Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species
Abstract
1. Introduction
2. Materials and Methods
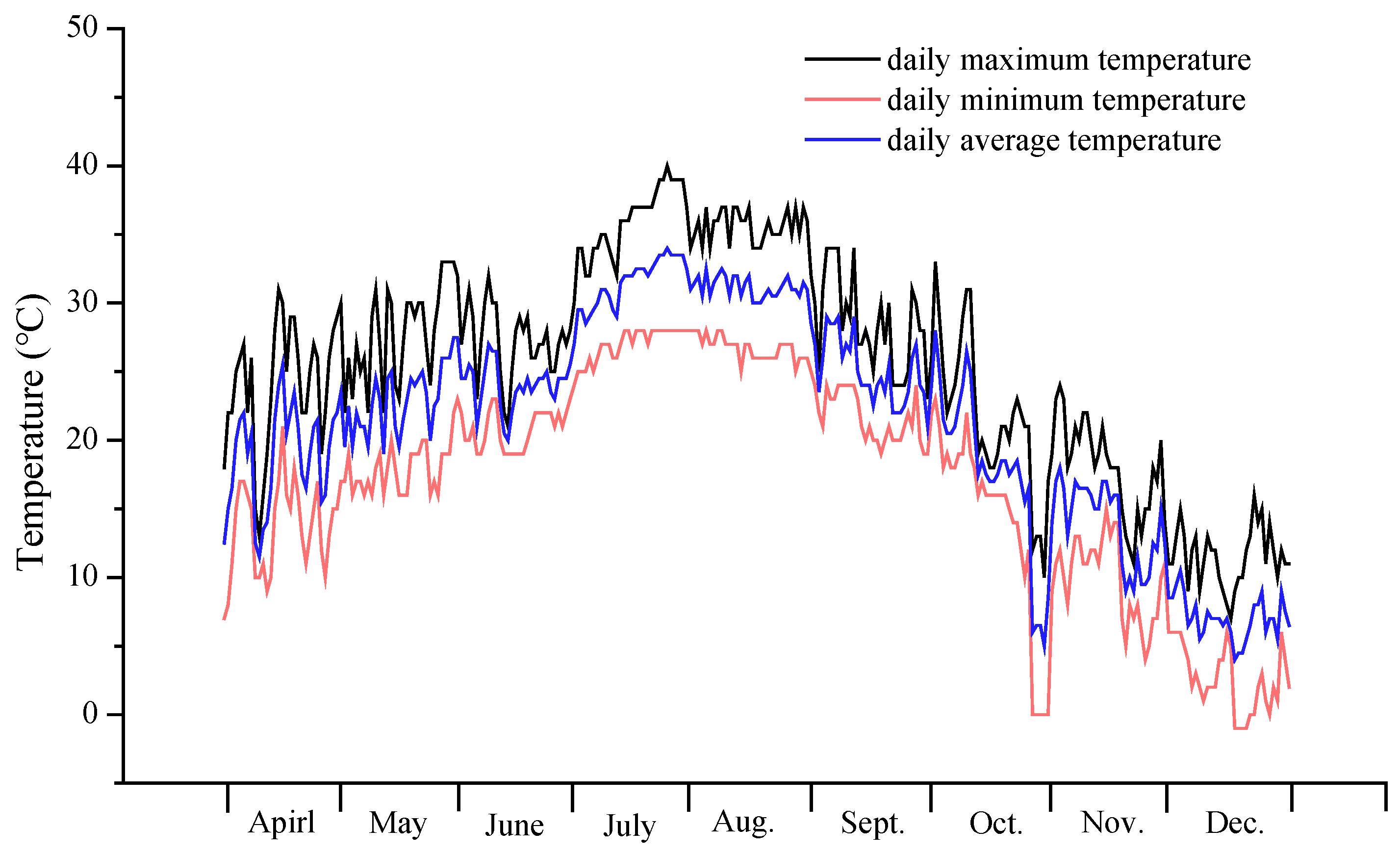
2.1. Experimental Setup
2.2. Harvest and Measurements
2.3. Statistical Analysis
3. Results
3.1. Effects of N Deposition on the Biomass Allocation of Moso Bamboo and Chinese Fir Seedlings
3.2. Effects of N Deposition on the Root Morphology and Architecture of Moso Bamboo and Chinese Fir Seedlings
3.3. Effects of N Deposition on the Allocation of Nutrient Elements and Non-Structural Carbohydrates of Moso Bamboo and Chinese Fir Seedlings
3.4. Effects of N Deposition on the Nutrient Accumulation Ratio in Different Organs of Moso Bamboo and Chinese Fir Seedlings
4. Discussion
4.1. Responses of Biomass Allocation to N Deposition
4.2. Responses of Root Morphology and Architecture to N Deposition
4.3. Responses of Nutrient Element and Non-Structural Carbohydrate Allocation to Different Soil N Deposition
4.4. Responses of Non-Structural Carbohydrate Allocation to Different Soil N Deposition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Nie, W.; Huang, X.; Ding, A.; Qin, B.; Fu, C. Atmospheric Reactive Nitrogen Deposition from 2010 to 2021 in Lake Taihu and the Effects on Phytoplankton. Environ. Sci. Technol. 2023, 57, 8075–8084. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Millet, D.B.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.F.; Tombeur, F.; Lambers, H.; Lu, X.F.; Lai, Y.; Kuang, Y.W. Atmospheric nitrogen deposition: What are the impacts on silicon dynamics in a subtropical forest? Plant Soil 2025, 509, 433–448. [Google Scholar] [CrossRef]
- Shen, H.; Dong, S.; DiTommaso, A.; Xiao, J.N.; Wen, L.; Zhi, Y.L. Nitrogen deposition shifts grassland communities through directly increasing dominance of graminoids: A3-Year case study from the Qinghai-Tibetan Plateau. Front. Plant Sci. 2022, 13, 811970. [Google Scholar] [CrossRef]
- Verkroost, A.W.M.; Wassen, M.J. A simple model for nitrogen-limited plant growth and nitrogen allocation. Ann. Bot. 2005, 96, 871–876. [Google Scholar] [CrossRef]
- Tian, D.; Lin, Q.H.; Zhao, C.T.; Ruide, Z.; Ma, S.H.; Yu, Q.S.; Ji, C.J.; Shen, H.H. Effects of nitrogen addition on the key carbon sequestration process in a tropical montane rainforest in Hainan Province of southern China: A case study of photosynthesis of dominant trees Cryptocarya chinensis and Gironniera subaequalis. J. Beijing For. Univ. 2022, 44, 93–101. [Google Scholar][Green Version]
- Guo, X.H.; Liu, H.; Ngosong, C.; Li, B.; Wang, Q.; Zhou, W.N.; Nie, M. Response of plant functional traits to nitrogen enrichment under climate change: A meta-analysis. Sci. Total Environ. 2022, 834, 155379. [Google Scholar] [CrossRef]
- Hong, S.B.; Gan, P.; Chen, A. Environmental controls on soil pH in planted forest and its response to nitrogen deposition. Environ. Res. 2019, 172, 159–165. [Google Scholar] [CrossRef]
- Staude, I.R.; Waller, D.M.; Bernhardt-Romermann, M.; Bjorkman, A.D.; Brunet, J.; De Frenne, P.; Háéšdl, R.; Jandt, U.; Lenoir, J.; Máliš, F.; et al. Replacements of small- by large-ranged species scale up to diversity loss in Europe’s temperate forest biome. Nat. Ecol. Evol. 2020, 4, 802–808. [Google Scholar] [CrossRef]
- Lu, G.; Xie, B.; Cagle, G.A.; Wang, X.H.; Han, G.X.; Wang, X.J.; Hou, A.X.; Guan, B. Effects of simulated nitrogen deposition on soil microbial community diversity in coastal wetland of the Yellow River Delta. Sci. Total Environ. 2021, 757, 143825. [Google Scholar] [CrossRef] [PubMed]
- Agren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef]
- Zheng, W.H.; Li, R.S.; Yang, Q.P.; Zhang, W.D.; Huang, K.; Guan, X.; Chen, L.C.; Yu, X.; Wang, Q.K.; Wang, S.L. Allocation patterns of nonstructural carbohydrates in response to CO2 elevation and nitrogen deposition in Cunninghamia lanceolata saplings. J. For. Res. 2023, 34, 87–98. [Google Scholar] [CrossRef]
- Ohse, B.; Jansen, D.; Härdtle, W.; Fichtner, A. Interactive effects of nitrogen deposition and climate change on a globally rare forest geophyte. Plant Biol. 2025, 27, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.Q.; Li, Q.; Li, Y.; Li, J.; Adomako, M.O.; Dai, Z.C.; Li, G.L.; Wan, L.Y.; Zhang, B.; Zou, C.B.; et al. The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 2019, 261, 151486. [Google Scholar] [CrossRef]
- Mao, J.H.; Xing, Y.J.; Ma, H.Y.; Wang, Q.G. Research progress of nitrogen deposition effect on plant growth. Chin. Agric. Sci. Bull. 2017, 33, 42–48. [Google Scholar]
- Ma, J.; Fan, W.G. Effects of different ratios of nitrate and ammonium on the dynamic kinetic and growth for Eriobotrya japonica Lindl. Seedlings. Sci. Agric. Sin. 2016, 49, 1152–1162. [Google Scholar]
- Liu, H.; Liu, Q.; Gao, X.H.; Fu, X.D. Role of nitrogen sensing and its integrative signaling pathways in shaping root system architecture. Front. Agric. Sci. Eng. 2022, 9, 316–332. [Google Scholar]
- Yuan, H.; Wang, J.Y. Effect of Nitrate Supply on Root Growth of Maize Inbred Line Zheng58. J. Agric. Sci. Technol. 2013, 15, 150–156. [Google Scholar]
- Zhang, H.F.; Huang, J.X.; Li, Y.M.; Zhao, J.L.; Mai, W.T.; Khan, L.; Zhang, M.; Zeng, C.Y.; Chen, X. Beyond nitrate transport: AtNRT2.4 responds to local and systemic nitrogen signaling in Arabidopsis. BMC Plant Biol. 2025, 25, 655. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; deVoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhao, J.C.; Ni, H.J.; Wang, H.; Zhou, B.Z. Three subtropical species adapt to drought by reallocating biomass and adjusting root architecture. Forests 2023, 14, 806. [Google Scholar] [CrossRef]
- Garcia, G.; Treccarichi, S.; Calì, R.; Arena, D.; Tribulato, A.; Branca, F. Nitrogen use efficiency of microbial and amino acid treatments for organic broccoli (Brassica oleracea L. var. italica Plenk) seed production. Horticulturae 2025, 11, 253. [Google Scholar] [CrossRef]
- Dong, J.L.; Han, X.; Xie, Y.X.; Chen, J.; Li, Y.P.; Lei, J. Effects of nitrogen addition on root morphology and nutrient content of roots and leaves of leguminous seedlings with different nitrogen requirements. Chin. J. Ecol. 2024, 43, 1255–1262. [Google Scholar]
- Sun, W.L.; Li, Y.N.; Xu, Z.H.; Bai, Y.F.; Bai, S.H. Biochar application for enhancing water and nitrogen use efficiency of understory acacia species in a suburban native forest subjected to nitrogen deposition in Southeast Queensland. Plant Soil 2024, 504, 607–624. [Google Scholar] [CrossRef]
- Teng, Q.M.; Lu, X.N.; Zhang, Q.Q.; Cai, L.L.; Sardar, M.F.; Li, Y.F.; Abbas, T.; Li, Y.; Chang, S.X.; Li, Y.C. Litterfall quality modulates soil ammonium and nitrate supply through altering microbial function in bamboo encroachment of broadleaf forests. Geoderma 2023, 437, 116592. [Google Scholar] [CrossRef]
- Zhong, S.Z.; Xu, Y.Q.; Meng, B.; Loik, M.E.; Ma, J.Y.; Sun, W. Nitrogen addition increases the sensitivity of photosynthesis to drought and re-watering differentially in C3 Versus C4 Grass Species. Front. Plant Sci. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.H.; Xing, Y.J.; Yan, G.Y.; Wang, Q.G. A meta-analysis of the response of terrestrial plant biomass allocation to simulated N deposition. Acta Ecol. Sin. 2018, 38, 3183–3194. [Google Scholar]
- Yu, G.C.; Chen, J.; Li, A.; Wang, S.H.; Song, L.; Shi, X.M.; Yan, J.H.; Xu, M.C.; Xue, Y.W.; Lu, X.K.; et al. Plant morphological and physiological traits are stable in a nitrogen-saturated tropical forest after 18-year nitrogen additions. Plant Soil 2025, 43, 06. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhou, B.Z.; Ge, X.G.; Cao, Y.H.; Brunner, I.; Shi, J.X.; Li, M.H. Species-Speciffc responses of root morphology of three co-existing tree species to nutrient patches reflect their root foraging strategies. Front. Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef]
- Song, X.Z.; Peng, C.H.; Ciais, P.; Li, Q.; Xiang, W.H.; Xiao, W.F.; Zhou, G.M.; Deng, L. Nitrogen addition increased CO2 uptake more than non-CO2 greenhouse gases emissions in a moso bamboo forest. Sci. Adv. 2020, 18, eaaw5790. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Stickland, T.R.; Harvey, M.L.; Wilson, G.W. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytol. 1991, 118, 375–382. [Google Scholar] [CrossRef]
- Bouma, T.J.; Nielsen, K.L.; Hal, J.V.; Koutstaal, B. Root system topology and diameter distribution of species from habitats differing in inundation frequency. Funct. Ecol. 2001, 15, 360–369. [Google Scholar] [CrossRef]
- Luo, F.; Gong, X.J.; Zhang, T.; Du, X. Effects of nitrogen, phosphorus and potassium on photo-biological characteristics and amino acid components of tea plants in spring. J. Plant Nutr. Fertil. 2015, 21, 147–155. [Google Scholar]
- Li, M.H.; Xiao, W.F.; Wang, S.G.; Cheng, G.W.; Cherubini, P.; Cai, X.H. Mobile carbohydrates in Himalayan treeline trees I. evidence for carbon gain limitation but not for growth limitation. Tree Physiol. 2008, 28, 1287–1296. [Google Scholar] [CrossRef]
- Zhang, C.C.; Gu, R.; Lin, L.X.; Russo, S.E. Functional traits and ecological niches as correlates of the interspecific growth–mortality trade-off among seedlings of 14 tropical tree species. Funct. Ecol. 2024, 38, 1888–1901. [Google Scholar] [CrossRef]
- Hoopen, F.T.; Cuin, T.A.; Pedas, P.; Hegelund, J.N.; Shabala, S.; Schjoerring, J.K.; Jahn, T.P. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: Molecular mechanisms and physiological consequences. J. Exp. Bot. 2010, 61, 2303–2315. [Google Scholar] [CrossRef]
- Kvakić, M.; Tzagkarakis, G.; Pellerin, S.; Ciais, P.; Goll, D.; Mollier, A.; Ringeval, B. Carbon and phosphorus allocation in annual plants: An optimal functioning approach. Front. Plant Sci. 2020, 11, 149. [Google Scholar] [CrossRef]
- Wang, S.Q.; Han, X.Z.; Qiao, Y.F.; Yan, J.; Li, X.H. Root morphology and nitrogen accumulation in soybean (Glycine max L.) under different nitrogen application levels. Chin. J. Eco-Agric. 2009, 17, 1069–1073. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.M.; Wei, S.C.; Wang, F.L.; Zhou, L.; Li, H.N. Effects of different N rates on the absorption, allocation and utilization of urea-15N in M. hupehensis Rehd. (Yan Fu3/M26) in the current year and next year. J. Plant Nutr. Fertil. 2014, 20, 407–413. [Google Scholar]
- Song, P.; Zhang, Y.; Zhang, R.; Zhou, Z.C.; Feng, Z.P. Responses of phosphorus efficiency to simulated nitrogen deposition under phosphorus deficiency in Pinus massoniana clones. J. Plant Nutr. Fertil. 2017, 23, 502–511. [Google Scholar]
- Li, M.Y.; Wang, J.; Wang, Z.X.; Wu, X.Y.; Huang, R.Z.; Zhu, J.M. Photosynthetic characteristics, biomass allocation, C, N and P distribution of Schima superbaseedlings in response to simulated nitrogen deposition. Acta Ecol. Sin. 2013, 33, 1569–1572. [Google Scholar]
- Liu, X.X.; Zhang, H.H.; Zhu, Q.Y.; Ye, J.Y.; Zhu, Y.X.; Jing, X.T.; Du, W.X.; Zhou, M.; Lin, X.Y.; Zheng, S.J.; et al. Phloem iron remodels root development in response to ammonium as the major nitrogen source. Nat. Commun. 2022, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, K.; Yu, S.Q.; Ruan, H.H.; Fan, H.; Yang, Y.; Xu, C.B.; Cao, G.H. Allocation of fine root biomass and its response to nitrogen deposition in poplar plantations with different stand ages. Chin. J. Ecol. 2014, 33, 583–591. [Google Scholar]
- Chen, M.; Cheng, H.Z.; Yao, X.D.; Cao, L.R.; Chen, R.; Chen, G.S.; Wang, X.H. Effects of soil warming and nitrogen addition on the morphological and chemical characteristics of fine roots in different order classes of the Chinese fir. Acta Ecol. Sin. 2023, 43, 1874–1883. [Google Scholar]
- Xin, W.; Zhang, L.; Gao, J.P.; Zhang, W.Z.; Yi, J.; Zhen, X.X.; Bi, C.Y.; He, D.; Liu, S.M.; Zhao, X.Y. Adaptation mechanism of roots to low and high nitrogen revealed by proteomic analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; Wirén, N. Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, W.; Ou, S.J.; Tang, J.Y.; Li, H.; Che, R.H.; Zhang, Z.H.; Chai, X.Y.; Wang, H.R.; Wang, Y.Q.; et al. Natural variation in NRT1.1B contributes to nitrate-use efficiency in rice. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Yang, J.J.; Liu, H.Y.; Sardans, J.; Zhang, Y.H.; Wang, X.B.; Wei, C.Z.; Lü, X.T.; Dijkstra, F.A.; Jiang, Y.; et al. Nitrogen enrichment buffers phosphorus limitation by mobilizing mineral-bound soil phosphorus in grasslands. Ecology 2022, 103, e3616. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Xiao, X.L.; Zhang, J.Q.; Satheesh, V.; Meng, F.X.; Gao, W.L.; Don, J.S.; Zheng, Z.; An, G.Y.; Nussaume, L.; Liu, D.; et al. SHORT-ROOT stabilizes PHOSPHATE1 to regulate phosphate allocation in Arabidopsis. Nat. Plants 2022, 8, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Wang, J.; Wu, H.H.; Yang, T.; An, Y.X.; Zhang, Y.L.; Bian, J.L.; Li, Y.; Ren, H.Y.; Lkhagva, A.; et al. Nitrogen addition alters aboveground C:N:P stoichiometry of plants but not for belowground in an Inner Mongolia grassland. J. Plant Ecol. 2024, 17, rtad041. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhang, Z.H.; Yang, R. Amount of irrigation and nitrogen application for maize grown on sandy farmland in the marginal oasis in the middle of Heihe river basion. Acta Agron. Sin. 2007, 33, 2007–2015. [Google Scholar]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.B.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.P.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Du, X.B.; Zhang, X.Y.; Xi, M.; Kong, L.C. Split application enhances sweetpotato starch production by regulating the conversion of sucrose to starch under reduced nitrogen supply. Plant Physiol. Biochem. 2020, 151, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Tarumoto, M.B.; Campos, M.; Momesso, L.; Nascimento, C.A.C.; Garcia, A.; Coscolin, R.B.S.C.; Martello, J.M.; Crusciol, C.A.C. Carbohydrate partitioning and antioxidant substances synthesis clarify the differences between sugarcane varieties on facing low phosphorus availability. Front. Plant Sci. 2022, 13, 888432. [Google Scholar] [CrossRef]
- Li, C.H.; Chang, Y.L.; Luo, Y.L.; Li, W.Q.; Jin, M.; Wang, Y.Y.; Cui, H.X.; Sun, S.F.; Li, Y.; Wang, Z.L. Nitrogen regulates stem lodging resistance by breaking the balance of photosynthetic carbon allocation in wheat. Field Crops Res. 2023, 296, 108908. [Google Scholar] [CrossRef]





| Indicators | Treatments | Periods | Species | Treatments × Periods | Treatments × Species | Periods × Species | Treatments × Periods × Species |
|---|---|---|---|---|---|---|---|
| RB | 4.02 * | 736.65 ** | 1457.26 ** | 2.776 * | 2.139 | 345.61 ** | 1.66 |
| SB | 3.93 * | 369.19 ** | 1428.21 ** | 4.06 * | 2.79 * | 244.06 ** | 3.33 * |
| LB | 24.59 ** | 832.05 ** | 3502.02 ** | 14.38 ** | 23.26 ** | 733.01 ** | 13.73 ** |
| Root–shoot ratio | 15.54 ** | 724.44 ** | 7128.97 ** | 19.67 ** | 4.56 ** | 335.20 ** | 39.49 ** |
| RL | 19.19 ** | 548.63 ** | 328.85 ** | 15.30 ** | 1.69 | 7.81 ** | 1.12 |
| RSA | 6.60 ** | 487.56 ** | 1020.43 ** | 4.67 ** | 5.51 ** | 118.14 ** | 4.61 ** |
| RD | 1.64 | 94.57 ** | 1691.84 ** | 2.58 | 3.59 * | 34.75 ** | 1.52 |
| SRL | 9.06 ** | 223.10 ** | 701.00 ** | 2.91 * | 13.135 ** | 115.35 ** | 0.94 |
| RT | 8.52 ** | 830.04 ** | 18.71 ** | 3.924 * | 19.91 ** | 37.41 ** | 16.03 ** |
| FD | 1.82 | 187.85 ** | 835.32 ** | 1.05 | 1.03 | 39.05 ** | 0.594 |
| RBA | 1.32 | 0.29 | 243.94 ** | 0.22 | 0.31 | 0.45 | 0.27 |
| TI | 5.15 ** | 3.32 | 21.42 ** | 0.51 | 0.31 | 0.32 | 0.61 |
| Leaves N | 112.24 ** | 11.94 ** | 354.04 ** | 50.01 ** | 17.35 ** | 12.65 ** | 0.92 |
| Stems N | 70.96 ** | 5.90 * | 76.71 ** | 6.67 ** | 12.95 ** | 4.34 * | 1.34 |
| Roots N | 57.35 ** | 0.04 | 95.93 ** | 9.57 ** | 2.04 | 4.86 * | 1.61 |
| Leaves P | 60.09 ** | 13.50 ** | 228.90 ** | 1.61 | 0.93 | 44.77 ** | 2.19 |
| Stems P | 59.99 ** | 36.08 ** | 22.99 ** | 1.04 | 1.55 | 1.42 | 0.99 |
| Roots P | 11.82 ** | 28.89 ** | 5.60 * | 0.01 | 3.36 * | 2.61 | 0.14 |
| Leaves N/P | 155.47 ** | 3.98 | 0.61 | 21.00 ** | 22.91 ** | 50.18 ** | 2.30 |
| Stems N/P | 118.01 ** | 35.54 ** | 101.00 ** | 7.33 ** | 18.28 ** | 2.55 | 1.63 |
| Roots N/P | 70.81 ** | 22.37 ** | 22.35 ** | 5.45 ** | 5.97 ** | 7.06 ** | 1.12 |
| Leaves SS | 11.15 ** | 23.52 ** | 1514.1 ** | 1.00 | 0.51 | 20.19 ** | 0.14 |
| Stems SS | 40.40 ** | 2.98 | 164.67 ** | 5.98 ** | 6.06 ** | 15.24 ** | 3.83 |
| Roots SS | 25.66 ** | 0.55 | 539.51 ** | 9.07 ** | 24.60 ** | 0.70 | 5.63 ** |
| Leaves ST | 24.81 ** | 27.07 ** | 272.65 ** | 0.684 | 0.647 | 4.91 * | 0.10 |
| Stems ST | 13.84 ** | 56.33 ** | 1853.57 ** | 1.04 | 5.05 ** | 0.003 | 0.397 |
| Roots ST | 47.24 ** | 19.38 ** | 366.57 ** | 1.04 | 7.84 ** | 2.13 | 0.76 |
| Leaves SS/ST | 50.18 ** | 62.01 ** | 1778.31 ** | 3.73 * | 5.38 ** | 19.62 ** | 1.14 |
| Stems SS/ST | 73.57 ** | 54.27 ** | 1862.53 ** | 6.42 ** | 16.50 ** | 1.50 | 5.42 ** |
| Roots SS/ST | 112.23 ** | 33.80 ** | 1253.38 ** | 13.52 ** | 33.02 ** | 8.66 ** | 4.83 ** |
| Leaves N accumulation ratio | 5.03 ** | 218.89 ** | 2322.66 ** | 0.71 | 3.47 * | 34.47 ** | 5.49 ** |
| Stems N accumulation ratio | 0.77 | 5.67 ** | 1.23 | 4.06 * | 3.01 * | 0.10 | 0.59 |
| Roots N accumulation ratio | 8.03 ** | 370.99 ** | 3069.56 ** | 3.03 * | 0.04 | 48.25 ** | 8.04 ** |
| Leaves P accumulation ratio | 9.12 ** | 314.66 ** | 2563.20 ** | 6.57 ** | 8.12 ** | 606.00 ** | 6.34 ** |
| Stems P accumulation ratio | 6.77 ** | 424.20 ** | 28.00 ** | 6.41 ** | 5.09 ** | 506.11 ** | 4.68 ** |
| Roots P accumulation ratio | 8.48 ** | 657.11 ** | 127.79 ** | 8.22 ** | 9.725 ** | 473.75 ** | 11.02 ** |
| Periods | Nutrient Content and Ratio | Treatments | Moso Bamboo | Chinese Fir | ||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Leaves | Stems | Roots | |||
| July | P (mg·g−1) | N0 | 1.174 ± 0.075 a | 0.626 ± 0.034 a | 0.696 ± 0.037 a | 1.090 ± 0.057 a | 0.605 ± 0.022 a | 0.739 ± 0.037 a |
| N1 | 1.098 ± 0.101 ab | 0.588 ± 0.048 ab | 0.639 ± 0.061 a | 0.983 ± 0.086 ab | 0.528 ± 0.035 b | 0.667 ± 0.022 b | ||
| N2 | 0.966 ± 0.055 bc | 0.537 ± 0.032 b | 0.624 ± 0.025 a | 0.867 ± 0.028 b | 0.500 ± 0.020 b | 0.641 ± 0.041 bc | ||
| N3 | 0.950 ± 0.047 c | 0.516 ± 0.040 b | 0.632 ± 0.069 a | 0.750 ± 0.023 c | 0.451 ± 0.029 c | 0.587 ± 0.056 c | ||
| N (mg·g−1) | N0 | 21.96 ±1.23 a | 7.37 ± 0.55 a | 9.85 ± 0.27 a | 14.64 ±0.55 c | 7.56 ± 0.55 b | 10.86 ± 0.41 c | |
| N1 | 22.26 ± 1.28 a | 7.45 ± 0.39 a | 10.18 ± 0.49 a | 16.20 ± 0.79 b | 8.89 ± 0.80 ab | 12.02 ± 0.57 b | ||
| N2 | 22.84 ± 1.03 a | 8.08 ± 0.75 a | 10.75 ± 0.66 a | 17.14 ± 0.42 b | 10.51 ± 1.29 a | 12.42 ± 0.77 ab | ||
| N3 | 21.86 ± 0.91 a | 8.01 ± 0.39 a | 10.52 ± 0.82 a | 18.78 ± 0.53 a | 11.06 ± 0.68 a | 13.42 ± 0.82 a | ||
| N/P | N0 | 18.73 ±0.96 b | 11.77 ± 0.72 b | 14.18 ± 0.65 b | 13.46 ±0.93 d | 12.49 ± 0.61c | 14.74 ± 1.17 c | |
| N1 | 20.39 ± 2.02 ab | 12.69 ± 1.01 b | 16.01 ± 1.17 a | 16.55 ± 1.30 c | 16.90 ± 1.66 b | 18.01 ± 0.72 b | ||
| N2 | 23.72 ±1.87 a | 15.02 ± 0.69 a | 17.22 ± 0.64 a | 19.79 ± 0.59 b | 21.04 ± 2.40 a | 19.41 ± 1.18 b | ||
| N3 | 23.06 ± 1.57 a | 15.55 ± 0.80 a | 16.73 ± 1.62 a | 25.06 ± 0.93 a | 24.66 ± 2.65 a | 23.01 ± 2.274 a | ||
| December | P (mg·g−1) | N0 | 1.270 ± 0.090 a | 0.602 ± 0.057 a | 0.613 ± 0.066 a | 0.872 ± 0.056 a | 0.574 ± 0.031 a | 0.700 ± 0.040 a |
| N1 | 1.075 ± 0.047 b | 0.491 ± 0.025 b | 0.571 ± 0.045 a | 0.797 ± 0.034 ab | 0.493 ± 0.031 b | 0.619 ± 0.028 b | ||
| N2 | 1.046 ± 0.054 b | 0.480 ± 0.046 b | 0.555 ± 0.071 a | 0.740 ± 0.075 bc | 0.460 ± 0.022 b | 0.595 ± 0.050 bc | ||
| N3 | 0.976 ± 0.072 b | 0.473 ± 0.029 b | 0.550 ± 0.031 a | 0.668 ± 0.069 c | 0.407 ± 0.015 c | 0.547 ± 0.042 c | ||
| N (mg·g−1) | N0 | 16.35 ± 0.72 c | 6.80 ± 0.41 c | 8.84 ± 0.48 c | 10.48 ± 1.02 d | 7.06 ± 0.43 c | 9.67 ± 0.70 c | |
| N1 | 20.10 ± 0.98 b | 8.30 ± 0.51 b | 10.05 ± 0.37 b | 14.60 ± 0.54 c | 8.12 ± 0.67 b | 11.30 ± 0.43 b | ||
| N2 | 21.35 ± 1.75 ab | 8.83 ± 0.81 ab | 11.33 ± 0.75 ab | 18.30 ± 1.45 b | 11.03 ± 1.10 a | 12.84 ± 0.59 a | ||
| N3 | 24.18 ± 1.55 a | 9.95 ± 0.42 a | 12.33 ± 1.32 a | 23.48 ± 1.50 a | 12.04 ± 0.79 a | 13.56 ± 0.95 a | ||
| N/P | N0 | 12.91 ± 1.00 c | 11.40 ± 1.56 c | 14.57 ± 2.05 c | 12.01 ± 0.64 d | 12.31 ± 0.94 d | 13.83 ± 1.18 d | |
| N1 | 18.72 ± 1.04 b | 16.97 ± 1.88 b | 17.67 ± 1.72 bc | 18.36 ± 1.30 c | 16.54 ± 1.76 c | 18.31 ± 1.34 c | ||
| N2 | 20.46 ± 1.95 ab | 18.57 ± 2.93 ab | 20.57 ± 1.73 ab | 24.99 ± 3.77 b | 24.07 ± 3.19 b | 21.70 ± 1.88 b | ||
| N3 | 24.90 ± 2.68 a | 21.11 ±1.89 a | 22.76 ± 2.52 a | 35.41 ± 3.63 a | 29.66 ± 2.42 a | 24.94 ± 3.09 a | ||
| Periods | NSCs Content and Ratio | Treatments | Moso Bamboo | Chinese Fir | ||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Leaves | Stems | Roots | |||
| July | soluble sugar (%) | N0 | 8.69 ± 0.50 a | 8.20 ± 0.56 a | 6.67 ± 0.37 a | 14.32 ± 0.85 a | 10.78 ± 0.50 a | 12.08 ± 0.93 a |
| N1 | 7.41 ± 0.75 b | 7.36 ± 0.44 b | 7.09 ± 0.39 a | 13.46 ± 0.85 a | 10.00 ± 0.48 ab | 10.85 ± 1.24 ab | ||
| N2 | 7.72 ± 0.28 b | 7.17 ± 0.23 b | 7.20 ± 0.50 a | 13.49 ± 0.91 a | 9.59 ± 1.01 b | 10.77 ± 0.38 b | ||
| N3 | 7.20 ± 0.55 b | 6.95 ± 0.54 b | 6.80 ± 0.40 a | 13.58 ± 1.01 a | 9.23 ± 0.75 b | 10.23 ± 0.73 b | ||
| Starch (%) | N0 | 8.08 ± 0.53 c | 11.81 ± 0.99 b | 8.46 ± 0.51 c | 5.92 ± 0.46 b | 6.86 ± 0.40 a | 6.55 ± 0.42 c | |
| N1 | 8.72 ± 0.79 b | 12.08 ± 0.61 ab | 9.93 ± 0.62 b | 6.65 ± 0.52 ab | 6.88 ± 0.34 a | 7.28 ± 0.48 bc | ||
| N2 | 8.92 ± 0.38 ab | 13.50 ± 0.37 a | 10.53 ± 1.20 ab | 6.93 ± 0.45 a | 7.04 ± 0.26 a | 7.63 ± 0.37 ab | ||
| N3 | 9.43 ± 0.65 a | 13.55 ± 0.81 a | 11.73 ± 1.05 a | 6.86 ± 0.58 a | 7.68 ± 0.70 a | 8.33 ± 0.41 a | ||
| Sugar/starch | N0 | 1.077 ± 0.044 a | 0.695 ± 0.035 a | 0.791 ± 0.068 a | 2.427 ± 0.194 a | 1.574 ± 0.082 a | 1.845 ± 0.112 a | |
| N1 | 0.851 ± 0.068 b | 0.611 ± 0.046 b | 0.715 ± 0.027 b | 2.025 ± 0.076 b | 1.454 ± 0.087 ab | 1.491 ± 0.129 b | ||
| N2 | 0.867 ± 0.053 b | 0.532 ± 0.026 c | 0.687 ± 0.040 b | 1.950 ± 0.121 b | 1.360 ± 0.094 b | 1.387 ± 0.062 bc | ||
| N3 | 0.764 ± 0.042 c | 0.515 ± 0.050 c | 0.582 ± 0.045 c | 1.990 ± 0.225 b | 1.207 ± 0.123 b | 1.230 ± 0.098 c | ||
| December | soluble sugar (%) | N0 | 8.76 ± 0.55 a | 9.08 ± 0.31 a | 7.28 ± 0.38 a | 16.12 ± 0.81 a | 11.81 ± 1.12 a | 13.98 ± 1.59 a |
| N1 | 7.82 ± 0.58 b | 8.33 ± 0.38 b | 7.32 ± 0.32 a | 15.60 ± 0.76 a | 10.47 ± 0.74 a | 11.89 ± 0.828 b | ||
| N2 | 7.63 ± 0.38 b | 8.20 ± 0.53 b | 7.39 ± 0.46 a | 14.95 ± 1.08 a | 8.49 ± 0.77 b | 9.50 ± 0.36 c | ||
| N3 | 7.07 ± 0.60 b | 7.40 ± 0.49 c | 6.83 ± 0.45 a | 14.64 ± 0.97 a | 7.53 ± 0.56 b | 8.31 ± 0.51 d | ||
| Starch (%) | N0 | 7.02 ± 0.53 b | 11.07 ± 0.69 c | 7.45 ± 0.65 c | 5.34 ±0.35 c | 6.05 ± 0.49 a | 5.80 ± 0.46 b | |
| N1 | 7.82 ± 0.34 a | 11.41 ± 0.62 b | 9.60 ± 0.80 b | 6.28 ± 0.42 b | 6.09 ± 0.27 a | 6.59 ± 0.25 a | ||
| N2 | 8.11 ± 0.60 a | 12.13 ± 0.38 b | 10.71 ± 1.03 ab | 6.47 ± 0.23 b | 6.16 ± 0.56 a | 6.81 ± 0.36 a | ||
| N3 | 8.72 ± 0.53 a | 12.39 ± 0.61 a | 11.08 ± 0.81 a | 6.87 ± 0.51 a | 6.27 ± 0.40 a | 7.00 ± 0.49 a | ||
| Sugar/starch | N0 | 1.249 ± 0.028 a | 0.822 ± 0.037 a | 0.981 ± 0.064 a | 3.027 ± 0.023 a | 1.954 ± 0.135 a | 2.415 ± 0.269 a | |
| N1 | 0.999 ± 0.040 b | 0.732 ± 0.052 b | 0.766 ± 0.069 b | 2.491 ± 0.192 b | 1.720 ± 0.082 b | 1.807 ± 0.149 b | ||
| N2 | 0.942 ± 0.034 b | 0.676 ± 0.028 b | 0.692 ± 0.030 bc | 2.316 ± 0.233 b | 1.381 ± 0.112 c | 1.397 ± 0.065 c | ||
| N3 | 0.811 ± 0.059 c | 0.597 ± 0.027 c | 0.618 ± 0.056 c | 2.137 ± 0.136 | 1.206 ± 0.138 c | 1.190 ± 0.095 d | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhou, B. Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species. Plants 2025, 14, 2153. https://doi.org/10.3390/plants14142153
Yang Z, Zhou B. Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species. Plants. 2025; 14(14):2153. https://doi.org/10.3390/plants14142153
Chicago/Turabian StyleYang, Zhenya, and Benzhi Zhou. 2025. "Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species" Plants 14, no. 14: 2153. https://doi.org/10.3390/plants14142153
APA StyleYang, Z., & Zhou, B. (2025). Effects of Simulated Nitrogen Deposition on the Physiological and Growth Characteristics of Seedlings of Two Typical Subtropical Tree Species. Plants, 14(14), 2153. https://doi.org/10.3390/plants14142153




