Abstract
Bract coloration in ornamental plants is a complex trait governed by diverse pigments (chlorophylls, anthocyanins, betalains, and carotenoids), their biosynthetic pathways, and regulatory networks. While previous research has primarily focused on floral pigmentation, studies on bract coloration—particularly in species where bracts serve as the primary ornamental feature—have received less attention until recent advances. This review synthesizes current understanding of bract color diversity, pigment biochemistry, and molecular regulation in key species including Bougainvillea, Euphorbia pulcherrima, Anthurium andraeanum, Curcuma alismatifolia, and Zantedeschia hybrida. Anthocyanins predominantly contribute to red-to-purple hues, while betalains generate red, purple, or yellow coloration through differential accumulation of betacyanins and betaxanthins. Developmental color transitions are mediated by chlorophyll degradation and carotenoid dynamics. The spatiotemporal regulation of pigment accumulation involves coordinated interactions between key structural genes (CHS, DFR, ANS for anthocyanins; DODA, CYP76AD1 for betalains), transcription factors (MYB, bHLH, WRKY), and plant growth regulators (BAP, GA, MeJA). Despite these advances, significant knowledge gaps remain in genetic inheritance patterns, epigenetic regulation, cross-pigment pathway crosstalk, and environmental modulation. Future research directions should integrate multi-omics approaches, wild germplasm resources, and gene-editing technologies to develop novel breeding strategies for bract color improvement.
1. Introduction
Bracts, as accessory organs of flowers or inflorescences, exhibit remarkable morphological diversity in color, size, and shape, while fulfilling diverse ecological functions [1]. Bracts are modified leaves that are considered to possess certain photosynthetic capabilities [1]. For instance, the albino bracts of Davidia involucrata exhibit limited photosynthetic activity, with their net photosynthetic rate, stomatal conductance, and transpiration rate being 96.38%, 84.49%, and 82.95% lower, respectively, compared to leaves [2]. However, exceptions exist under specific conditions; in cotton, bracts demonstrate superior photosynthetic performance, higher chlorophyll and rubisco content, and greater net photosynthesis than leaves under water stress [3]. In contrast, brightly colored ornamental bracts lack chloroplasts and, thus, are non-photosynthetic [4,5].
Beyond protecting flowers, fruits, or seeds from herbivory, bracts may deter predators through warning signals or camouflage [6]. This morphological and functional diversity results from natural selection, enabling adaptation to various ecological niches and fostering complex interactions within ecosystems [1,7]. For instance, in Castilleja coccinea, bract color polymorphism is linked to reproductive trade-offs: scarlet individuals exhibit higher seed production, whereas yellow individuals benefit from reproductive assurance, collectively maintaining population polymorphism [8]. Similarly, in species such as Davidia involucrata, bract color variation correlates with reproductive functions (e.g., pollen protection), enhancing pollen viability by up to 662% [9]. The large bracts of hummingbird-pollinated plants have been shown to attract hummingbirds [10]. Additionally, in Thunia alba, large bracts serve a protective role by deterring nectar robbers and enhancing the plant’s reproductive fitness [11].
Brightly colored and large bracts also play a crucial role in pollinator attraction [12]. However, their ecological significance extends beyond pollination. Bracts provide essential protection for reproductive structures, such as shielding flowers from harmful ultraviolet (UV) radiation, which can impair reproductive organs and reduce seed yield [13]. This protective function is particularly critical in habitats with high environmental stress [1]. In addition to their resistance to environmental stressors, bracts can also protect seeds and nectar from predation [14].
Additionally, bracts serve as the primary ornamental feature in many horticultural species. Although flower coloration has been extensively studied [15,16], research on ornamental bract pigmentation remains limited. Recent advances have been made in understanding bract color traits in key ornamental species, including Bougainvillea, Euphorbia pulcherrima, Anthurium andraeanum, Curcuma alismatifolia, and Zantedeschia hybrida, among others. Figure 1 shows the main pigments in the ornamental bracts of these key plant species.
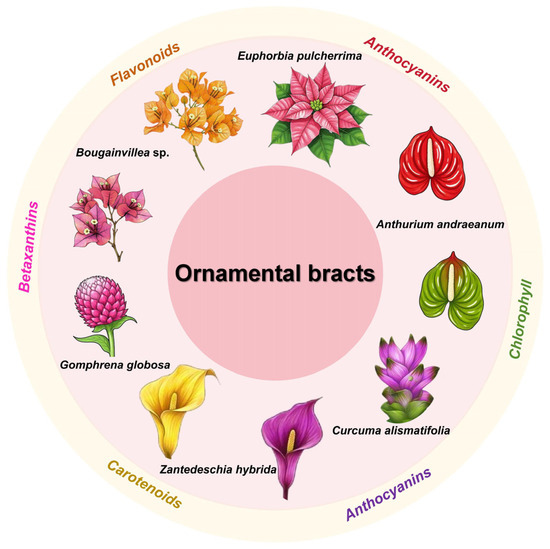
Figure 1.
Major pigment composition in ornamental bracts of key ornamental plant species.
2. Diversity of Color Phenotypes and Key Pigments in Ornamental Plants Bracts
2.1. The Color Phenotypic Diversity of Ornamental Plant Bracts
Bract coloration exhibits remarkable diversity across species and cultivars, with significant variation in both phenotypic expression and underlying pigments. In Bougainvillea, bracts display a wide color spectrum including red, purple, magenta, white, green, and orange [17,18,19,20]. Similar diversity is observed in Curcuma alismatifolia (white, pink, red, purple) [21], Euphorbia pulcherrima (red, pink, white, orange) [22,23,24], Anthurium andraeanum (red, pink, purple, white, green) [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29], and Zantedeschia hybrida (black, purple-red, red, orange, yellow, pink, white, gold) [30,31]. Other species with colorful bracts include Gomphrena globosa (orange, red, purple) [32], Globba (white, pink, red, purple) [33,34], Musella lasiocarpa (yellow and red) [35], Alpinia hainanensis (pink) [36], Telopea speciosissima (white, green, red) [37], Heliconia (yellow, orange-yellow, orange, pink, red) [38,39], Guzmania (red) [40], and Calathea crotalifera (yellow and red) [41].
2.2. The Influence of Pigment Distribution and Cellular Structure on Bract Coloration
In most cases, petal pigments are primarily localized in the upper epidermal cells, though they may also occur in the palisade and lower epidermal tissues of darker-colored petals [42,43]. Notably, green petals are exceptionally rare in flowering plants [44]. In contrast, bracts of many plant species exhibit green pigmentation, while those displaying color diversity are often cultivated as ornamental specimens. The coloration of most ornamental bracts predominantly results from pigments concentrated in epidermal and parenchyma cells. For instance, anthocyanins are distributed in both upper and lower epidermis as well as adjacent mesophyll cells (including palisade and spongy tissues) [45]. Similarly, studies on Zantedeschia cultivars demonstrate that pigments responsible for bract coloration are mainly localized in epidermal and parenchyma cells [30].
Interestingly, beyond pigment composition itself, epidermal cell morphology significantly influences color presentation. Compared to flat cells, conical epidermal cells enhance light penetration and pigment absorption, thereby intensifying floral coloration [46]. Ornamental bracts exhibit interspecific variation in epidermal cell morphology. Microscopic analysis of 27 colored Zantedeschia spathes revealed uniformly flattened epidermal cells in all cultivars [30]. Conversely, in Davidia involucrata, pink bracts display irregularly shaped upper epidermal cells with higher anthocyanin content, whereas white bracts feature flatter cells with lower pigment concentrations [47]. Noda et al. documented that when magenta Antirrhinum mutants turned pink, their conical epidermal cells flattened—a transformation regulated by MYB-family transcription factors [48]. Despite these findings, research on the cellular mechanisms underlying coloration in ornamental bracts remains limited and warrants further investigation.
2.3. Key Pigments and Their Mechanisms in Bract Coloration
The primary pigments responsible for this coloration include chlorophylls, betalains, flavonoids (particularly anthocyanins), and carotenoids, with their relative contributions varying significantly among species and cultivars. Betalains play a dominant role in Bougainvillea and Gomphrena globosa, where betaxanthins and betacyanins produce yellow and red hues, respectively, with red bracts containing significantly higher betacyanin concentrations than yellow ones [18,49,50]. Anthocyanins are the primary pigments in Curcuma alismatifolia [21,51], Globba [33,34], Anthurium andraeanum [26], Alpinia hainanensis [36], and Zantedeschia hybrida [30]. Specific anthocyanins identified include cyanidin-3-O-glucosylrutinoside and cyanidin-3-O-rutinoside-5-O-glucoside in Alpinia hainanensis red bracts [36], malvidin 3-rutinoside, delphinidin-3-O-rutinoside, and peonidin-3-O-rutinoside in Curcuma alismatifolia [21], and cyanidin derivatives in Zantedeschia hybrida purple-red bracts [30]. Furthermore, anthocyanin accumulation patterns in Anthurium andraeanum ‘Sonate’ mutants exhibit distinct phenotypic correlations: the dark-green mutant displays elevated anthocyanin levels in bracts, while the reddish-brown mutant shows maximal anthocyanin accumulation in leaves. In contrast, chlorotic and albino mutants demonstrate significantly reduced anthocyanin content [28]. In Euphorbia pulcherrima bracts, cyanidin and pelargonidin derivatives determine deep-red and orange-red coloration, respectively [22,24].
Pigment accumulation and color manifestation in bracts are significantly influenced by developmental stages across multiple species. Young bracts typically exhibit high chlorophyll content, appearing green due to active photosynthesis. The dynamic balance between chlorophyll, flavonol, and carotenoid accumulation or degradation regulates bract color changes during development. In white Bougainvillea bracts, chlorophyll content progressively decreases during development, resulting in white coloration [17]. Carotenoid levels similarly influence color transitions at the bract tips of Curcuma alismatifolia [51]. Zantedeschia bracts demonstrate a remarkable chlorophyll increase (10-fold for Chl a/b) accompanied by 20% carotenoid reduction and chloroplast proliferation during post-maturation regreening [52,53,54]. Euphorbia pulcherrima exhibits stage-specific pigment profiles: chlorophyll dominates early development, while cyanidin-3-galactoside, cyanidin-3-glucoside, cyanidin-3-rutinoside, pelargonidin-3-glucoside, and pelargonidin-3-rutinoside become predominant pigments in later stages [55]. In Calathea crotalifera, the concentrations of chlorophylls, carotenoids, and anthocyanins in the bracts undergo dynamic changes during inflorescence development, contributing to their ornamental appearance [41]. The white bracts of Davidia involucrata primarily result from significantly reduced chlorophyll and carotenoid levels compared to green leaves [2]. However, some cultivars like Zantedeschia pentlandii ‘Best Gold’ maintain orange bracts through lutein, violaxanthin, and β-carotene accumulation [52].
Current research demonstrates that betalains and anthocyanins serve as the dominant pigments responsible for color diversity in ornamental bracts of major decorative species, while chlorophylls and carotenoids primarily function as auxiliary pigments that modulate coloration through stage-specific accumulation or degradation during bract development. As systematically summarized in Table 1, these pigment combinations create distinct color categories in bracts that serve as primary ornamental features across key horticultural plants.

Table 1.
Color categories of main ornamental bracts in different plants and their primary contributing pigments.
2.4. Instrumental Methods for Pigment Detection and Quantification
In most studies investigating pigments in ornamental bracts, spectrophotometry and high-performance liquid chromatography-mass spectrometry (HPLC-MS) remain the primary analytical methods. Spectrophotometry serves as a fundamental method for extracting and quantifying various plant pigments, including chlorophylls, carotenoids, anthocyanins, and betalains, and has been widely employed in studies of ornamental bract pigmentation [17,25,26,27,28,38,41,47,50,51,53]. However, this approach typically only measures total pigment content without distinguishing specific structural variants. Recent advances in chromatography and mass spectrometry, coupled with reduced costs, have established high-performance liquid chromatography (HPLC) and its tandem mass spectrometry (MS) configurations as powerful tools for plant pigment analysis. For instance, researchers have identified diverse betalains (betaxanthins, betacyanins, and Σ-betalains) in Bougainvillea bracts using HPLC [55], Ion-Pair High-Speed Countercurrent Chromatography/Electrospray Ionization-Tandem Mass Spectrometry (IP-HSCCC/ESI-MS-MS) [56], high-performance liquid chromatography-Diode Array Detector-Tandem Mass Spectrometry (HPLC-DAD-MS/MS) [50], and High-Performance Liquid Chromatography-Diode Array Detector-Electrospray Ionization-Multistage Mass Spectrometry (HPLC-DAD-ESI-MSn) [32]. Similarly, anthocyanins and flavonoids have been characterized in Euphorbia pulcherrima bracts through HPLC and HPLC/MS [24,57,58], and in Curcuma alismatifolia, Globba, and Alpinia hainanensis bracts via Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) [21,34,36]. These chromatographic and mass spectrometric methods enable precise structural identification of pigments and determination of key chromogenic compounds. Notably, certain structurally unique pigments, such as acyl-oligosaccharide-linked betacyanins in Bougainvillea bracts, require specialized detection methods like IP-HSCCC/ESI-MS-MS [56]. However, studies on pigment separation and methodological development for ornamental bracts remain limited, highlighting the need for interdisciplinary collaboration.
The time-consuming processes involved in pigment extraction and analysis pose significant challenges for large-scale genetic investigations. To overcome this limitation, researchers have developed colorimeter-based predictive models. Studies on Gerbera flower coloration [59], Caladium leaf pigmentation [60], as well as bract color in Curcuma alismatifolia [21] and Globba [34] have revealed strong correlations between specific colorimetric parameters (a* values with anthocyanin levels; b* values with carotenoid concentrations), validating their utility as robust proxies for pigment content. Expanding these non-destructive, high-throughput approaches to additional ornamental bract species would significantly enhance the efficiency of pigment quantification in breeding and genetic studies.
3. Metabolic Pathway of Pigments in Ornamental Bracts
3.1. Chlorophyll Metabolism
Chlorophyll biosynthesis is a multi-step enzymatic process primarily occurring in plant chloroplasts. The key enzymes involved in chlorophyll synthesis include: glutamyl-tRNA reductase (HEMA1), which catalyzes the conversion of glutamate to 5-aminolevulinic acid (ALA); ALA dehydratase (ALAD), responsible for condensing ALA to form porphobilinogen (PBG); magnesium chelatase subunits (CHLH/CHLI), which insert Mg2+ to generate Mg-protoporphyrin IX; protochlorophyllide oxidoreductase (POR), facilitating the reduction in protochlorophyllide to chlorophyllide; and chlorophyll a oxygenase (CAO), a Rieske-type non-heme iron oxygenase that converts chlorophyll a to chlorophyll b [61,62]. The chlorophyll degradation pathway involves several critical enzymes: chlorophyllase (CLH), which hydrolyzes the phytol side chain of chlorophyll; pheophorbide a oxygenase (PAO), catalyzing the ring-opening of pheophorbide a to produce red chlorophyll catabolite (RCC); red chlorophyll catabolite reductase (RCCR), reducing RCC to colorless primary fluorescent chlorophyll catabolite (pFCC); and STAY-GREEN protein (SGR), regulating the degradation of chlorophyll-protein complexes [61,62].
In Curcuma alismatifolia, reduced expression of CAO in bracts leads to decreased chlorophyll content, resulting in red bracts, a finding consistent with the observed CAO expression pattern in white bracts [63]. Similarly, mutations in the OsCAO1 gene in rice cause pale green leaves, demonstrating the crucial role of CAO activity in pigment accumulation [64]. Studies on Bougainvillea bracts identified multiple chlorophyll metabolism-related genes. Protochlorophyllide oxidoreductase A gene BgPORA, which catalyzes the reduction in protochlorophyllide to chlorophyllide, is highly expressed during early bract development but declines as bracts transition from green to white. Concurrently, increased expression of the chlorophyll degradation-related gene BgSGR (STAY-GREEN protein) accelerates chlorophyll breakdown, promoting bract whitening. Additionally, BgPPH (pheophytinase), BgPAO (pheophorbide a oxygenase, chloroplastic), and BgRCCR (accelerated cell death) contribute to chlorophyll degradation, further influencing bract color changes [17].
Dysregulation of photosynthetic pigment metabolism genes can disrupt chloroplast structure and impair pigment accumulation. In Davidia involucrata bracts, significantly lower levels of chlorophylls a, b, and carotenoids compared to leaves were attributed to downregulation of chlorophyll biosynthesis-related proteins (PPR and AARS) and elevated PAO activity [2]. Similarly, studies on Guzmania bracts identified chlorophyll synthesis-related enzymes (GTS and UROS) and the key degradation enzyme PPH, with PPH playing a pivotal role in chlorophyll breakdown [40].
3.2. Anthocyanins Biosynthesis
Anthocyanins are water-soluble pigments belonging to the flavonoid family that play a crucial role in determining plant coloration, exhibiting red, purple, and blue hues depending on environmental pH [16,65]. Their biosynthesis occurs through the phenylpropanoid and flavonoid pathways. The phenylpropanoid pathway involves key enzymes including phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumarate-CoA ligase (4CL), while the flavonoid pathway requires chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavonoid 3′-hydroxylase (F3′H), flavonoid 3′5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR), and leucoanthocyanidin dioxygenase (LDOX/ANS) [15,16,66]. Subsequent modifications by glycosyltransferases (UFGT), acyltransferases (AT), and methyltransferases (OMT) through glycosylation, acylation, and methylation are essential for producing stable anthocyanin derivatives [15,67].
In Curcuma alismatifolia, significantly reduced expression of DFR (gene25158) and ANS (gene437) in white-bracted ‘Country Snow’ cultivars contrasts with their high expression in pink-bracted ‘Chiang Mai Pink’ varieties, correlating with anthocyanin accumulation patterns [63]. Similarly, low ANS and DFR expression in Bougainvillea redirects pigment synthesis towards carotenoids and betalains [18]. White bracts in Euphorbia pulcherrima result from downregulation of anthocyanin modification (UGT79B10) and transport (GSTF11) genes [23,55], while pink bracts in Alpinia hainanensis accumulate cyanidin and peonidin derivatives through upregulated AhF3′5′H and AhUGT77B2-mediated glycosylation and methylation [36]. Musaceae bract color diversity correlates with F3′5′H gene expansion, potentially regulated by transposable elements, methylation patterns, and expression levels [35]. CRISPR/Cas9-mediated F3′H knockout in E. pulcherrima reduces cyanidin and pelargonidin content, shifting bract color from red to orange-red without direct anthocyanin synthesis effects [24], whereas DFR overexpression enhances anthocyanin production in Arabidopsis thaliana [68].
Glycosylation (UGT) and transport (GST) genes critically influence anthocyanin stability, solubility, and cellular distribution. In E. pulcherrima, the hypervariable GST gene Bract1—phylogenetically similar to known anthocyanin transporters—contains a 4 bp repeat mutation causing white phenotypes. This mutation exhibits X-ray-induced instability, generating frequent color variants [69]. Anthurium spathe coloration depends on stage-specific expression of CHI and CYP genes, with F3′H expression strongly correlating with anthocyanin content and color intensity [70]. Conversely, low CHI2 and DFR1/DFR2 expression in Zantedeschia limits anthocyanin synthesis, producing pink and white spathes [31]. These findings demonstrate that tightly regulated expression of flavonoid biosynthesis genes governs anthocyanin accumulation and subsequent bract coloration.
3.3. Betalains Biosynthesis
Betalains are water-soluble pigments primarily found in Caryophyllales plants, including crops such as Beta vulgaris, Chenopodium quinoa, and Amaranthus hypochondriacus. These pigments are classified into red-violet betacyanins and yellow-orange betaxanthins [71]. The betalain biosynthesis pathway originates from tyrosine metabolism, involving several key enzymatic steps: (1) Tyrosine hydroxylase (TYRH) catalyzes the hydroxylation of tyrosine to form L-DOPA. (2) DOPA 4,5-dioxygenase (DOD) then cleaves L-DOPA to produce betalamic acid and cyclo-DOPA. (3) UDP-glucosyltransferase (UGT) mediates the glycosylation of cyclo-DOPA to form cyclo-DOPA-5-O-glucoside. (4) Finally, betalamic acid condenses with cyclo-DOPA-5-O-glucoside to yield betanin [72].
The DODA gene encodes a tyrosine decarboxylase that serves as a key regulatory enzyme in betalain biosynthesis by catalyzing the conversion of tyrosine to dopamine, representing the initial step in this pathway. Upregulation of DODA expression positively correlates with betalain accumulation in Bougainvillea bracts, particularly influencing betanidin and betacyanin production [50]. Conversely, reduced expression of BpCYP76AD1 leads to decreased betalain synthesis, resulting in lighter bract coloration in Bougainvillea ‘Thimma’. Notably, heterologous expression of BpCYP76AD1 and BpDODA1 in tobacco significantly enhances anthocyanin accumulation in leaves [73].
The final step in betalain biosynthesis involves glucosyltransferases encoded by Betanidin 5GT/6GT genes, which catalyze the transfer of glucosyl groups to specific hydroxyl positions (C5 or C6) of betanidin to form stable pigments such as betanin and isobetanin [19,67]. In white-bracted Bougainvillea ‘BXGZ’, reduced expression of Betanidin 5GT/6GT contributes to diminished betalain accumulation [18]. The color expression in Gomphrena globosa and Bougainvillea inflorescences is modulated by the ratio of betacyanins to betaxanthins (including arginine-, lysine-, and putrescine-betaxanthins), which is influenced by glycosylation patterns and acylation degrees [32]. Comparative genomic analyses reveal that betalain biosynthesis genes in Bougainvillea × buttiana ‘Mrs Butt’ primarily expanded through whole-genome triplication (WGT) events, while other cultivars underwent alternative expansion mechanisms such as tandem duplication. These duplication events increase gene copy numbers and expression levels, indirectly affecting bract coloration. Correlation analyses suggest that both betalains and flavonoids play major roles in color development in Bougainvillea × buttiana ‘Mrs Butt’ bracts [18]. Among 13 BbCYPs identified BbCYPs, BbCYP40, and BbCYP220 show the strongest correlations with betalain and flavonoid accumulation, respectively [74]. Functional studies demonstrate that overexpression of BpCYP76AD15 can induce betalain production in Bougainvillea callus cultures [75], further confirming the crucial role of cytochrome P450 enzymes in betalain biosynthesis.
3.4. Carotenoids Biosynthesis
Carotenoid biosynthesis initiates in plastids through the methylerythritol phosphate (MEP) pathway, which produces the precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These substrates are subsequently converted into various carotenoids and xanthophylls via sequential enzymatic reactions, including condensation, isomerization/dehydrogenation, hydroxylation, and oxidation [15,76]. Although carotenoids play a key role in color modulation in the bracts of some ornamental plants, research on how carotenoid biosynthesis pathways regulate bract pigmentation remains limited. Current studies have identified several correlations between gene expression and carotenoid accumulation. In Curcuma alismatifolia, high expression levels of the carotenoid biosynthesis gene ZEP correlate with elevated carotenoid content in variegated bract regions [51]. Transcriptomic analysis of Bougainvillea bracts identified 35 carotenoid biosynthesis-related unigenes [75]. Moreover, another research found that BbCYP85 showed a strong association with carotenoid content in Bougainvillea bracts [74]. In green spathes of Anthurium, the high expression of five carotenoid biosynthesis genes—PDS (Unigene54090), two LUT5 (Unigene14810 and Unigene58790), CrtR-b (Unigene54067), and ZEP (Unigene8259)—coincides with substantial carotenoid accumulation [27]. However, these findings remain correlative, lacking functional validation through genetic manipulation or mechanistic studies.
4. Transcriptional Regulation
The formation of bract coloration is regulated by transcription factors (TFs) that bind to promoters of pigment biosynthesis genes. The MYB TF family, the largest among eukaryotes, serves as the most prevalent regulator of anthocyanin biosynthesis pathways [77,78,79,80]. In Anthurium, spathe color variation is determined by stage-specific expression of CHI and CYP genes, where AaMYB2 activate CHS, F3H, and ANS to promote anthocyanin accumulation [25,26,27,70]. The bHLH family, the second-largest plant TF group, forms functional complexes with MYB through conserved N-terminal domains to regulate downstream targets [81]. In Zantedeschia, bHLH1 potentially acts as a positive regulator of ANS expression to enhance anthocyanin synthesis [31]. Multi-omics analyses of Curcuma alismatifolia bracts identified MYB and bHLH TFs co-expressed with anthocyanin biosynthesis genes, suggesting their regulatory roles in pigment accumulation [21,63]. MYB and WRKY TFs also regulate betalain biosynthesis. In Beta vulgaris, BvMYB activates BvDODA1 and BvCYP76AD1 to promote betalain production [19]. The group IIb WRKY protein HmoWRKY40 transcriptionally represses HmoCYP76AD1 to modulate betalain synthesis in pitaya [82], while HuMYB9 inhibits betalain biosynthesis by binding super-enhancers of HuCYP76AD1-1, HuADH1, and HuDODA1 [83]. MADS-box (BgAP1, BgFULL) and SBP (BgSPL16, BgCMB1, BgDEFA) family genes are critical during Bougainvillea bract development from green to white [17].
Interestingly, several transcription factors involved in anthocyanin biosynthesis regulation in bracts have demonstrated the capacity to modulate petal pigmentation when ectopically expressed. For example, The anthocyanin biosynthesis pathway in white Cymbidium orchid petals was successfully reconstituted through biolistic co-transformation of AaMYB1 (A. andraeanum) with its cognate bHLH partners [84]. Similarly, heterologous expression of A. andraeanum AaMYB2 in tobacco leaves induced anthocyanin accumulation accompanied by upregulation of both early and late biosynthetic genes in the anthocyanin pathway [26]. Furthermore, expression of the bract-specific transcription factor AaFUL1 from Anthurium in tobacco resulted in petal fading phenotypes [85]. These findings collectively suggest that the regulatory functions of MYB and FUL family transcription factors in anthocyanin biosynthesis exhibit considerable conservation across different plant tissues. However, the extent to which this functional conservation applies broadly to other transcription factor families remains to be systematically investigated through comprehensive molecular genetic studies.
5. Plant Growth Regulators
Phytohormones interact with pigment biosynthesis genes to modulate coloration [86]. MeJA, GA, and BAP significantly influence pigment accumulation in bracts. In Zantedeschia ‘Best Gold’, cytokinin (BAP) and gibberellin (GA3) delay bract regreening by suppressing chlorophyll metabolism, extending ornamental value [52,53,54]. Pitaya bracts treated with 1.0% chitosan/0.2% κ-carrageenan coatings plus 50 mg L−1 GA3 or 0.1 mM MeJA retain higher chlorophyll content and color stability [87]. In Bougainvillea, upregulated JAZ genes may mediate JA signaling to promote white bract formation, while their downregulation induces purple bracts [20]. These findings underscore the coordinated interplay between transcriptional regulatory networks and hormonal signaling pathways in determining bract pigmentation patterns.
6. Conclusions
Bract coloration in ornamental plants represents a complex phenotypic trait governed by intricate interactions among four major pigment classes (chlorophylls, anthocyanins, betalains, and carotenoids), their biosynthetic pathways, transcriptional regulation networks, and hormonal signaling cascades. The remarkable color spectrum observed in ornamental bracts stems from species-specific pigment profiles, where anthocyanins predominantly contribute to red-to-purple hues, while betalains produce red, purple, yellow, or orange coloration depending on the relative ratios of betacyanins to betaxanthins. Developmental color transitions are further modulated by chlorophyll degradation and carotenoid dynamics, as exemplified by the whitening process in Bougainvillea and regreening phenomenon in Zantedeschia. The metabolism of these pigments is precisely regulated through: (1) biosynthesis genes (e.g., CHS, DFR, and ANS for anthocyanins; DODA and CYP76AD1 for betalains; CAO for chlorophylls), (2) degradation enzyme genes (e.g., PAO and SGR for chlorophyll catabolism), and (3) transcriptional control mediated by MYB, bHLH, and WRKY transcription factors in conjunction with plant growth regulators (BAP, GA, MeJA). These regulatory mechanisms collectively determine the spatiotemporal patterns of pigment accumulation.
Despite significant progress, including the functional validation of key genes influencing bract coloration (Table 2), critical knowledge gaps persist regarding (1) the complete genetic architecture underlying bract coloration, (2) epigenetic regulation mechanisms, and (3) cross-talk between different pigment pathways. Furthermore, current research remains largely limited to a few model species (e.g., Bougainvillea, Zantedeschia, Euphorbia pulcherrima, Anthurium andraeanum, and Curcuma alismatifolia) and their selected cultivars. This narrow focus presents substantial challenges in elucidating the universal principles governing pigment accumulation and genetic regulation across diverse ornamental species.

Table 2.
Recently cloned and functionally validated genes in ornamental bracts.
7. Prospects and Future Research Directions
Despite significant progress in understanding the molecular mechanisms underlying bract pigmentation, several key scientific questions remain unresolved. First, the genetic basis of pigment biosynthesis in bracts remains incompletely characterized, particularly with respect to systematic genetic mapping studies. Although four quantitative trait loci (QTLs) associated with bract color have been identified in globe artichoke [88], genetic linkage maps for most ornamental species have yet to be established, hindering the elucidation of inheritance patterns and the application of marker-assisted breeding.
The environmental regulation of pigment biosynthesis during bract development is also poorly understood. Factors such as temperature, light intensity, spectral quality, pH, sugar signaling, mineral elements, oxidative stress, and CO2 concentration can modulate the biosynthesis of these plant pigments [86,89]. For instance, in Telopea, chlorophyll, carotenoid, and anthocyanin levels in bracts are significantly influenced by light conditions, with shading treatments effectively maintaining pigment content [37]. Under blue light-deficient conditions, the bracts of Guzmania turn yellow [90]. Shading treatment significantly reduces chlorophyll, anthocyanin, and carotenoid contents in Calathea crotalifera bracts [41]. However, similar studies in other ornamental bracts remain scarce. Additionally, copigments such as flavonoids can enhance color intensity (hyperchromic effect) and induce spectral shifts (bathochromic shift) through interactions with anthocyanins [91]. A notable example is the formation of blue complexes in hydrangeas, where delphinidin-3-glucoside binds with 5-O-acylquinic acids in the presence of aluminum ions [92]. While flavonoids have been detected in bracts of Bougainvillea [50] and colored spathes of Zantedeschia [30], their specific roles in bract pigmentation require further investigation.
Multi-omics approaches remain underutilized in bract pigment research. Recent advancements, including chromosome-level genome assemblies for Anthurium [93], Curcuma [58,94], Bougainvillea [18], and Zantedeschia elliottiana [95], provide a robust foundation for systematically dissecting the molecular mechanisms of bract pigmentation. Future studies should leverage these genomic resources to employ high-throughput genetic analyses (e.g., GWAS, BSA-seq) for elucidating the genetic basis of bract color variation. Integrating transcriptomic, metabolomic, proteomic, and epigenomic data will further enable the construction of comprehensive gene regulatory networks, revealing coordinated control across pigment biosynthesis pathways.
Furthermore, current research predominantly focuses on cultivated varieties, with limited exploration of unique color variations in wild genetic resources. Expanding the collection and evaluation of wild germplasm, coupled with comparative genomic analyses, may uncover novel regulatory genes in pigment biosynthesis, thereby enriching the genetic toolkit for ornamental plant breeding. Functional validation of candidate genes using CRISPR/Cas9 and other gene-editing technologies will accelerate targeted breeding efforts for bract color improvement.
Based on current research advances, we propose the following priority areas for future investigation of ornamental bract coloration:
- (1)
- Comparative pigment accumulation mechanisms: Systematic comparison of pigmentation processes between ornamental bracts, vegetative bracts, leaves, and petals to identify tissue-specific regulatory patterns.
- (2)
- Environmental regulation of coloration: Molecular-level characterization of bract color variation in response to abiotic factors (light intensity, temperature, water availability) and biotic stresses.
- (3)
- Copigmentation effects: Comprehensive analysis of copigment-pigment interactions and their impacts on color stability and diversity in ornamental bracts.
- (4)
- Multi-omics integration: Combined application of genomics, transcriptomics, metabolomics, and proteomics with forward genetics approaches to elucidate key coloration factors and their regulatory networks.
- (5)
- Advanced pigment separation technologies: Development of novel techniques for precise isolation and identification of key chromogenic compounds in bracts.
- (6)
- High-throughput phenotyping systems: Develop efficient systems for detecting or predicting pigment content in ornamental bracts to enhance the phenotypic screening efficiency of bract pigment content for large-scale breeding programs.
- (7)
- Molecular breeding tools: Development of functional markers through high-throughput sequencing and association mapping in diverse genetic populations to enable marker-assisted selection.
- (8)
- Genetic transformation systems: Establishment of efficient, stable transformation protocols for targeted modification of coloration pathways in ornamental bract species.
Author Contributions
Conceptualization, X.L. and Y.Z.; software, Y.L.; formal analysis, X.L., Y.L. and Y.Z.; investigation, Z.C. and Y.L.; writing—original draft preparation, X.L. and Y.Z.; writing—review and editing, Y.L., Z.C. and Y.Z.; visualization, Y.L.; supervision, Z.C. and Y.Z.; project administration, Z.C. and Y.Z.; funding acquisition, Y.L., Z.C. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangdong Basic and Applied Basic Research Foundation, grant number 2023A1515140021; National Natural Science Foundation of China, grant number 32402620; Shenzhen Science and Technology Program, grant number RCBS20221008093306011; Project of Shenzhen Polytechnic, grant number 6022310012K; and Open Research Project of Guangdong Provincial Key Laboratory of Ornamental Plant Germplasm Innovation and Utilization, grant number HY202201.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Song, B.; Chen, J.; Lev Yadun, S.; Niu, Y.; Gao, Y.; Ma, R.; Armbruster, W.S.; Sun, H. Multifunctionality of angiosperm floral bracts: A review. Biol. Rev. Camb. Philos. Soc. 2024, 99, 1100–1120. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Li, Y.; Xu, L.; Xu, W.; Vetukuri, R.R.; Xu, X.; Sveriges, L. Proteome and metabolome analyses of albino bracts in Davidia involucrata. Plants 2025, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhan, D.X.; Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Photorespiration and photoinhibition in the bracts of cotton under water stress. Photosynthetica 2016, 54, 12–18. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H. The bracts of Saussurea velutina (asteraceae) protect inflorescences from fluctuating weather at high elevations of the hengduan mountains, southwestern china. Arct. Antarct. Alp. Res. 2009, 41, 515–521. [Google Scholar] [CrossRef]
- Omori, Y.; Takayama, H.; Ohba, H. Selective light transmittance of translucent bracts in the himalayan giant glasshouse plant Rheum nobile Hook. F. & thomson (polygonaceae). Bot. J. Linn. Soc. 2000, 132, 19–27. [Google Scholar]
- Armbruster, W.S.; Lee, J.; Baldwin, B.G. Macroevolutionary patterns of defense and pollination in Dalechampia vines: Adaptation, exaptation, and evolutionary novelty. Proc. Natl. Acad. Sci. USA 2009, 106, 18085–18090. [Google Scholar] [CrossRef]
- Kim, E.S.; Zaya, D.N.; Fant, J.B.; Ashley, M.V. Reproductive trade-offs maintain bract color polymorphism in scarlet indian paintbrush (Castilleja coccinea). PLoS ONE 2019, 14, e209176. [Google Scholar] [CrossRef]
- Sun, J.; Gong, Y.; Renner, S.S.; Huang, S.; Henry, M.W. Multifunctional bracts in the dove tree Davidia involucrata (nyssaceae: Cornales): Rain protection and pollinator attraction. Am. Nat. 2008, 171, 119–124. [Google Scholar] [CrossRef]
- Whitney, K.D.; Smith, A.K.; White, T.E.; Williams, C.F. Birds perceive more intraspecific color variation in bird-pollinated than bee-pollinated flowers. Front. Plant Sci. 2020, 11, 590347. [Google Scholar] [CrossRef]
- Bergamo, P.J.; Wolowski, M.; Telles, F.J.; De Brito, V.L.G.; Varassin, I.G.; Sazima, M. Bracts and long-tube flowers of hummingbird-pollinated plants are conspicuous to hummingbirds but not to bees. Biol. J. Linn. Soc. 2019, 126, 533–544. [Google Scholar] [CrossRef]
- Wu, S.; Gao, J. The conspicuously large bracts influence reproductive success in Thunia alba (Orchidaceae). J. Plant Ecol. 2024, 17, rtad036. [Google Scholar] [CrossRef]
- Titulaer, M.; Melgoza-Castillo, A.; Macías-Duarte, A.; Panjabi, A.O. Seed size, bill morphology, and handling time influence preferences for native vs. Nonnative grass seeds in three declining sparrows. Wilson J. Ornithol. 2018, 130, 445–456. [Google Scholar] [CrossRef]
- Song, B.; Gao, Y.; Stöcklin, J.; Song, M.; Sun, L.; Sun, H. Ultraviolet screening increases with elevation in translucent bracts of Rheum nobile (polygonaceae), an alpine ‘Glasshouse’ plant from the high himalayas. Bot. J. Linn. Soc. 2020, 193, 276–286. [Google Scholar] [CrossRef]
- Armbruster, W.S. Exaptations link evolution of plant-herbivore and plant-pollinator interactions: A phylogenetic inquiry. Ecology 1997, 78, 1661. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Ng, J.; Freitas, L.B.; Smith, S.D. Stepwise evolution of floral pigmentation predicted by biochemical pathway structure. Evolution 2018, 72, 2792–2802. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Zeng, Q.; Ma, Y.; Liu, J.; Huang, Y.; Yu, X.; Luo, J.; Li, Y.; Li, M.; et al. Transcriptomic profiling and gene network analysis revealed regulatory mechanisms of bract development in Bougainvillea glabra. BMC Plant Biol. 2024, 24, 543. [Google Scholar] [CrossRef]
- Lan, L.; Zhao, H.; Xu, S.; Kan, S.; Zhang, X.; Liu, W.; Liao, X.; Tembrock, L.R.; Ren, Y.; Reeve, W.; et al. A high-quality Bougainvillea genome provides new insights into evolutionary history and pigment biosynthetic pathways in the caryophyllales. Hortic. Res. 2023, 10, uhad124. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Q.; Lin, J.; Ma, X.; Dong, F.; Yan, H.; Zhong, W.; Lu, Y.; Yao, Y.; Shen, X.; et al. Transcriptome analyses shed light on floral organ morphogenesis and bract color formation in Bougainvillea. BMC Plant Biol. 2022, 22, 97. [Google Scholar] [CrossRef]
- Huang, H.; Ji, H.; Ju, S.; Lin, W.; Li, J.; Lv, X.; Lin, L.; Guo, L.; Qiu, D.; Yan, J.; et al. Pantranscriptome combined with phenotypic quantification reveals germplasm kinship and regulation network of bract color variation in Bougainvillea. Front. Plant Sci. 2022, 13, 1018846. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Tan, J.; Huang, L.; Zhu, G.; Ye, Y. Role of anthocyanin metabolic diversity in bract coloration of Curcuma alismatifolia varieties. Plant Physiol. Bioch. 2024, 216, 109156. [Google Scholar] [CrossRef] [PubMed]
- Vilperte, V.; Boehm, R.; Debener, T.; Flachowsky, H. Development of a multiplex amplicon-sequencing assay to detect low-frequency mutations in poinsettia (Euphorbia pulcherrima) breeding programmes. Plant Breed. 2021, 140, 497–507. [Google Scholar] [CrossRef]
- Vilperte, V.; Lucaciu, C.R.; Halbwirth, H.; Boehm, R.; Rattei, T.; Debener, T. Hybrid de novo transcriptome assembly of poinsettia (Euphorbia pulcherrima willd. Ex Klotsch) bracts. BMC Genom. 2019, 20, 900. [Google Scholar]
- Nitarska, D.; Boehm, R.; Debener, T.; Lucaciu, R.C.; Halbwirth, H. First genome edited poinsettias: Targeted mutagenesis of flavonoid 3′-hydroxylase using CRISPR/CAS9 results in a colour shift. Plant Cell Tissue Organ Cult. 2021, 147, 49–60. [Google Scholar] [CrossRef]
- Gopaulchan, D.; Lennon, A.M.; Umaharan, P. Expression analysis of the anthocyanin genes in pink spathes of Anthurium with different color intensities. J. Am. Soc. Hortic. Sci. 2015, 140, 480–489. [Google Scholar] [CrossRef]
- Li, C.; Qiu, J.; Yang, G.; Huang, S.; Yin, J. Isolation and characterization of a R2R3-MYB transcription factor gene related to anthocyanin biosynthesis in the spathes of Anthurium andraeanum (Hort.). Plant Cell Rep. 2016, 35, 2151–2165. [Google Scholar] [CrossRef]
- Li, J.; Tan, Q.; Yi, M.; Yu, Z.; Xia, Q.; Zheng, L.; Chen, J.; Zhou, X.; Zhang, X.Q.; Guo, H.R. Identification of key genes responsible for green and white colored spathes in Anthurium andraeanum (Hort.). Front. Plant Sci. 2023, 14, 1208226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Xu, B.; Li, Y.; Ma, Y.; Wang, G. Phenotype and transcriptome analysis reveals chloroplast development and pigment biosynthesis together influenced the leaf color formation in mutants of Anthurium andraeanum ‘Sonate’. Front. Plant Sci. 2015, 6, 139. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Fu, Y.; Gao, Y.; Lu, H.; Xu, L. Transcriptome profiling in the spathe of Anthurium andraeanum ‘Albama’ and its anthocyanin-loss mutant ‘xueyu’. Sci. Data 2018, 5, 180247. [Google Scholar] [CrossRef]
- Lei, T.; Song, Y.; Jin, X.; Su, T.; Pu, Y. Effects of pigment constituents and their distribution on spathe coloration of Zantedeschia hybrida. Hortscience 2017, 52, 1840–1848. [Google Scholar] [CrossRef]
- Fang, Y.; Lei, T.; Wu, Y.; Jin, X. Mechanism underlying color variation in calla lily spathes based on transcriptomic analysis. J. Am. Soc. Hortic. Sci. 2021, 146, 387–398. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterisation of betalain patterns of differently coloured inflorescences from Gomphrena globosa L. and Bougainvillea sp. By HPLC-DAD-ESI-MSN. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, Y.; Tan, J.; Huang, L.; Xu, Y. Globba hongman, a cut-flower cultivar with red bract color and long vase life. Hortscience 2024, 59, 1550–1552. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, J.; Huang, L.; Xu, Y.; Ye, Y. Decoding the color palette in the ornamental bracts of Globba spp.: Insights from phenotypic, metabolomic, and transcriptomic analyses. Planta 2025, 262, 11. [Google Scholar] [CrossRef]
- Cui, D.; Xiong, G.; Ye, L.; Gornall, R.; Wang, Z.; Heslop-Harrison, P.; Liu, Q. Genome-wide analysis of flavonoid biosynthetic genes in musaceae (Ensete, Musella, and Musa species) reveals amplification of flavonoid 3′,5′-hydroxylase. AoB Plants 2024, 16, plae49. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, Q.; Liu, H.; Wei, Z. Comparative metabolomic and transcriptomic analyses uncover variation in pigment accumulation profiles in Alpinia hainanensis bracts. Horticulturae 2025, 11, 266. [Google Scholar] [CrossRef]
- Martyn, A.J.; Larkum, A.W.D.; Mcconchie, R.; Offord, C.A. Photoinhibition and changes in pigments associated with bract browning in waratahs (Telopea spp., Proteaceae). J. Hortic. Sci. Biotechnol. 2008, 83, 367–373. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, K.P.; García-Nava, J.R.; Leyva-Ovalle, O.R.; Peña-Valdivia, C.B.; Trejo, C.; Ybarra-Moncada, M.C. Chitosan coating effect on vase life of flowering stems of Heliconia bihai (L.) L. Cv. Halloween. Postharvest Biol. Tec. 2017, 132, 179–187. [Google Scholar] [CrossRef]
- Jagtap, A.Y.; Jadhav, P.R.; Safeena, S.A.; Solanke, A.U.; Pagariya, M.C.; Prasad, K.V.; Kawar, P.G. Integrating molecular and phenotypic approaches to assess genetic diversity in Heliconia genotypes. Genet. Resour. Crop Evol. 2024, 72, 5409–5427. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, G.F.; Cai, S.Y.; Hu, S.Q.; Wang, W.Y. Mechanism of bract reddening Guzmania. Pak. J. Bot. 2023, 55, 313–320. [Google Scholar]
- Rozali, S.E.; Rashid, K.A.; Farzinebrahimi, R. Effects of shading treatments on pigmentation and inflorescence quality of Calathea crotalifera bracts. Int. J. Agric. Biol. 2016, 18, 549–556. [Google Scholar] [CrossRef]
- Qi, Y.; Lou, Q.; Li, H.; Yue, J.; Liu, Y.; Wang, Y. Anatomical and biochemical studies of bicolored flower development in Muscari latifolium. Protoplasma 2013, 250, 1273–1281. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, N.; Momonoi, K.; Kawachi, M.; Katou, K.; Okazaki, Y.; Uozumi, N.; Maeshima, M.; Kondo, T. Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly blue. Proc. Jpn. Acad. Ser. B 2009, 85, 187–197. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Whittall, J.B. Painting the green canvas:how pigments produce flower colours. Biochemistry 2021, 43, 6–12. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ros formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Baumann, K.; Perez-Rodriguez, M.; Bradley, D.; Venail, J.; Bailey, P.; Jin, H.; Koes, R.; Roberts, K.; Martin, C. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development 2007, 134, 1691–1701. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Y.Q.; Chen, X.H. Anatomical structure and pigment content of Davidia involucrata leaves and Bracts with different colors. Acta Bot. Boreali-Occident. Sin. 2020, 40, 1539–1548. (In Chinese) [Google Scholar]
- Noda, K.; Glover, B.J.; Linstead, P.; Martin, C. Flower colour intensity depends on specialized cell shape controlled by a MYB-related transcription factor. Nature 1994, 369, 661–664. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, Y.; Zhang, T.; Wang, P.; Liu, W.; Zhang, Z.; Yu, W.; Wang, J.; Wang, J.; Zhou, Y. Integrated metabolome, full-length sequencing, and transcriptome analyses unveil the molecular mechanisms of color formation of the canary yellow and red bracts of Bougainvillea × Buttiana ‘Chitra’. Plant J. 2023, 116, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tu, S.; Ke, L.; Lu, L.; Yu, H. Transcriptome analysis revealed the anabolic regulation of chlorophyll and carotenoids in Curcuma alismatifolia bracts. Biochem. Genet. 2024, 1–14. [Google Scholar] [CrossRef]
- Chen, J.; Funnell, K.A.; Lewis, D.H.; Eason, J.R.; Woolley, D.J. Cytokinin and gibberellin delay regreening of spathe tissue of Zantedeschia ‘Best gold’. Postharvest Biol. Technol. 2013, 84, 61–65. [Google Scholar] [CrossRef]
- Chen, J.; Funnell, K.A.; Lewis, D.H.; Eason, J.R.; Woolley, D.J. Relationship between changes in colour and pigment content during spathe regreening of Zantedeschia ‘Best gold’. Postharvest Biol. Technol. 2012, 67, 124–129. [Google Scholar] [CrossRef]
- Chen, J.; Funnell, K.A.; Lewis, D.H.; Eason, J.R.; Woolley, D.J. Regreening in spathes of Zantedeschia after anthesis: Its physiology and control by fructification and hormones. Physiol. Plant. 2015, 154, 128–141. [Google Scholar] [CrossRef]
- Heuer, S.; Richter, S.; Metzger, J.W.; Wray, V.; Nimtz, M.; Strack, D. Betacyanins from bracts of Bougainvillea glabra. Phytochemistry 1994, 37, 761. [Google Scholar] [CrossRef]
- Jerz, G.; Wybraniec, S.; Gebers, N.; Winterhalter, P. Target-guided separation of Bougainvillea glabra betacyanins by direct coupling of preparative ion-pair high-speed countercurrent chromatography and electrospray ionization mass-spectrometry. J. Chromatogr. A 2010, 1217, 4544–4554. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Stampar, F.; Schmitzer, V. Anthocyanin and chlorophyll content during poinsettia bract development. Sci. Hortic. 2013, 150, 142–145. [Google Scholar] [CrossRef]
- Nitarska, D.; Stefanini, C.; Haselmair-Gosch, C.; Miosic, S.; Walliser, B.; Mikulic-Petkovsek, M.; Regos, I.; Slatnar, A.; Debener, T.; Terefe-Ayana, D.; et al. The rare orange-red colored Euphorbia pulcherrima cultivar ‘harvest orange’ shows a nonsense mutation in a flavonoid 3′-hydroxylase allele expressed in the bracts. BMC Plant Biol. 2018, 18, 216. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, M.; Abbas, F.; Sun, Y.; Gao, T.; Yan, F.; Li, X.; Yu, Y.; Yue, Y.; Yu, R.; et al. Classification and association analysis of gerbera (Gerbera hybrida) flower color traits. Front. Plant Sci. 2022, 12, 779288. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Zhu, G.; Tan, J.; Lin, J.; Huang, L.; Ye, Y.; Liu, J. Pigment diversity in leaves of Caladium × hortulanum Birdsey and transcriptomic and metabolic comparisons between red and white leaves. Int. J. Mol. Sci. 2024, 25, 605. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shen, X.; Li, Y.; Xu, Y.; Chen, Y.; Chen, Y.; Hu, X.; Li, X.; Sun, X.; Gong, J. Regulation of chlorophyll and carotenoid metabolism in citrus fruit. Hortic. Plant J. 2025, 11, 951–962. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-translational coordination of chlorophyll biosynthesis and breakdown by bcms maintains chlorophyll homeostasis during leaf development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef]
- Liao, X.; Ye, Y.; Zhang, X.; Peng, D.; Hou, M.; Fu, G.; Tan, J.; Zhao, J.; Jiang, R.; Xu, Y.; et al. The genomic and bulked segregant analysis of Curcuma alismatifolia revealed its diverse bract pigmentation. aBIOTECH 2022, 3, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Feng, P.; Li, Y.; Yu, P.; Yu, G.; Sang, X.; Ling, Y.; Zeng, X.; Li, Y.; Huang, J.; et al. Virescent-albino leaf 1 regulates leaf colour development and cell division in rice. J. Exp. Bot. 2018, 69, 4791–4804. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163. [Google Scholar] [CrossRef]
- Sun, L.; Huo, J.; Liu, J.; Yu, J.; Zhou, J.; Sun, C.; Wang, Y.; Leng, F. Anthocyanins distribution, transcriptional regulation, epigenetic and post-translational modification in fruits. Food Chem. 2023, 411, 135540. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, H.; Yang, R.; Ran, M. Identification of DFR as a promoter of anthocyanin accumulation in poinsettia (Euphorbia pulcherrima, Willd. Ex klotzsch) bracts under short-day conditions. Sci. Hortic. 2018, 236, 158–165. [Google Scholar] [CrossRef]
- Vilperte, V.; Boehm, R.; Debener, T. A highly mutable GST is essential for bract colouration in Euphorbia pulcherrima willd. Ex klotsch. BMC Genom. 2021, 22, 208–216. [Google Scholar] [CrossRef]
- Osorio-Guarin, J.A.; Gopaulchan, D.; Quanckenbush, C.; Lennon, A.M.; Umaharan, P.; Cornejo, O.E. Comparative transcriptomic analysis reveals key components controlling spathe color in Anthurium andraeanum (Hort.). PLoS ONE 2021, 16, e261364. [Google Scholar] [CrossRef]
- Borjan, D.; Šeregelj, V.; Andrejč, D.C.; Pezo, L.; Šaponjac, V.T.; Knez, Ž.; Vulić, J.; Marevci, M.K. Green techniques for preparation of red beetroot extracts with enhanced biological potential. Antioxidants 2022, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation mechanism of plant pigments biosynthesis: Anthocyanins, carotenoids, and betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Makishima, R.; Doi, M. Post-transcriptional gene silencing of CYP76AD controls betalain biosynthesis in bracts of bougainvillea. J. Exp. Bot. 2021, 72, 6949–6962. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, S.; Zheng, Y. Genome-wide identification of the pigment formation-regulating CYP450 family gives new insights into color improvement in Bougainvillea. Sci. Hortic. 2025, 341, 113997. [Google Scholar] [CrossRef]
- Ohno, S.; Kokado, R.; Makishima, R.; Doi, M. BpCYP76AD15 is involved in betaxanthin biosynthesis in Bougainvillea callus. Planta 2023, 258, 47. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Wang, F.; Yao, G.; Li, J.; Zhu, W.; Li, Z.; Sun, Z.; Xin, P. Mining and expression analysis of color related genes in Bougainvillea glabra bracts based on transcriptome sequencing. Sci. Rep. 2024, 14, 24491. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. Myb-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Chen, L.; Hu, B.; Qin, Y.; Hu, G.; Zhao, J. Advance of the negative regulation of anthocyanin biosynthesis by myb transcription factors. Plant Physiol. Bioch. 2019, 136, 178–187. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in arabidopsis. The Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef]
- Chen, C.; Xie, F.; Shah, K.; Hua, Q.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Genome-wide identification of wrky gene family in pitaya reveals the involvement of HmoWRKY42 in betalain biosynthesis. Int. J. Mol. Sci. 2022, 23, 10568. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Shah, K.; Chen, C.; Sabir, I.A.; Chen, J.; Chen, J.; Chen, J.; Qin, Y. Unraveling betalain suppression in pitaya: Insights from co-activator HuMYB9 binding at HuCYP76AD1-1, HuADH1, and HuDODA1 super-enhancers. Food Qual. Saf. 2024, 8, fyae016. [Google Scholar] [CrossRef]
- Albert, N.W.; Arathoon, S.; Collette, V.E.; Schwinn, K.E.; Jameson, P.E.; Lewis, D.H.; Zhang, H.; Davies, K.M. Activation of anthocyanin synthesis in cymbidium orchids: Variability between known regulators. Plant Cell Tissue Organ Cult. 2010, 100, 355–360. [Google Scholar] [CrossRef]
- Ma, G.; Zou, Q.; Shi, X.; Tian, D.; Sheng, Q. Ectopic expression of the aaful1 gene identified in Anthurium andraeanum affected floral organ development and seed fertility in tobacco. Gene 2019, 696, 197–205. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Boonyaritthongchai, P.; Buanong, M.; Supapvanich, S.; Wongs-Aree, C. Chitosan- and κ-carrageenan-based composite coating on dragon fruit (Hylocereus undatus) pretreated with plant growth regulators maintains bract chlorophyll and fruit edibility. Sci. Hortic. 2021, 281, 109916. [Google Scholar] [CrossRef]
- Portis, E.; Mauro, R.P.; Acquadro, A.; Moglia, A.; Mauromicale, G.; Lanteri, S. Inheritance of bract pigmentation and fleshy thorns on the globe artichoke capitulum. Euphytica 2015, 206, 523–531. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- De Keyser, E.; Dhooghe, E.; Christiaens, A.; Van Labeke, M.; Van Huylenbroeck, J. Led light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Aoki, D.; Fukushima, K.; Yoshida, K. Direct mapping of hydrangea blue-complex in sepal tissues of Hydrangea macrophylla. Sci. Rep. 2019, 9, 5450. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Sim, S.B.; Geib, S.M.; Imamura, J.S.L.; Corpuz, B.L.; Corpuz, R.L.; Kauwe, A.N.; Simmonds, T.J.; Arakawa, C.N.; Myers, R.Y.; et al. Chromosome-level genome assembly and annotation of Anthurium amnicola. Sci. Data 2025, 12, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zou, Q.; Mao, L.; Tian, D.; Hu, W.; Cao, X.; Ding, H. The chromosome-scale assembly of the Curcuma alismatifolia genome provides insight into anthocyanin and terpenoid biosynthesis. Front. Plant Sci. 2022, 13, 899588. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Wang, D.; Gou, R.; Jiang, Y.; Zhang, G.; Zheng, Y.; Gao, D.; Chen, L.; Zhang, X.; et al. Chromosome level genome assembly of colored calla lily (Zantedeschia elliottiana). Sci. Data 2023, 10, 605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).