Secondary Metabolites in Plants
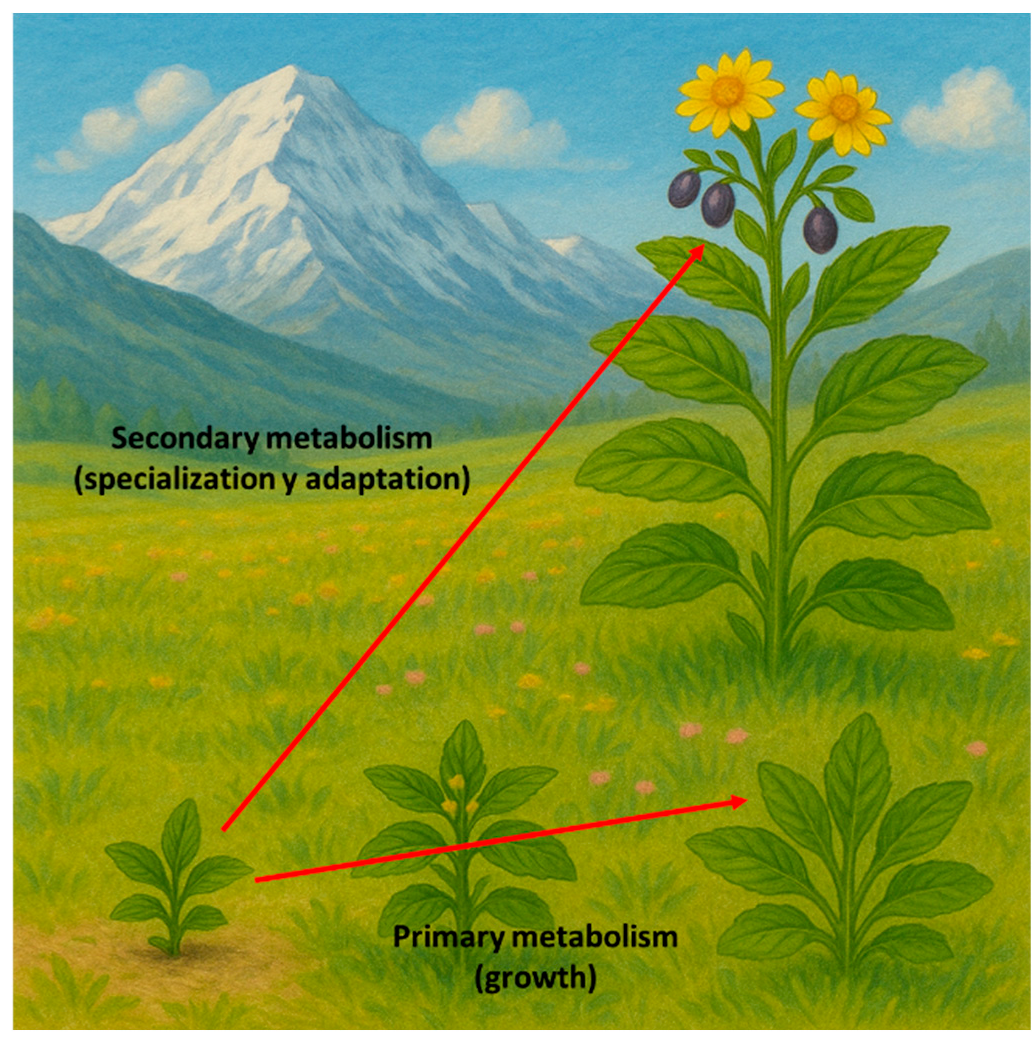
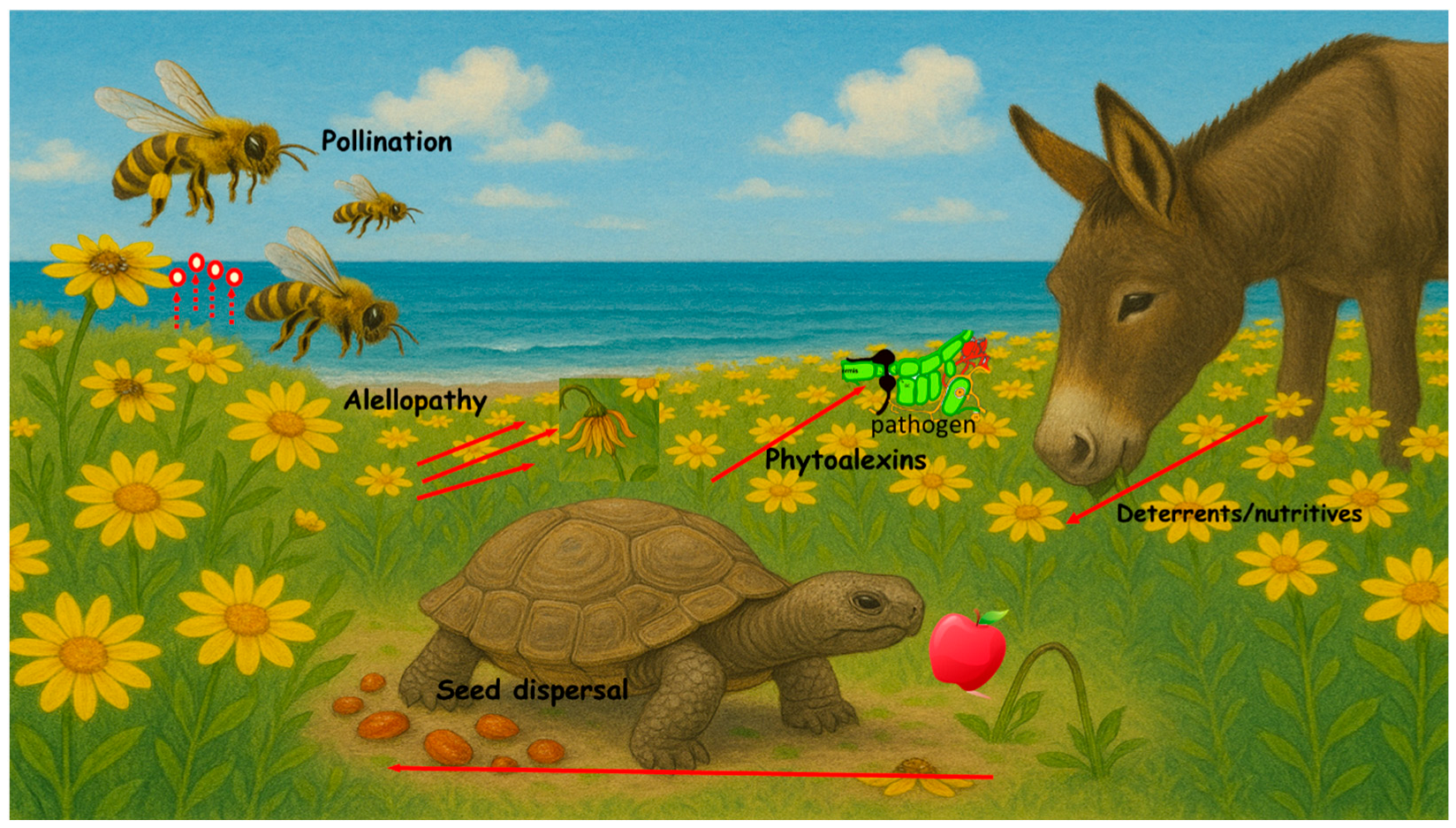
1. Introduction
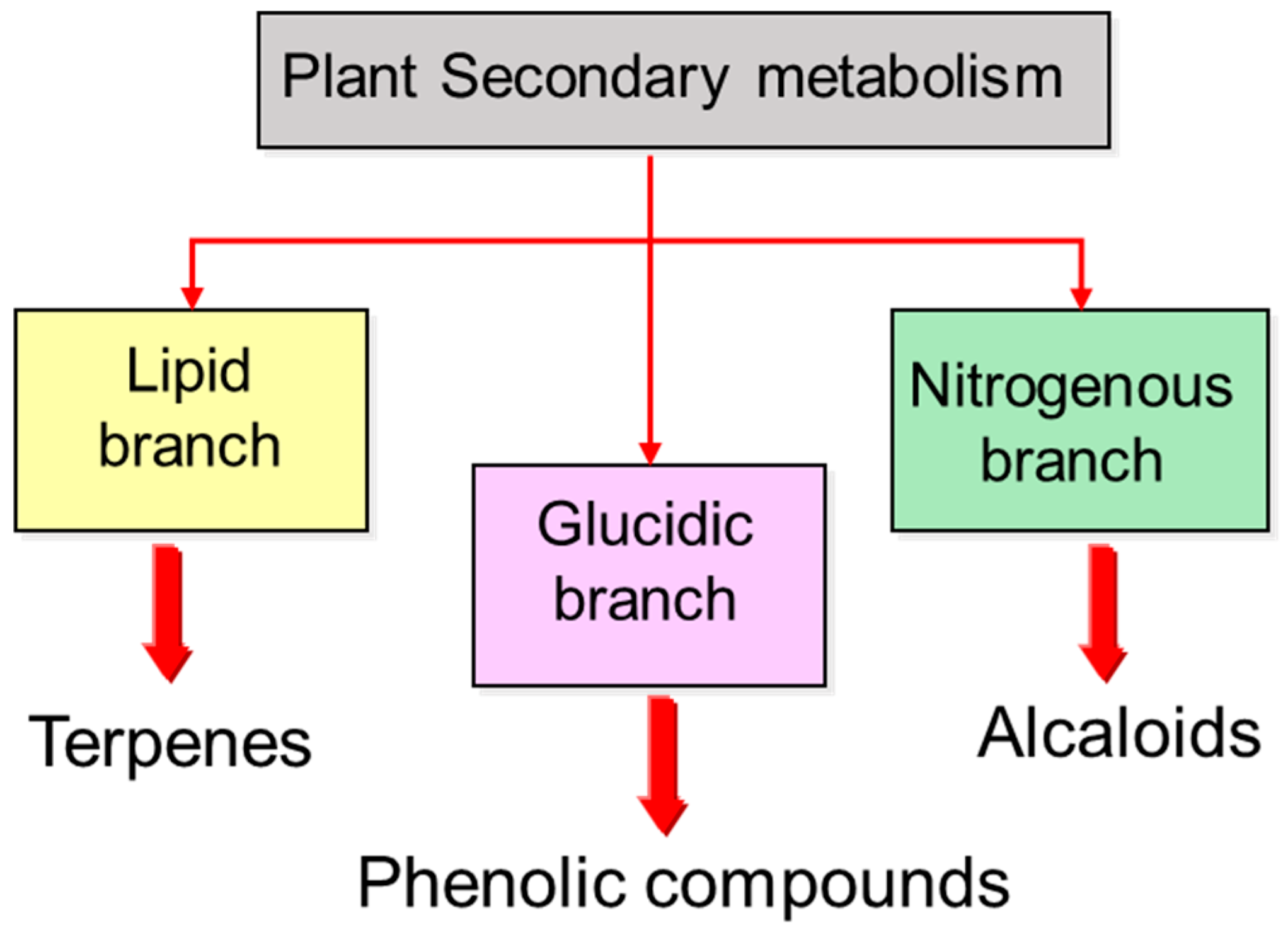
2. Special Issue Overview
2.1. Alkaloid Metabolism
2.2. Phenol Metabolism
2.3. Terpene Metabolism
2.4. Volatile Compounds
3. Concluding Remarks
Conflicts of Interest
References
- Li, H.; Chen, N.; Zhang, H.; Xu, D. Multidimensional regulation of transcription factors: Decoding the comprehensive signals of plant secondary metabolism. Front. Plant Sci. 2025, 16, 1522278. [Google Scholar] [CrossRef]
- Basarllo, O.; Lucido, A.; Sorribas, A.; Marin-Sanguino, A.; Vilaprinyo, E.; Martinez, E.; Eleiwa, A.; Alves, R. Modeling the effect of daytime duration on the biosynthesis of terpenoid precursors. Front. Plant Sci. 2024, 15, 1465030. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. Enhancement of Phytosterol and Triterpenoid Production in Plant Hairy Root Cultures—Simultaneous Stimulation or Competition? Plants 2021, 10, 2028. [Google Scholar] [CrossRef]
- Yang, M.; Wang, M.; Zhou, M.; Zhang, Y.; Yu, K.; Wang, T.; Bu, T.; Tang, Z.; Zheng, T.; Chen, H. ABA and SA Participate in the Regulation of Terpenoid Metabolic Flux Induced by Low-Temperature within Conyza blinii. Life 2023, 13, 371. [Google Scholar] [CrossRef]
- Bapat, V.A.; Kavi Kishor, P.B.; Jalaja, N.; Jain, S.M.; Penna, S. Plant Cell Cultures: Biofactories for the Production of Bioactive Compounds. Agronomy 2023, 13, 858. [Google Scholar] [CrossRef]
- Devi, A.M.; Khedashwori, D.; Prem, D.; Das, S. Metabolic engineering of plant secondary metabolites: Prospects and its technological challenges. Front. Plant Sci. 2023, 14, 1171154. [Google Scholar] [CrossRef]
- Zhang, Y.; Wiese, L.; Fang, H.; Alseekh, S.; de Souza, L.P.; Scossa, F.; Fernie, A. Synthetic biology identifies the minimal gene set required for paclitaxel biosynthesis in a plant chassis. Mol. Plant 2023, 16, 1951–1961. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Su, Z.; Xiong, L.; Wang, P.; Lei, W.; Yan, X.; Ma, D.; Zhao, G.; Zhou, Z. Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol. Nat. Commun. 2024, 15, 2339. [Google Scholar] [CrossRef]
- Májer, P.; Németh, É.Z. Alkaloid Accumulation and Distribution within the Capsules of Two Opium Poppy (Papaver somniferum L.) Varieties. Plants 2024, 13, 1640. [Google Scholar] [CrossRef]
- Petrova, M.; Geneva, M.; Trendafilova, A.; Miladinova-Georgieva, K.; Dimitrova, L.; Sichanova, M.; Nikolova, M.; Ivanova, V.; Dimitrova, M.; Sozoniuk, M. Antioxidant Capacity and Accumulation of Caffeoylquinic Acids in Arnica montana L. In Vitro Shoots After Elicitation with Yeast Extract or Salicylic Acid. Plants 2025, 14, 967. [Google Scholar] [CrossRef]
- Li, S.; Yin, M.; Wang, P.; Gao, L.; Lv, F.; Yang, R.; Li, Y.; Wang, Q.; Li, L.; Liu, Y.; et al. Phenolic Compounds and Antioxidant Capacity Comparison of Wild-Type and Yellow-Leaf gl1 Mutant of Lagerstroemia indica. Plants 2024, 13, 315. [Google Scholar] [CrossRef]
- Zhou, J.; Zou, X.; Deng, Z.; Duan, L. Analysing a Group of Homologous BAHD Enzymes Provides Insights into the Evolutionary Transition of Rosmarinic Acid Synthases from Hydroxycinnamoyl-CoA:Shikimate/Quinate Hydroxycinnamoyl Transferases. Plants 2024, 13, 512. [Google Scholar] [CrossRef]
- Tan, X.; Chen, J.; Zhang, J.; Guo, G.; Zhang, H.; Zhao, X.; Lv, S.; Xu, H.; Hou, D. Gene Expression and Interaction Analysis of FsWRKY4 and FsMAPK3 in Forsythia suspensa. Plants 2023, 12, 3415. [Google Scholar] [CrossRef]
- Suprun, A.R.; Kiselev, K.V.; Aleynova, O.A.; Manyakhin, A.Y.; Ananev, A.A. Analysis of Phenolic Compounds of Reynoutria sachalinensis and Reynoutria japonica Growing in the Russian Far East. Plants 2024, 13, 3330. [Google Scholar] [CrossRef]
- Sharma, A.R.; Gajurel, G.; Abdel-Karim, S.; Alam, M.A.; Shields, R.C.; Medina-Bolivar, F. Production of Malheuran A, a Geranylated Flavonoid with Antimicrobial and Anti-Inflammatory Activities, in Hairy Root Cultures of Dalea purpurea. Plants 2025, 14, 259. [Google Scholar] [CrossRef]
- Morante-Carriel, J.; Živković, S.; Nájera, H.; Sellés-Marchart, S.; Martínez-Márquez, A.; Martínez-Esteso, M.J.; Obrebska, A.; Samper-Herrero, A.; Bru-Martínez, R. Prenylated Flavonoids of the Moraceae Family: A Comprehensive Review of Their Biological Activities. Plants 2024, 13, 1211. [Google Scholar] [CrossRef]
- Khamsaw, P.; Sommano, S.R.; Wongkaew, M.; Willats, W.G.T.; Bakshani, C.R.; Sirilun, S.; Sunanta, P. Banana Peel (Musa ABB cv. Nam Wa Mali-Ong) as a Source of Value-Adding Components and the Functional Properties of Its Bioactive Ingredients. Plants 2024, 13, 593. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Palazon, J.; Bonfill, M.; Hidalgo-Martinez, D. Enhancing Centelloside Production in Centella asiatica Hairy Root Lines through Metabolic Engineering of Triterpene Biosynthetic Pathway Early Genes. Plants 2023, 12, 3363. [Google Scholar] [CrossRef]
- Cheng, R.; Yang, S.; Wang, D.; Qin, F.; Wang, S.; Meng, S. Advances in the Biosynthesis of Plant Terpenoids: Models, Mechanisms, and Applications. Plants 2025, 14, 1428. [Google Scholar] [CrossRef]
- Shakeel, A.; Noor, J.J.; Jan, U.; Gul, A.; Handoo, Z.; Ashraf, N. Saponins, the Unexplored Secondary Metabolites in Plant Defense: Opportunities in Integrated Pest Management. Plants 2025, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Nurcholis, W.; Rahmadansah, R.; Astuti, P.; Priosoeryanto, B.P.; Arianti, R.; Kristóf, E. Comparative Analysis of Volatile Compounds and Biochemical Activity of Curcuma xanthorrhiza Roxb. Essential Oil Extracted from Distinct Shaded Plants. Plants 2024, 13, 2682. [Google Scholar] [CrossRef]
- Sun, Y.-D.; Wallis, C.M.; Krugner, R.; Yokomi, R. Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition. Plants 2025, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Charoimek, N.; Phusuwan, S.; Petcharak, C.; Huanhong, K.; Prasad, S.K.; Junmahasathien, T.; Khemacheewakul, J.; Sommano, S.R.; Sunanta, P. Do Abiotic Stresses Affect the Aroma of Damask Roses? Plants 2023, 12, 3428. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazon, J.; Alcalde, M.A. Secondary Metabolites in Plants. Plants 2025, 14, 2146. https://doi.org/10.3390/plants14142146
Palazon J, Alcalde MA. Secondary Metabolites in Plants. Plants. 2025; 14(14):2146. https://doi.org/10.3390/plants14142146
Chicago/Turabian StylePalazon, Javier, and Miguel Angel Alcalde. 2025. "Secondary Metabolites in Plants" Plants 14, no. 14: 2146. https://doi.org/10.3390/plants14142146
APA StylePalazon, J., & Alcalde, M. A. (2025). Secondary Metabolites in Plants. Plants, 14(14), 2146. https://doi.org/10.3390/plants14142146




