Suppression of LPS-Induced Inflammation by Phragmites communis Young Leaf Extract via Multi-Target Inhibition of IκB, AP-1, and STAT1/3 Pathways in RAW 264.7 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantification of Flavonoids and Phenolics
2.2. Effects of PCE on Cell Viability and LPS-Induced Nitric Oxide Production in Macrophages
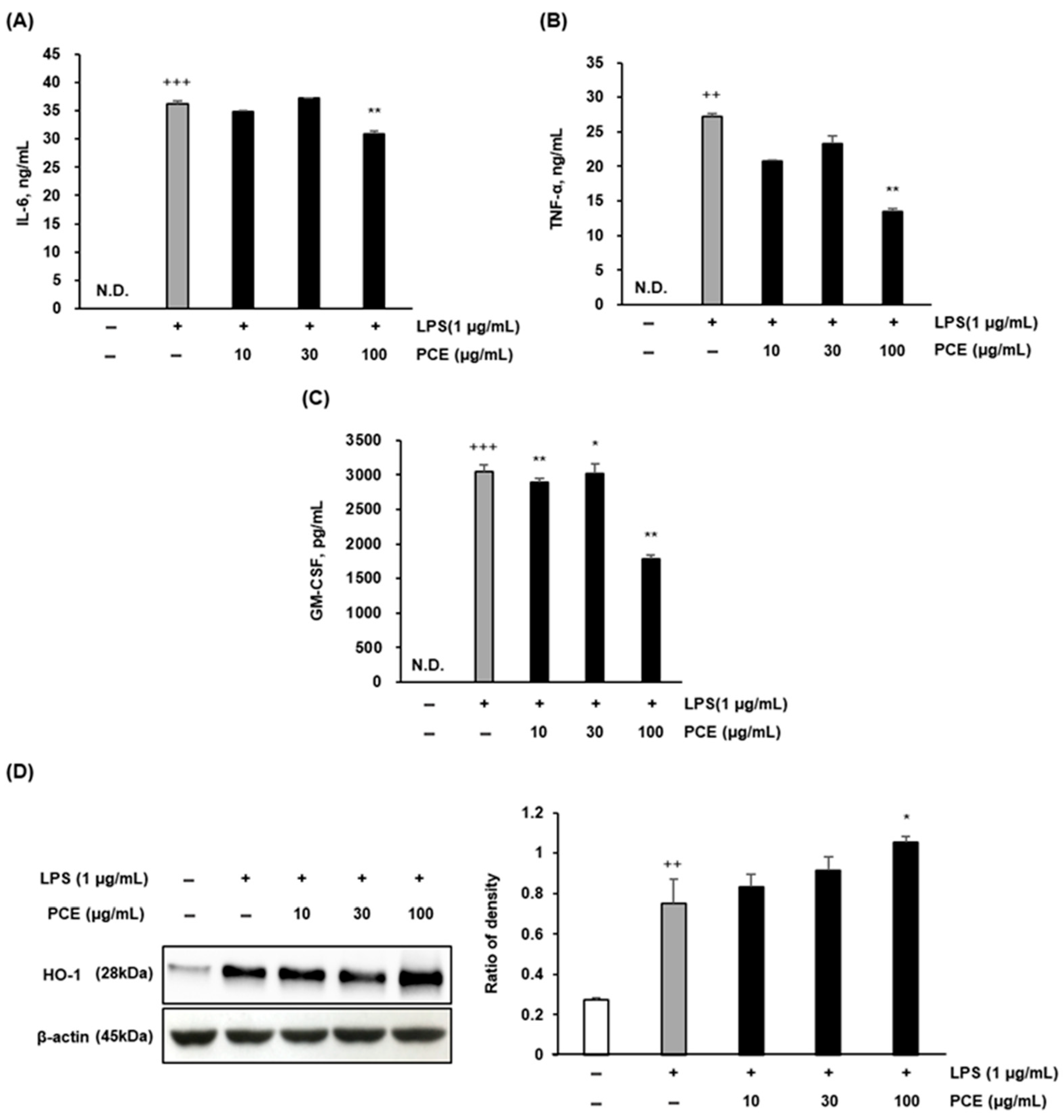
2.3. PCE Attenuates LPS-Induced Inflammatory Cytokine Production and Enhances HO-1 Expression in Macrophages
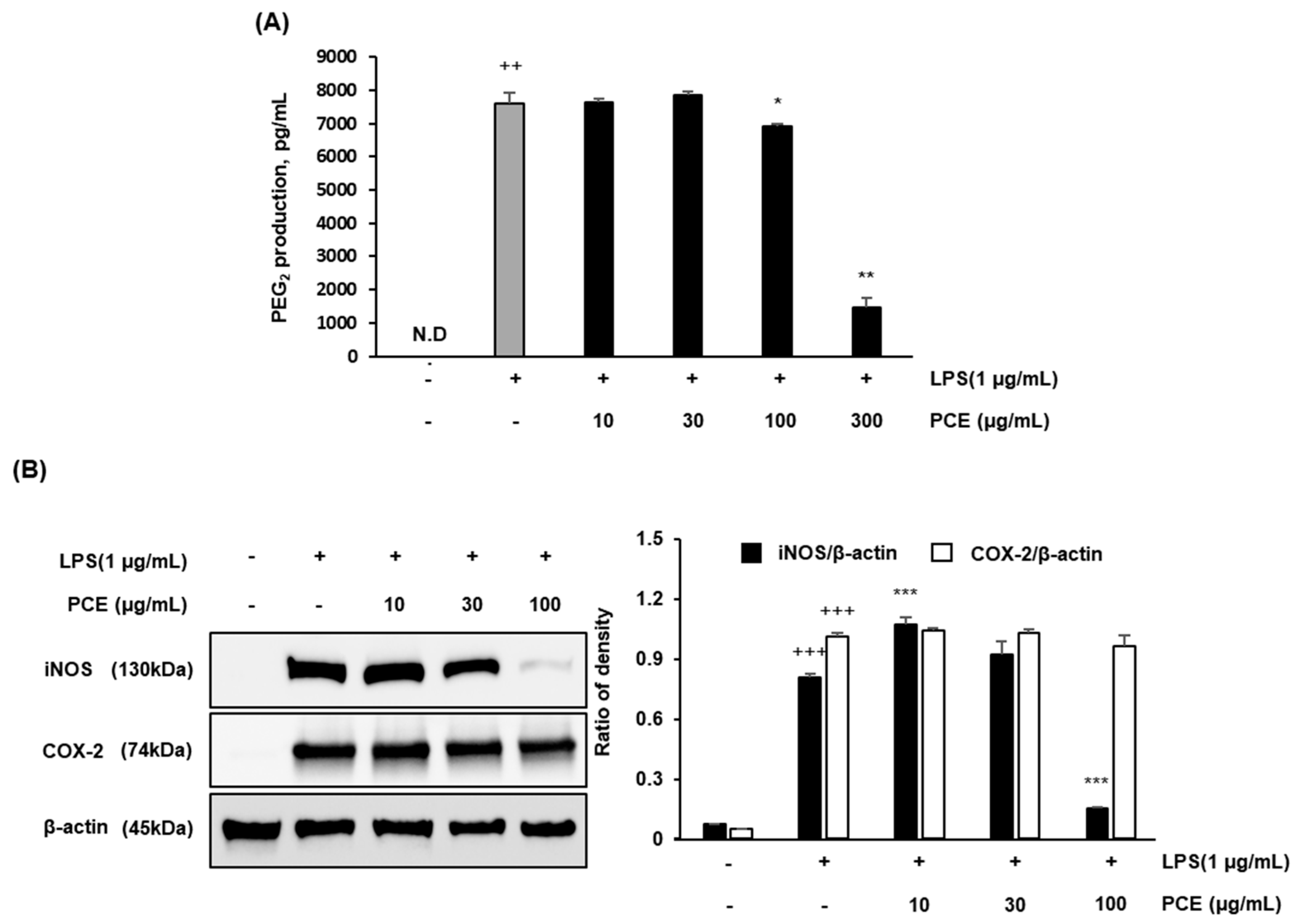
2.4. PCE Inhibits LPS-Induced PGE2 Production and Downregulates iNOS and COX-2 Expression in Macrophages
2.5. PCE Suppresses Inflammation via IκB Pathway Inhibition in Macrophages
2.6. PCE Inhibits Inflammation via STAT Pathway Suppression in Macrophages
2.7. PCE Inhibits Inflammation via AP-1 and MAPKs Pathway Suppression in Macrophages
3. Materials and Methods
3.1. Pretreatment and Extraction of Samples
3.2. Quantification of Condensed Tannins, Flavonoids, and Phenolics
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. Measurement of Nitric Oxide (NO) Production
3.6. Measurement of Pro-Inflammatory Cytokines and Prostaglandin E2
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef][Green Version]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Lee, K.H.; Chow, Y.L.; Sharmili, V.; Abas, F.; Alitheen, N.B.M.; Shaari, K.; Israf, D.A.; Lajis, N.H.; Syahida, A. BDMC33, A Curcumin Derivative Suppresses Inflammatory Responses in Macrophage-Like Cellular System: Role of Inhibition in NF-κB and MAPK Signaling Pathways. Int. J. Mol. Sci. 2012, 13, 2985–3008. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, D.; Ye, Z.; Huang, Y.; Zhang, N.; Lui, E.M.; Xue, C.; Xiao, M. Fucoidan isolated from Saccharina japonica inhibits LPS-induced inflammation in macrophages via blocking NF-κB, MAPK and JAK-STAT pathways. Mar. Drugs 2020, 18, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, J.Y. NF-κB in biology and targeted therapy. Signal Transduct. Target Ther. 2023, 8, 707. [Google Scholar]
- Zhou, Y.; Chen, Y.; He, Y.; Cui, X. Phytochemical constituents and pharmacological activities of Phragmites communis: A review. J. Ethnopharmacol. 2016, 189, 79–92. [Google Scholar]
- Kim, H.J.; Kim, S.C.; Kang, K.S. Anti-inflammatory and antioxidant properties of young leaves of Phragmites communis Trin. in lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2021, 266, 113430. [Google Scholar]
- Sim, M.-O.; Ham, J.R.; Lee, M.-K. Young leaves of reed (Phragmites communis) suppress melanogenesis and oxidative stress in B16F10 melanoma cells. Biomed. Pharmacother. 2017, 93, 165–171. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.J.; Kim, Y.A.; Cho, E.-S.; Huh, E.; Bang, O.-S.; Kim, N.S. Aqueous extract of Phragmitis rhizoma ameliorates myelotoxicity of docetaxel in vitro and in vivo. BMC Complement. Altern. Med. 2017, 17, 393. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Li, Y. Evaluation of antioxidant activities and bioactive compounds in young and mature leaves of Phragmites australis. Ind. Crops Prod. 2020, 147, 112249. [Google Scholar]
- Yang, J.; Han, M.; Zhang, L.; Wu, Q. Young leaves of reed (Phragmites communis) suppress inflammatory factors and activate antioxidative HO-1 in LPS-stimulated RAW 264.7 macrophages. Immunopharmacol. Immunotoxicol. 2018, 40, 425–434. [Google Scholar]
- Kim, T.W.; Shin, J.S.; Chung, K.S.; Lee, Y.G.; Baek, N.I.; Lee, K.T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-κB, and JAK/STAT Signaling. Cells 2019, 8, 1163. [Google Scholar] [CrossRef] [PubMed]
- Park, W. A combination extract of Gardeniae Fructus and Perillae Folium exerts anti-inflammatory effects on LPS-activated RAW 264.7 mouse macrophages via an ER stress-induced CHOP pathway. Processes 2021, 9, 1632. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, H.J. Anti-inflammatory activity of extracts and pure compounds derived from medicinal plants targeting macrophages. Int. J. Mol. Sci. 2020, 21, 9605. [Google Scholar]
- Ahmed, H.A.; El-Kholy, D.S.; Khalifa, H.O.; El-Metwally, M.A.; Abou-ElWafa, G.S.; Hassan, M.M. Phytochemical profiling of Phragmites australis leaf extract and its nano-structural antioxidant, antimicrobial, and anticancer activities. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4509–4523. [Google Scholar]
- Lee, H.; Kim, J. Flavonoid profiles of Miscanthus sinensis and their antioxidant potential. Plant Foods Hum. Nutr. 2019, 74, 180–188. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jun, C.-D.; Suk, K.; Choi, B.-J.; Lim, H.; Park, S.; Ho Lee, S.; Shin, H.-Y.; Kim, D.-K.; Shin, T.-Y. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Lee, T.S.; Chau, L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Liu, F.; Zhang, Y.H.; Liu, J.; Zhang, Y.; Xu, L.P.; Bai, B.; Du, L.J. Phenethylferulate as a natural inhibitor of inflammation in LPS-stimulated RAW 264.7 macrophages: Focus on NF-κB, Akt and MAPK signaling pathways. BMC Complement. Med. Ther. 2023, 23, 234. [Google Scholar]
- Lee, H.-S.; Kwon, Y.-J.; Seo, E.-B.; Kim, S.-K.; Lee, H.; Lee, J.-T.; Chang, P.-S.; Choi, Y.J.; Lee, S.-H.; Ye, S.-K. Anti-inflammatory effects of Allium cepa L. peel extracts via inhibition of JAK-STAT pathway in LPS-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 317, 116851. [Google Scholar] [CrossRef]
- Yoo, H.-S.; Yoon, Y.-S.; Shin, J.-W.; Choi, S.-I.; Son, S.H.; Jang, Y.H.; Yang, Y.-S.; Kim, S.-Y.; Kim, Y.-R.; Chung, K.-S.; et al. In vitro and in vivo anti-inflammatory and antinociceptive activities of a synthetic hydrangenol derivative: 5-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one. Int. Immunopharmacol. 2025, 148, 114175. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Plumlee, C. Inteferons pen the JAK-STAT pathway. Semin. Cell Dev. Biol. 2008, 19, 311–318. [Google Scholar]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–247. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-κB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Le, B.; Androutsopoulos, V.P.; Tsukamoto, C.; Shin, T.-S.; Tsatsakis, A.M.; Chung, G. Anti-inflammatory effects of soyasapogenol I-αa via downregulation of the MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2018, 113, 211–217. [Google Scholar] [CrossRef]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and MyD88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediator production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef]




| Type of Sample | Polyphenols (GAE) a | Flavonoids (QE) b |
|---|---|---|
| PCE | 4.14 ± 0.60 | 10.18 ± 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-Y.; Park, K.-W. Suppression of LPS-Induced Inflammation by Phragmites communis Young Leaf Extract via Multi-Target Inhibition of IκB, AP-1, and STAT1/3 Pathways in RAW 264.7 Cells. Plants 2025, 14, 2178. https://doi.org/10.3390/plants14142178
Kang K-Y, Park K-W. Suppression of LPS-Induced Inflammation by Phragmites communis Young Leaf Extract via Multi-Target Inhibition of IκB, AP-1, and STAT1/3 Pathways in RAW 264.7 Cells. Plants. 2025; 14(14):2178. https://doi.org/10.3390/plants14142178
Chicago/Turabian StyleKang, Kyung-Yun, and Kyung-Wuk Park. 2025. "Suppression of LPS-Induced Inflammation by Phragmites communis Young Leaf Extract via Multi-Target Inhibition of IκB, AP-1, and STAT1/3 Pathways in RAW 264.7 Cells" Plants 14, no. 14: 2178. https://doi.org/10.3390/plants14142178
APA StyleKang, K.-Y., & Park, K.-W. (2025). Suppression of LPS-Induced Inflammation by Phragmites communis Young Leaf Extract via Multi-Target Inhibition of IκB, AP-1, and STAT1/3 Pathways in RAW 264.7 Cells. Plants, 14(14), 2178. https://doi.org/10.3390/plants14142178






