Evaluation of Stress-Tolerant Serratia and Enterobacter as PGPR for Nutrient Solubilization and Dose-Dependent Bioformulation to Enhance Tomato Seedlings
Abstract
1. Introduction
2. Results
2.1. Isolation and Biochemical Characterization of Rhizobacteria
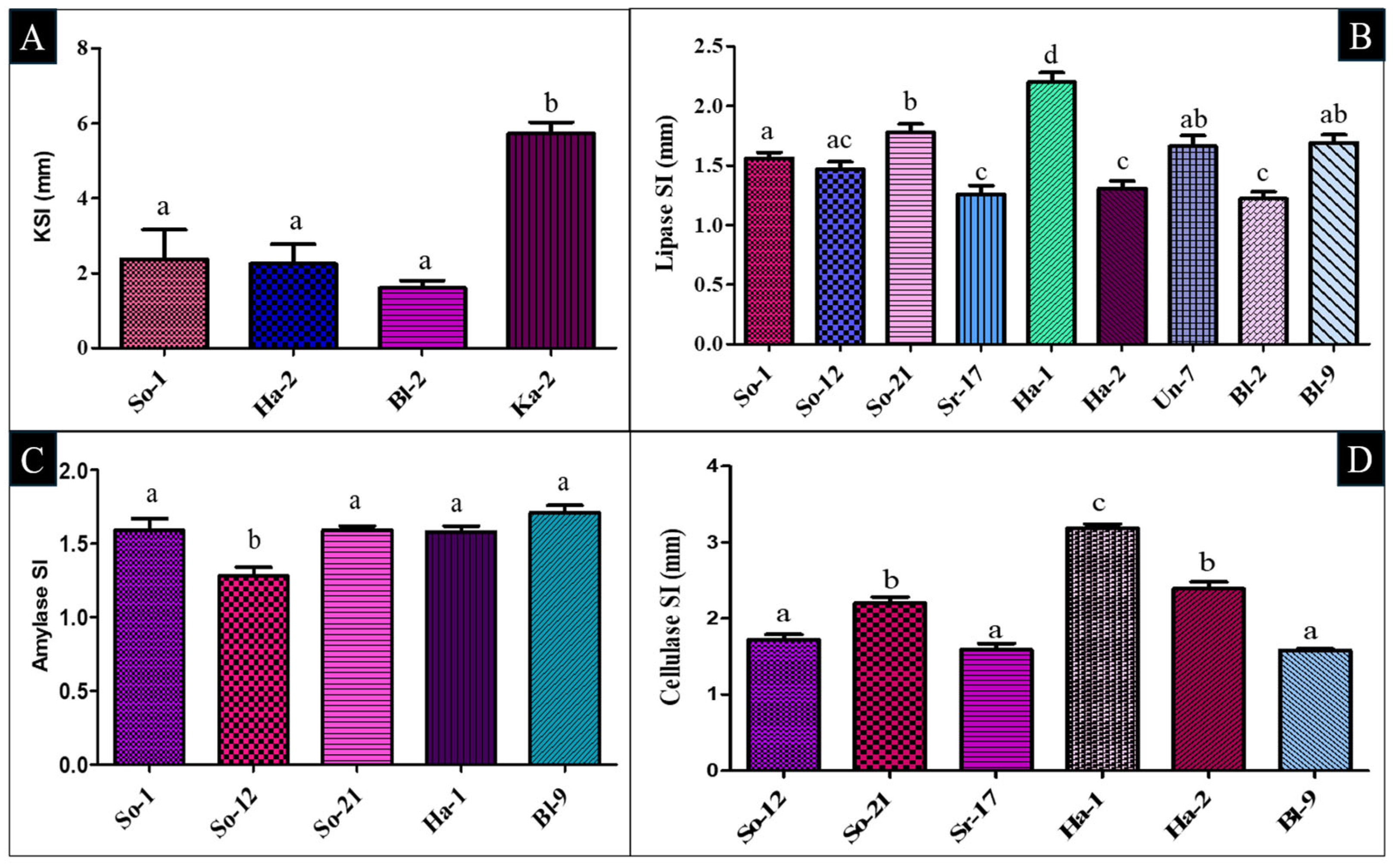
2.2. Characterization of Isolates for Their PGP Attributes
2.3. Evaluation of Antimicrobial Activity
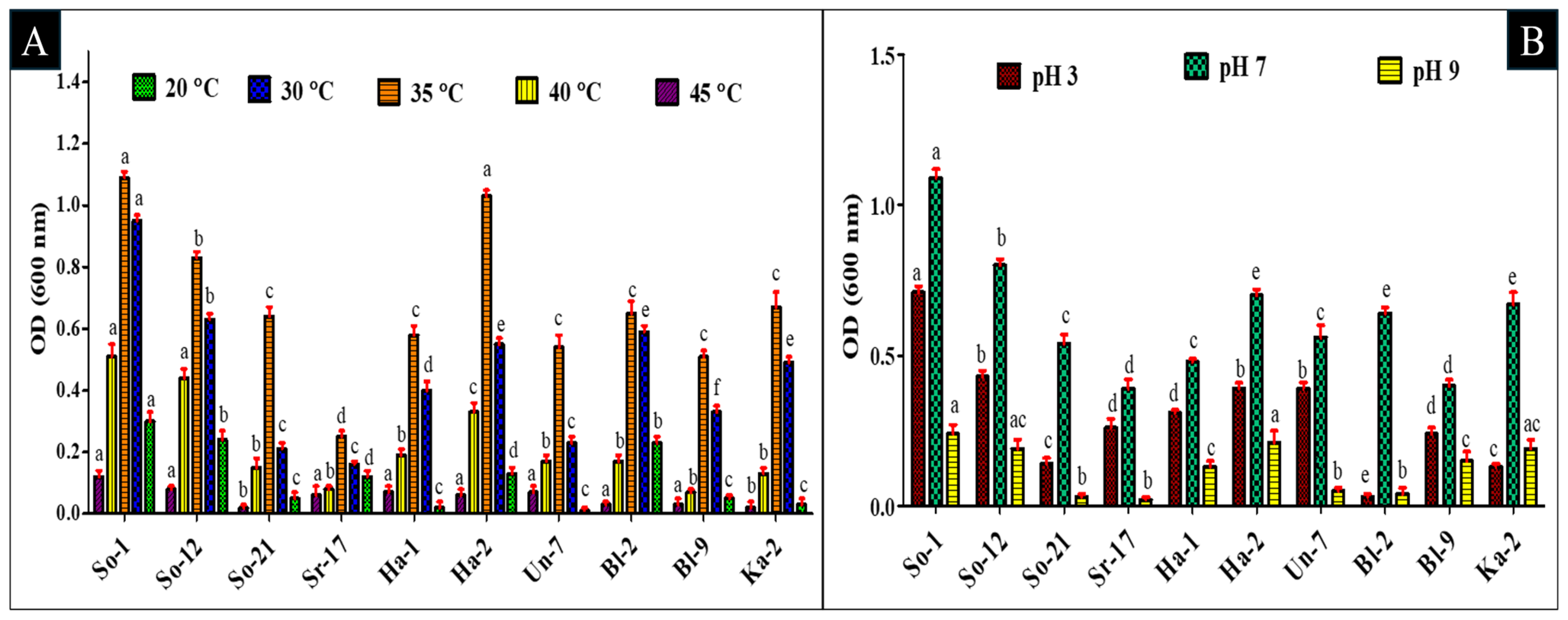
2.4. Screening of Bacterial Isolates for pH and Heat Tolerance
2.5. Molecular Characterization
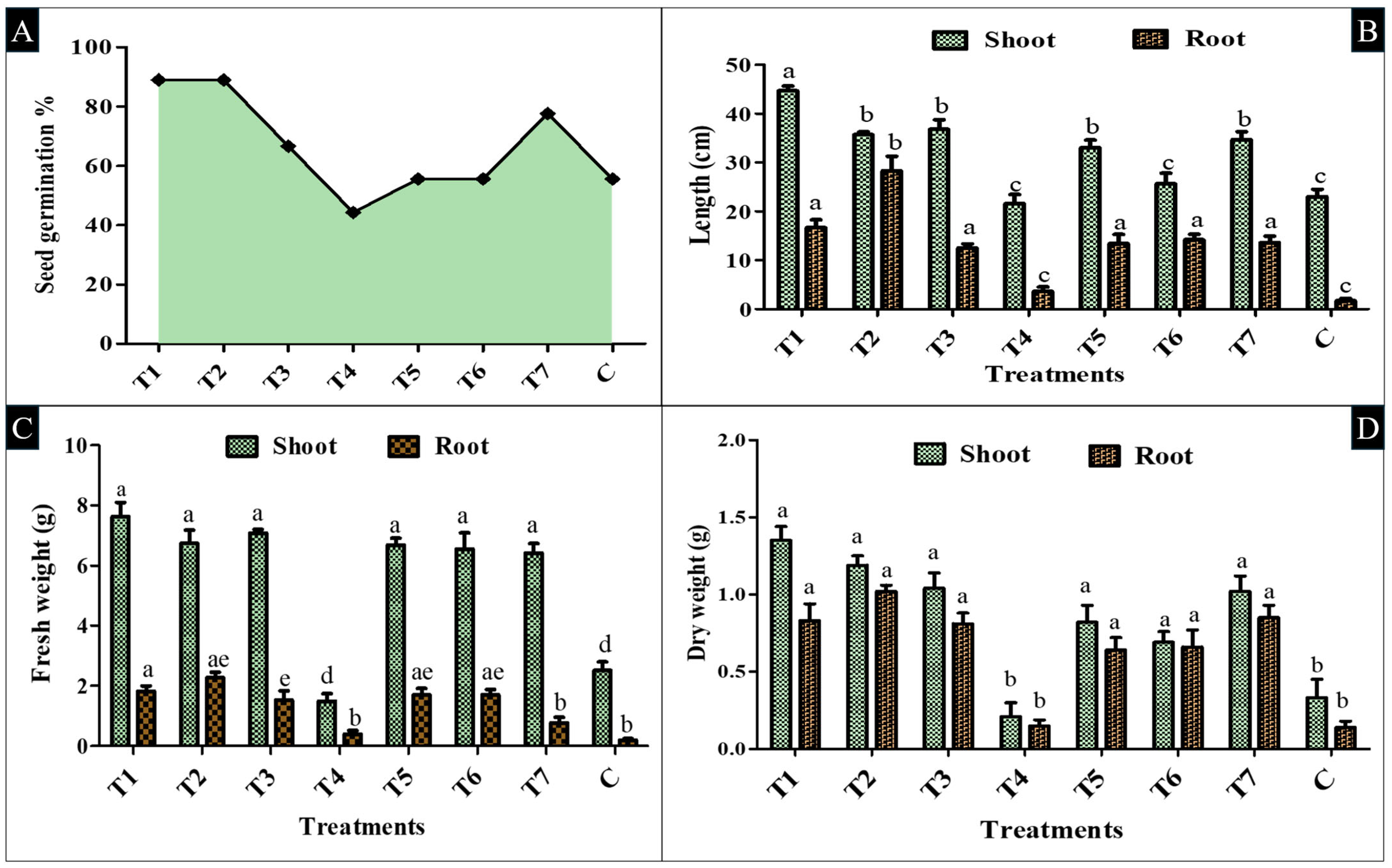
2.6. In Vitro Evaluation on Germination and Growth Parameters of Tomato Seedlings
2.7. In Vivo Polyhouse Screening and Measuring the Growth Parameters of Tomato Seedlings
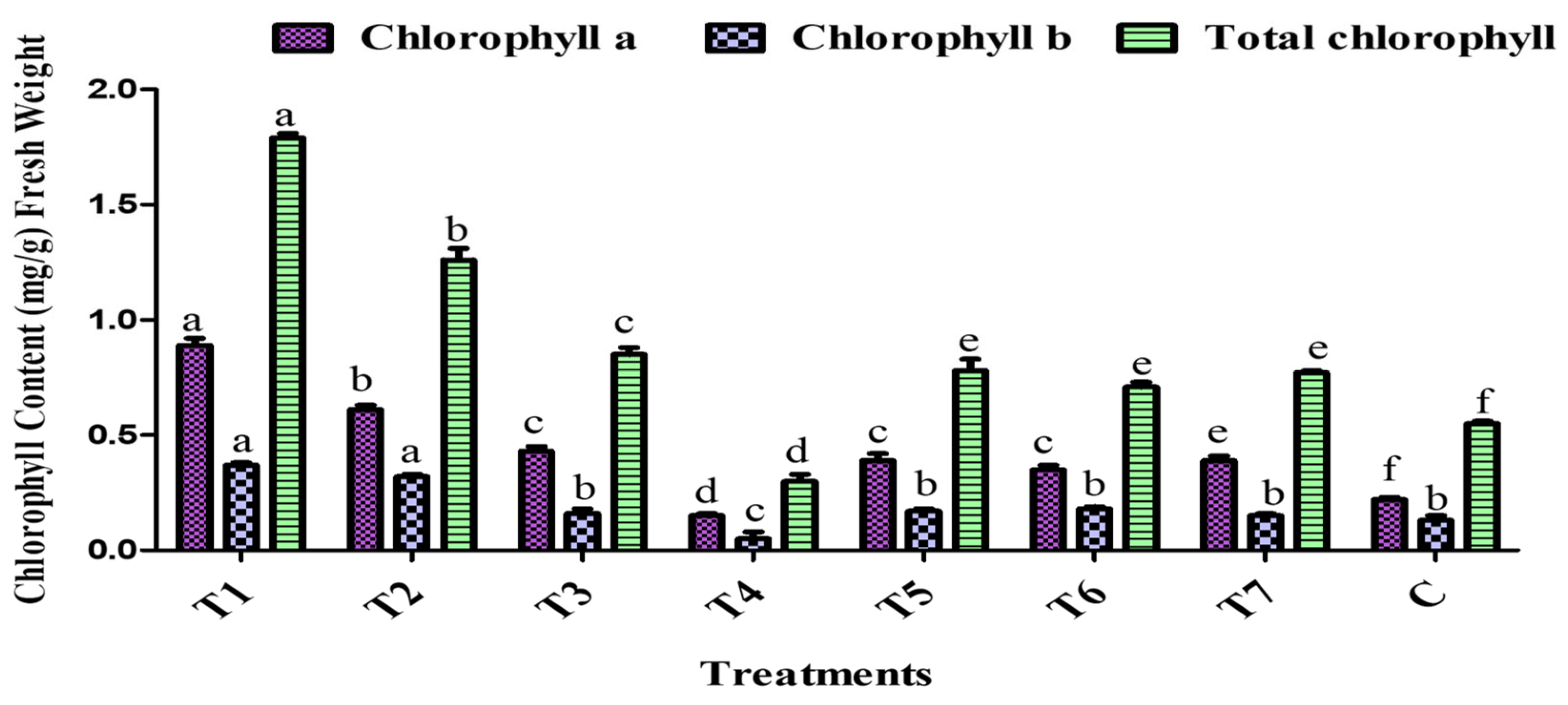
2.8. Chlorophyll Estimation
3. Discussion
3.1. Evaluation of In Vitro Environmental Stress Tolerance of Bacterial Isolates
3.2. Antagonistic Activity of the Isolates
3.3. Bacterial Identification and Its Pathogenicity
3.4. In Vitro- and In Vivo-Level Investigation of PGPR Inoculant to Enhance Tomato Plant Growth
3.5. Future Recommendations
4. Materials and Methods
4.1. Soil Sampling and Rhizobacterial Isolation
4.1.1. Description of Study Area
4.1.2. Collection and Isolation of Rhizobacteria
4.2. Biochemical Characterization
4.2.1. Catalase and Oxidase Test
4.2.2. Mannitol Fermentation Test
4.2.3. Citrate Utilization Test
4.2.4. Urease Test
4.2.5. TSI Test
4.2.6. MR Test
4.3. In Vitro Bioassays to Evaluate Growth-Promoting and Biocontrol Attributes of the Rhizobacterial Isolates
4.3.1. Characterization of PGPRs Based on Their PGP Capabilities
Qualitative Screening of K Solubilization Activity
Qualitative Estimation of Ammonia Synthesis
Screening of EPS Formation
4.3.2. Characterization of PGPRs Based on Their Biocontrol Activity
4.3.3. Qualitative Characterization of PGPRs Based on Their Hydrolytic Enzyme Production Activity
Lipase Activity
Amylase Activity
Cellulase Activity
4.3.4. Characterization of PGPRs Based on Their Phytohormone Production Activity
4.4. Evaluation of Antimicrobial Activity
4.5. Bacterial Tolerance Response to Different pH and Heat
4.6. Molecular Characterization of Bacterial PGPR Strain
4.7. Preparation of Bacterial Inoculum
4.8. In Vitro Response of the Bacterial Isolates on the Growth of Tomato Seedlings
4.9. Preparation of Cocopeat-Based Bioformulation and Evaluation of In Vivo Pot Trial
4.9.1. Preparation of Cocopeat-Based Bioformulation
4.9.2. Soil Characteristics Used for Experiment
4.9.3. Response of PGPR Bioformulation in Tomato Plant
4.10. Chlorophyll Estimation
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGPR | Plant Growth-Promoting Rhizobacteria |
| SVI | Seed Vigor Index |
| PGP | Plant Growth Promoting |
| CFU | Colony-Forming Unit |
| CMC | Carboxymethyl cellulose |
| IAA | Indole-3-Acetic Acid |
| SI | Solubilizing Index |
References
- Hasan, A.; Tabassum, B.; Hashim, M.; Khan, N. Role of plant growth promoting rhizobacteria (PGPR) as a plant growth enhancer for sustainable agriculture: A review. Bacteria 2024, 3, 59–75. [Google Scholar] [CrossRef]
- Bhardwaj, I.; Kumar, V.; Bhardwaj, N.; Verma, R.; Bhardwaj, Y.; Kumari, T. A glimpse into the performance and synthesis of microbial nanoparticles and its new advances in soil enrichment and plant nutrition: A review. Nanotechnol. Environ. Eng. 2023, 8, 943–964. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Yadav, A.; Sahoo, A.; Kumari, P.; Singh, L.A.; Swapnil, P.; Meena, M.; Kumar, S. Microalgal-based sustainable bio-fungicides: A promising solution to enhance crop yield. Discov. Sustain. 2025, 6, 39. [Google Scholar] [CrossRef]
- Goswami, S.K.; Singh, V.; Chakdar, H.; Choudhary, P. Harmful effects of fungicides—Current status. Int. J. Agric. Environ. Biotechnol. 2018, 11, 1011–1019. [Google Scholar]
- Singh, V.K.; Meena, M.; Zehra, A.; Tiwari, A.; Dubey, M.K.; Upadhyay, R.S. Fungal toxins and their impact on living systems. In Microbial Diversity and Biotechnology in Food Security; Springer: Cham, Switzerland, 2014; pp. 513–530. [Google Scholar] [CrossRef]
- Kürklü, A.; Pearson, S.; Felek, T. Climate change impacts on tomato production in high-tech soilless greenhouses in Türkiye. BMC Plant Biol. 2025, 25, 339. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Cheema, A.; Paliyath, G. Solanaceous fruits including tomato, eggplant, and peppers. In Encyclopedia of Food and Health; Elsevier: Oxford, UK, 2016; pp. 24–32. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 25 June 2025).
- DesiKheti. Top 10 Tomato-Producing States in India: A Detailed Statewise Overview. 2024. Available online: https://knowledge.desikheti.com/top-10-tomato-producing-states-in-india-a-detailed-statewise-overview/ (accessed on 27 June 2025).
- District Solan, Government of Himachal Pradesh. Mushroom City of India. Available online: https://hpsolan.nic.in/ (accessed on 11 May 2025).
- Times of India. Off-Season Tomatoes a Hit in HP, Plan to Hike Production. 2022. Available online: https://timesofindia.indiatimes.com/city/shimla/off-season-tomatoes-a-hit-in-hp-plan-to-hike-production/articleshow/92686994.cms (accessed on 11 May 2025).
- Kumar, V.; Sharma, H.; Sharma, D. Profile and problems of tomato cultivation in Bilaspur district of Himachal Pradesh. Him. J. Agric. Res. 2018, 44, 60–67. [Google Scholar]
- National Horticulture Board. Tomato—October 2018. 2018. Available online: https://nhb.gov.in/statistics/Reports/TomatoOctober2018.pdf (accessed on 11 May 2025).
- CEIC. India Production of Horticulture Crops in Major States: Vegetables: Tomato. 2025. Available online: https://www.ceicdata.com/en/india/production-of-horticulture-crops-in-major-states-vegetables-tomato (accessed on 2 June 2025).
- Shubham; Rao, A.; Kaushal, S. The Multifaceted Soils of Himachal Pradesh, India: Types and Characteristics. Arch. Curr. Res. Int. 2024, 24, 145–160. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Davino, S.; Bertacca, S.; Cravero, V.; Catara, A.; Garibaldi, A.; Minuto, A. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Khlode, R.S.; Abdel-Gawad, T.I.; El-Bana, A.A.; Galal, A.A. Fusarium oxysporum f. sp. lycopersici (fol) race 1 and 3 as wilt-incitants to tomato plants growing at El-Minia Governorate, Egypt. Minia J. Agric. Res. Dev. 2016, 36, 229–244. [Google Scholar]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth-promoting rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Aksoy, A.; Kaymak, H.Ç. Tomato production quantity estimates for 2023–2027 with ARIMA model: Evidence from leading producing countries including Turkey. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2024, 24, 41–46. [Google Scholar]
- Prasad, R.; Kumar, M.; Varma, A. Role of PGPR in Soil Fertility and Plant Health. In Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants; Egamberdieva, D., Shrivastava, S., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2015; Volume 42, pp. 247–260. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Amoo, A.E.; Babalola, O.O. Characterization of plant growth-promoting rhizobacterial isolates associated with food plants in South Africa. Antonie Van Leeuwenhoek 2021, 114, 1683–1708. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Gupta, R.; Solanki, M.K.; Saini, S.; Saxena, A.K. Identification, characterization and optimization of phosphate-solubilizing rhizobacteria (PSRB) from rice rhizosphere. Saudi J. Biol. Sci. 2022, 29, 35–42. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.A.; Babar, M. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Arora, N.K.; Tewari, S.; Singh, R. Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In Plant Microbe Symbiosis: Fundamentals and Advances; Arora, N., Ed.; Springer: New Delhi, India, 2013; pp. 411–449. [Google Scholar] [CrossRef]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.; Bejai, S.; Meijer, J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 2017, 245, 15–30. [Google Scholar] [CrossRef]
- Ummara, U.; Noreen, S.; Afzal, M.; Ahmad, P. Bacterial bioaugmentation enhances hydrocarbon degradation, plant colonization and gene expression in diesel-contaminated soil. Physiol. Plant. 2021, 173, 58–66. [Google Scholar] [CrossRef]
- Odelade, K.A.; Babalola, O.O. Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int. J. Environ. Res. Public Health 2019, 16, 3873. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Aydogdu, M.; Ates, F.; Oguz, A.; Ilkme, G.; Yildirim, H.; Ozer, G. Microbial-based production system: A novel approach for plant growth and pest and disease management in greenhouse-grown peppers (Capsicum annuum L.). J. Agric. Sci. Technol. 2018, 18, 371–386. [Google Scholar]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Chromkaew, Y.; Kaeomuangmoon, T.; Mawan, N.; Mukjang, N.; Khongdee, N. Is coconut coir dust an efficient biofertilizer carrier for promoting coffee seedling growth and nutrient uptake? PeerJ 2023, 11, e15530. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic pathways and functions of indole-3-acetic acid in microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P. Screening and characterization of native Pseudomonas sp. as plant growth-promoting rhizobacteria in chickpea (Cicer arietinum L.) rhizosphere. Afr. J. Microbiol. Res. 2013, 7, 1465–1474. [Google Scholar] [CrossRef]
- Kalimuthu, R.; Suresh, P.; Varatharaju, G.; Balasubramanian, N.; Rajasekaran, K.M.; Shanmugaiah, V. Isolation and characterization of indole acetic acid (IAA) producing tomato rhizobacterium Pseudomonas sp. VSMKU4050 and its potential for plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 443–455. [Google Scholar] [CrossRef]
- Lata, D.L.; Abdie, O.; Rezene, Y. IAA-producing bacteria from the rhizosphere of chickpea (Cicer arietinum L.): Isolation, characterization, and their effects on plant growth performance. Heliyon 2024, 10, e27742. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Khan, I.; Mohyuddin, S.G.; Sohail Zaman, S.; Qadir, M.; Guo, J.; Li, G. Enhancing growth in Vigna radiata through the inhibition of charcoal rot disease: A strategic approach using plant growth-promoting rhizobacteria. Microorganisms 2024, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Alonazi, M.A.; Alwathnani, H.A.; Al-Barakah, F.N.; Alotaibi, F. Native plant growth-promoting rhizobacteria containing ACC deaminase promote plant growth and alleviate salinity and heat stress in maize (Zea mays L.) plants in Saudi Arabia. Plants 2025, 14, 1107. [Google Scholar] [CrossRef] [PubMed]
- Azizah, H.; Rahajeng, S.M.; Jatmiko, Y.D. Isolation and screening of phosphate and potassium solubilizing endophytic bacteria in maize (Zea mays L.). J. Exp. Life Sci. 2020, 10, 3. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, H.; Zhang, J.; Chen, Y.; Dhital, Y.P.; Zhao, T.; Wang, Z. Isolation and characterization of potassium-solubilizing rhizobacteria (KSR) promoting cotton growth in saline–sodic regions. Microorganisms 2024, 12, 1474. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Mekonnen, H.; Kibret, M.; Assefa, F. Plant growth promoting rhizobacteria for biocontrol of tomato bacterial wilt caused by Ralstonia solanacearum. Int. J. Agron. 2022, 2022, 1489637. [Google Scholar] [CrossRef]
- Yao, X.; Lan, X.; Jin, Y.; Li, C. Screening, identification, and characterization of plant growth-promoting rhizobacterium strains from alpine grassland as biocontrol agents against Fusarium oxysporum. Agronomy 2024, 14, 2856. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Patel, S.K.; Singh, S.; Benjamin, J.C.; Singh, V.R.; Bisht, D.; Lal, R.K. Plant growth-promoting activities of Serratia marcescens and Pseudomonas fluorescens on Capsicum annuum L. plants. Ecol. Front. 2024, 44, 654–663. [Google Scholar] [CrossRef]
- Chakraborty, U.; Chakraborty, B.N.; Chakraborty, A.P. Influence of Serratia marcescens TRS-1 on growth promotion and induction of resistance in Camellia sinensis against Fomes lamaoensis. J. Plant Interact. 2010, 5, 261–272. [Google Scholar] [CrossRef]
- Zaheer, A.; Mirza, B.S.; Mclean, J.E.; Yasmin, S.; Shah, T.M.; Malik, K.A.; Mirza, M.S. Association of plant growth-promoting Serratia spp. with the root nodules of chickpea. Res. Microbiol. 2016, 167, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Abreo, E.; Altier, N. Pangenome of Serratia marcescens strains from nosocomial and environmental origins reveals different populations and the links between them. Sci. Rep. 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Chakdar, H.; Vaishnav, A.; Srivastava, A.K.; Khan, N.; Bansal, Y.K.; Kaushik, R. Multifarious plant growth-promoting rhizobacterium Enterobacter sp. CM94-mediated systemic tolerance and growth promotion of chickpea (Cicer arietinum L.) under salinity stress. Front. Biosci.-Landmark 2023, 28, 241. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, J.; Hao, X.; Feng, G.; Shen, Y.; Wang, G.; Chen, Z. Serratia marcescens LYGN1 reforms the rhizosphere microbial community and promotes cucumber and pepper growth in plug seedling cultivation. Plants 2024, 13, 592. [Google Scholar] [CrossRef]
- Almaghrabi, O.A.; Massoud, S.I.; Abdelmoneim, T.S. Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J. Biol. Sci. 2013, 20, 57–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, Y.; Wang, P.; Gou, Y.; Zeng, R.; Zhang, Q.; Chen, D.; Song, Y. Assembly of tomato rhizobacteria from different functional groups improves seedling photosynthesis and growth. Plants 2023, 12, 4000. [Google Scholar] [CrossRef]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of seed with plant growth-promoting bacteria for improved germination and seedling growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar] [CrossRef]
- González-Ista, N.S.; Castro-Mercado, E.; de la Cruz, H.R.; Campos-García, J.; López-Bucio, J.; García-Pineda, E. Comparison of the rhizobacteria Serratia sp. H6 and Enterobacter sp. L7 on Arabidopsis thaliana growth promotion. Curr. Microbiol. 2023, 80, 117. [Google Scholar] [CrossRef]
- Mengistie, G.Y.; Awlachew, Z.T. Evaluation of the plant growth promotion effect of Bacillus species on different varieties of tomato (Solanum lycopersicum L.) seedlings. Adv. Agric. 2022, 2022, 1771147. [Google Scholar] [CrossRef]
- Singh, A.; Yadav, V.K.; Chundawat, R.S.; Soltane, R.; Awwad, N.S.; Ibrahium, H.A.; Yadav, K.K.; Vicas, S.I. Enhancing plant growth promoting rhizobacterial activities through consortium exposure: A review. Front. Bioeng. Biotechnol. 2023, 11, 1099999. [Google Scholar] [CrossRef]
- Rosier, A.; Beauregard, P.B.; Bais, H.P. Quorum quenching activity of the PGPR Bacillus subtilis UD1022 alters nodulation efficiency of Sinorhizobium meliloti on Medicago truncatula. Front. Microbiol. 2021, 11, 596299. [Google Scholar] [CrossRef] [PubMed]
- Abou Jaoudé, R.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Effect of plant growth-promoting rhizobacteria synthetic consortium on growth, yield, and metabolic profile of lettuce (Lactuca sativa L.) grown under suboptimal nutrient regime. Horticulturae 2025, 11, 64. [Google Scholar] [CrossRef]
- Mekonnen, H.; Kibret, M.; Assefa, F.; Kabtimer, N. Potential of plant growth-promoting rhizobacteria for enhancement of tomato growth. Agrosyst. Geosci. Environ. 2025, 8, e70036. [Google Scholar] [CrossRef]
- He, S.; Li, L.; Lv, M.; Wang, R.; Wang, L.; Yu, S.; Gao, Z.; Li, X. PGPR: Key to enhancing crop productivity and achieving sustainable agriculture. Curr. Microbiol. 2024, 81, 377. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manag. Sci. 2012, 68, 404–411. [Google Scholar] [CrossRef]
- Patani, A.; Prajapati, D.; Ali, D.; Kalasariya, H.; Yadav, V.K.; Tank, J.; Bagatharia, S.; Joshi, M.; Patel, A. Evaluation of the growth-inducing efficacy of various Bacillus species on the salt-stressed tomato (Lycopersicon esculentum Mill.). Front. Plant Sci. 2023, 14, 1168155. [Google Scholar] [CrossRef]
- Dhole, S.M.; Amnerkar, N.D.; Khedekar, P.B. Comparison of UV spectrophotometry and high performance liquid chromatography methods for the determination of repaglinide in tablets. Pharm. Methods 2012, 3, 68–72. [Google Scholar] [CrossRef]
- Hegyi, A.; Nguyen, T.B.K.; Posta, K. Metagenomic analysis of bacterial communities in agricultural soils from Vietnam with special attention to phosphate solubilizing bacteria. Microorganisms 2021, 9, 1796. [Google Scholar] [CrossRef]
- Brar, B.; Kumar, R.; Sharma, D.; Sharma, A.K.; Thakur, K.; Mahajan, D.; Kumar, R. Metagenomic analysis reveals diverse microbial community and potential functional roles in Baner rivulet, India. J. Genet. Eng. Biotechnol. 2023, 21, 147. [Google Scholar] [CrossRef]
- Qingwei, Z.; Lushi, T.; Yu, Z.; Yu, S.; Wanting, W.; Jiangchuan, W.; Xiaolei, D.; Xuejiao, H.; Bilal, M. Isolation and characterization of phosphate-solubilizing bacteria from rhizosphere of poplar on road verge and their antagonistic potential against various phytopathogens. BMC Microbiol. 2023, 23, 221. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing unculturable bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.C.; Dogra, S. Characterization of the soils of lower Himalayas of Himachal Pradesh, India. Nat. Environ. Pollut. Technol. 2011, 10, 439–446. [Google Scholar]
- Mengesha, A.S.; Legesse, N.H. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of lentil (Lens culinaris M.) collected from Hagere Mariam district, Central Ethiopia. PLoS ONE 2024, 19, e0308915. [Google Scholar] [CrossRef] [PubMed]
- Arjyal, C.; Kc, J.; Neupane, S. Prevalence of Methicillin-Resistant Staphylococcus aureus in Shrines. Int. J. Microbiol. 2020, 2020, 7981648. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Krishnasamy, C. Isolation and characterization of salt-stress-tolerant rhizosphere soil bacteria and their effects on plant growth-promoting properties. Sci. Rep. 2024, 14, 24909. [Google Scholar] [CrossRef]
- Hiranmayee, G.; Marik, D.; Sadhukhan, A.; Reddy, G.S. Isolation of plant growth-promoting rhizobacteria from the agricultural fields of Tattiannaram, Telangana. J. Genet. Eng. Biotechnol. 2023, 21, 159. [Google Scholar] [CrossRef]
- Pallavi; Mishra, R.K.; Sahu, P.K.; Mishra, V.; Jamal, H.; Varma, A.; Tripathi, S. Isolation and characterization of halotolerant plant growth promoting rhizobacteria from mangrove region of Sundarbans, India for enhanced crop productivity. Front. Plant Sci. 2023, 14, 1122347. [Google Scholar] [CrossRef]
- Sherpa, M.T.; Lepcha, S.R.; Rai, P.; Arora, D.K. Isolation and characterization of plant growth promoting rhizobacteria isolated from organically grown high yielding pole type native pea (Pisum sativum L.) variety Dentami of Sikkim, India. Curr. Res. Microb. Sci. 2021, 2, 100068. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, H.J.; Zhang, H.; Mukhtar, H.; Hussain, S.; Siddique, M.H.; Haseeb, M.; Mehmood, K.; Muneer, M.A.; Ismail, M. Contribution of potassium solubilizing bacteria in improved potassium assimilation and cytosolic K+/Na+ ratio in rice (Oryza sativa L.) under saline-sodic conditions. Front. Microbiol. 2023, 14, 1196024. [Google Scholar] [CrossRef]
- Alotaibi, F.; St-Arnaud, M.; Hijri, M. In-depth characterization of plant growth promotion potentials of selected alkanes-degrading plant growth-promoting bacterial isolates. Front. Microbiol. 2022, 13, 863702. [Google Scholar] [CrossRef]
- Shreshtha, K.; Rai, B.; Sharma, R.; Pradhan, A. Isolation and characterization of plant growth promoting rhizobacteria from cacti root under drought condition. Curr. Res. Microb. Sci. 2025, 8, 100319. [Google Scholar] [CrossRef] [PubMed]
- Vasant, G.; Bhatt, S.; Raghav, R. Isolation and molecular characterization of plant growth promoting rhizobacteria from groundnut (Arachis hypogaea L.) rhizosphere. Curr. Agric. Res. J. 2023, 11, 30. [Google Scholar] [CrossRef]
- Tsegaye, Z.; Woldesenbet, F.; Mulaw, T.; Sahile, S. Isolation and biochemical characterization of plant growth promoting (PGP) bacteria colonizing the rhizosphere of Tef crop during the seedling stage. J. Plant Sci. Phytopathol. 2019, 14, 13–27. [Google Scholar] [CrossRef]
- Yasmin, H.; Naz, R.; Nosheen, A.; Hassan, M.N.; Ilyas, N.; Sajjad, M.; Anjum, S.; Gao, X.; Geng, Z. Identification of new biocontrol agent against charcoal rot disease caused by Macrophomina phaseolina in soybean (Glycine max L.). Sustainability 2020, 12, 6856. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.I.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Ganesh, J.; Hewitt, K.; Devkota, A.R.; Wilson, T.; Kaundal, A. IAA-producing plant growth-promoting rhizobacteria from Ceanothus velutinus enhance cutting propagation efficiency and Arabidopsis biomass. Front. Plant Sci. 2024, 15, 1374877. [Google Scholar] [CrossRef]
- Agarwal, M.; Dheeman, S.; Dubey, R.C.; Kumar, P.; Maheshwari, D.K.; Bajpai, V.K. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017, 205, 40–47. [Google Scholar] [CrossRef]
- Divyanshu, K.; Kumari, A.; Dixit, S.; Singh, S.K.; Yadav, A.K.; Pandey, V.; Srivastava, M. Molecular identification and characterization of plant growth-promoting rhizobacteria and their effect on seed germination and vigour index of barley (Hordeum vulgare L.). J. Pure Appl. Microbiol. 2022, 16, 21. [Google Scholar] [CrossRef]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes faecalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int. J. Phytoremediation 2016, 18, 200–209. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust estimation of bacterial cell count from optical density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Chabbi, N.; El Harchli, E.H.; El Yamani, M.; Rahou, Y.; Rahou, A.; El Farissi, M.; Ouhammou, A. Plant-growth-promoting rhizobacteria improve seeds germination and growth of Argania spinosa. Plants 2024, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Dhar, H.; Swarnkar, M.K.; Soni, R.; Sharma, K.C.; Singh, A.K.; Gulati, A.; Sud, R.K.; Gulati, A. Understanding the molecular mechanism of PGPR strain Priestia megaterium from tea rhizosphere for stress alleviation and crop growth enhancement. Plant Stress 2024, 12, 100494. [Google Scholar] [CrossRef]
- Abd El, F.E.Z.A.; Bashandy, S.R. Dose-dependent effects of Pseudomonas trivialis rhizobacteria and synergistic growth stimulation effect with earthworms on the common radish. Rhizosphere 2019, 10, 100156. [Google Scholar] [CrossRef]
- Kurabachew, H.; Wydra, K. Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacearum. Biol. Control 2013, 67, 75–83. [Google Scholar] [CrossRef]
- CSIR-North East Institute of Science and Technology. Development of Commercial Bioformulation of PGPR Using Coir Pith as a Carrier; Project Report Supported by Central Coir Research Institute, Coir Board, Kalavoor, Ministry of MSME; Govt. of India: Kalavoor, India, 2018; Available online: https://www.ccriindia.org/pdf/NIEST.pdf (accessed on 13 May 2025).
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; Abd_Allah, E.F. Impact of soil salinity on the plant-growth–promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 2017, 24, 1601–1608. [Google Scholar] [CrossRef]
- Meena, P.; Rai, A.K. Biochemical analysis of PGPR and its effect on chlorophyll, ascorbic acid, starch & total polyphenolic content (TPC) of different varieties of wheat (Triticum aestivum). Pharma Innov. 2017, 6 Pt E, 310. [Google Scholar]






| Isolate Name | Growth-Promoting Activities | Biocontrol Activity | Antagonistic Effect of F. oxysporum (SR266-9) | ||
|---|---|---|---|---|---|
| Ammonia Production | EPS Production | HCN | Fungal Growth Diameter (cm) | Growth Inhibition (%) | |
| Control | − | − | − | 4.6 | 0 |
| So-1 | ++++ | + | +++ | 3.2 | 30.4 ± 0.85 a |
| So-12 | ++++ | + | + | − | − |
| So-21 | − | + | + | − | − |
| Sr-17 | ++ | + | − | 3.1 | 32.6 ± 1.15 a |
| Ha-1 | + | − | − | − | − |
| Ha-2 | +++ | + | ++ | 2.9 | 36.9 ± 1.00 b |
| Un-7 | + | − | − | − | − |
| Bl-2 | ++ | − | ++++ | − | − |
| Bl-9 | ++ | + | − | − | − |
| Ka-2 | − | − | − | − | − |
| Treatment Code | PGPR Strain/Formulation | Description |
|---|---|---|
| T1 | Serratia marcescens So-1 | Single isolate |
| T2 | Enterobacter sp. So-12 | Single isolate |
| T3 | Enterobacter cloacae Ha-2 | Single isolate |
| T4 | So-1 + So-12 + Ha-2 | Consortium |
| T5 | So-1 + So-12 | Dual combination |
| T6 | So-1 + Ha-2 | Dual combination |
| T7 | So-12 + Ha-2 | Dual combination |
| Control (C) | --- | Uninoculated control |
| Shoot Length (cm) | Root Length (cm) | Fresh Weight of Shoot (g) | Fresh Weight of Root (g) | Dry Weight of Shoot (g) | Dry Weight of Root (g) | |
|---|---|---|---|---|---|---|
| Shoot length (cm) | 1 | |||||
| Root length (cm) | 0.633 | 1 | ||||
| Fresh weight of shoot (g) | 0.812 | 0.733 | 1 | |||
| Fresh weight of root (g) | 0.621 | 0.855 | 0.797 | 1 | ||
| Dry weight of shoot (g) | 0.920 | 0.796 | 0.893 | 0.744 | 1 | |
| Dry weight of root (g) | 0.784 | 0.874 | 0.908 | 0.793 | 0.924 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, I.; Kumar, V.; Singh, S.; Sharma, A.J.; Kumari, S.; Bhardwaj, N.; Dulta, K.; Peter, L.; Verma, R.; Kumar, N.; et al. Evaluation of Stress-Tolerant Serratia and Enterobacter as PGPR for Nutrient Solubilization and Dose-Dependent Bioformulation to Enhance Tomato Seedlings. Plants 2025, 14, 2154. https://doi.org/10.3390/plants14142154
Bhardwaj I, Kumar V, Singh S, Sharma AJ, Kumari S, Bhardwaj N, Dulta K, Peter L, Verma R, Kumar N, et al. Evaluation of Stress-Tolerant Serratia and Enterobacter as PGPR for Nutrient Solubilization and Dose-Dependent Bioformulation to Enhance Tomato Seedlings. Plants. 2025; 14(14):2154. https://doi.org/10.3390/plants14142154
Chicago/Turabian StyleBhardwaj, Indu, Vijay Kumar, Somvir Singh, Arti Jamwal Sharma, Shikha Kumari, Nidhi Bhardwaj, Kanika Dulta, Lukas Peter, Richa Verma, Nitesh Kumar, and et al. 2025. "Evaluation of Stress-Tolerant Serratia and Enterobacter as PGPR for Nutrient Solubilization and Dose-Dependent Bioformulation to Enhance Tomato Seedlings" Plants 14, no. 14: 2154. https://doi.org/10.3390/plants14142154
APA StyleBhardwaj, I., Kumar, V., Singh, S., Sharma, A. J., Kumari, S., Bhardwaj, N., Dulta, K., Peter, L., Verma, R., Kumar, N., Ahlawat, Y. K., Malik, A., Okla, M. K., Porcel, R., Mulet, J. M., & Jayabalan, K. (2025). Evaluation of Stress-Tolerant Serratia and Enterobacter as PGPR for Nutrient Solubilization and Dose-Dependent Bioformulation to Enhance Tomato Seedlings. Plants, 14(14), 2154. https://doi.org/10.3390/plants14142154










