 g) | Total Phenols mg (GAE/g DW) | Total Flavonoids (mg CE/g DW) | Condensed Tannins Leucocyanidin (%) | DPPH Scavenging Activity (%) | B-Carotene Linoleic Acid (%) | |
| Gravel film technique hydroponic production (shade-net) | |||||||
| Basil | 13.8 b | 36.7 ab | 17.1 b | 5.4 ab | 0.09 de | 78.4 b | 55.3 h |
| Coriander | 8.3 c | 35.9 ab | 7.0 de | 5.1 abc | 0.15 abc | 73.6 b | 85.5 bcd |
| Fennel | 7.9 c | 33.8 abc | 8.7 d | 5.0 abc | 0.06 e | 86.9 a | 87.9 abc |
| Parsley | 7.9 c | 35.3 ab | 3.9 h | 2.6 d | 0.07 e | 87.6 a | 73.2 ef |
| Rocket | 7.9 c | 36.3 ab | 7.5 de | 3.5 bcd | 0.1 cd | 86.44 a | 88.0 abc |
| In-oilless production (Plastic tunnel) | |||||||
| Basil | 8.3 c | 24.6 e | 13.2 c | 5.3 ab | 0.07 e | 59.1 c | 79.7 de |
| Coriander | 9.2 c | 27.6 cde | 7.3 de | 5.5 ab | 0.17 a | 89.3 a | 69.6 fg |
| Fennel | 8.0 c | 25.2 de | 8.4 de | 3.0 d | 0.07 e | 89.3 a | 75.7 ef |
| Parsley | 8.1 c | 25.7 de | 5.9 ef | 5.0 abc | 0.07 e | 61.02 c | 83.0 cd |
| Rocket | 9.5 c | 29.8 bcde | 5.9 ef | 4.0 bcd | 0.07 e | 76.9 b | 65.8 g |
| In-soil production (Shade-net) | |||||||
| Basil | 18.6 a | 39.9 a | 25.6 a | 6.9 a | 0.13 bc | 88.6 a | 92.3 ab |
| Coriander | 8.0 c | 31.5 bcd | 9.1 d | 5.5 ab | 0.12 cd | 74.7 b | 91.6 ab |
| Fennel (SB) | 7.9 c | 32.3 bcd | 7.5 de | 3.4 bcd | 0.14 bc | 88.3 a | 94.2 a |
| Parsley (SP) | 8.1 c | 36.6 ab | 7.9 de | 4.3 bcd | 0.13 bc | 87.2 a | 90.3 ab |
| Rocket (SR) | 12.4 b | 32.7 bc | 8.9 d | 4.8 abcd | 0.15 ab | 87.7 a | 89.7 abc |
| Treatment | Leaf Mineral Content (mg/100 g) | Yield (g/Plant) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Calcium | Iron | Potassium | Magnesium | Manganese | Sodium | Phosphorus | Zinc | ||
| Gravel film technique production (Shade-net) | |||||||||
| Basil | 1211 bc | 10.9 f | 2180 fg | 339.3 bcd | 8.6 bc | 68.7 g | 717.3 a | 4.3 d | 122 b |
| Coriander | 651 f | 35.7 c | 3453 d | 322 cd | 5.8 de | 122 ef | 554.7 bc | 3.7 ef | 67.24 ef |
| Fennel | 1116 bc | 35.5 c | 3950 bcd | 317 cde | 6.1 de | 463 b | 428.7 ef | 3.4 fg | 78.49 d |
| Parsley | 835 def | 77.9 b | 5087 a | 237 e | 11.2 a | 151.3 cd | 467.3 cdef | 4.3 de | 51.18 hi |
| Rocket | 1868 a | 31.8 cd | 3280 de | 447 a | 3.5 fg | 125 e | 396.7 f | 2.9 g | 41.47 ij |
| In soilless production (Plastic tunnel) | |||||||||
| Basil | 1267 b | 12.9 ef | 2440 fg | 343.3 bcd | 6.7 d | 98.6 f | 691.3 a | 6.2 b | 109.6 c |
| Coriander | 745 ef | 28.7 cde | 3833 cd | 326 bcd | 6.3 de | 133.2 de | 528.7 bcd | 7 a | 70.53 de |
| Fennel | 858 def | 14.4 ef | 3833 cd | 285 de | 4.8 ef | 469.7 b | 431.3 ef | 4.6 d | 55.9 gh |
| Parsley | 983 def | 65.5 b | 4433 abc | 297.3 de | 8.3 c | 165 c | 480.7 cdef | 5.6 c | 51.1 hi |
| Rocket | 1894 a | 31.6 cd | 2630 ef | 392 abc | 2.5 g | 126.3 de | 298.5 g | 6.5 ab | 57.fghi |
| In-soil production (Shade-net) | |||||||||
| Basil | 1193 bc | 19.5 cdef | 2287 fg | 405 ab | 8.3 c | 97.9 f | 495.3 bcde | 3 g | 156.1 a |
| Coriander | 735 f | 128.4 a | 3407 d | 318 cde | 8.9 bc | 144 cde | 583.3 b | 3.4 fg | 58.7 fgh |
| Fennel | 991 cd | 18.3 def | 3680 d | 311.3 cde | 5.2 de | 567.3 a | 458 def | 3 g | 62.4 efg |
| Parsley | 1023 cd | 119.4 b | 4610 ab | 294.7 de | 10 ab | 167.2 c | 509.3 bcde | 4.5 d | 36.3 j |
| Rocket | 1109 bc | 61.5 cd | 1853 g | 307.3 de | 3 g | 130.1 de | 230.1 g | 2.1 h | 49.1 hi |
| Microclimate | Temperature (°C) | Relative Humidity (%) | Light Quality | |
|---|---|---|---|---|
| Maximum | Minimum | PAR (400–700 nm) (μmol m−2 s−1) | ||
| Non-temperature-controlled tunnel | 36.5 | 14 | 66.9 | 418 |
| 40% White shade-net structure | 33.4 | 13 | 63.7 | 683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mampholo, B.M.; Truter, M.; Maboko, M.M. Yield, Phytonutritional and Essential Mineral Element Profiles of Selected Aromatic Herbs: A Comparative Study of Hydroponics, Soilless and In-Soil Production Systems. Plants 2025, 14, 2179. https://doi.org/10.3390/plants14142179
Mampholo BM, Truter M, Maboko MM. Yield, Phytonutritional and Essential Mineral Element Profiles of Selected Aromatic Herbs: A Comparative Study of Hydroponics, Soilless and In-Soil Production Systems. Plants. 2025; 14(14):2179. https://doi.org/10.3390/plants14142179
Chicago/Turabian StyleMampholo, Beverly M., Mariette Truter, and Martin M. Maboko. 2025. "Yield, Phytonutritional and Essential Mineral Element Profiles of Selected Aromatic Herbs: A Comparative Study of Hydroponics, Soilless and In-Soil Production Systems" Plants 14, no. 14: 2179. https://doi.org/10.3390/plants14142179
APA StyleMampholo, B. M., Truter, M., & Maboko, M. M. (2025). Yield, Phytonutritional and Essential Mineral Element Profiles of Selected Aromatic Herbs: A Comparative Study of Hydroponics, Soilless and In-Soil Production Systems. Plants, 14(14), 2179. https://doi.org/10.3390/plants14142179








