Breeding Wheat (Triticum aestivum L.) for Pre-Harvest Sprouting Tolerance in South Africa: Current Status and Future Prospects
Abstract
1. Introduction
2. Overview of South African Wheat Production and Pre-Harvest Sprouting Tolerance
3. Mechanisms of Pre-Harvest Sprouting Tolerance
4. Breeding Strategies for Pre-Harvest Sprouting Tolerance
4.1. Conventional Breeding
4.2. Marker-Assisted Selection (MAS)
4.3. Genomic Selection
4.4. Integrated Approaches
5. Challenges and Limitations in Breeding for Pre-Harvest Sprouting Tolerance
6. Future Prospects and Research Directions
6.1. Climate-Resilient Breeding
6.2. Technological Advances
6.3. Collaboration and Policy Support
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| ARC-Small Grain | Agricultural Research Council—Small Grain Institute |
| COW | Class Other Wheat |
| CRISPR-Cas9 | clustered regularly interspaced palindromic repeats-associated Cas-9 protein |
| CIMMYT | International Maize and Wheat Improvement Centre |
| DH | doubled-haploid |
| DNA | Deoxyribonucleic acid |
| DSI | Department of Science and Innovation |
| GA | gibberellic acid |
| GEBVs | genomic estimated breeding values |
| GP | genomic prediction |
| GWASs | genome-wide association studies |
| ICARDA | International Centre for Agricultural Research in the Dry Areas |
| ISPHSC | International Symposium on Pre-Harvest Sprouting in Cereals |
| KASP | Kompetitive allele-specific PCR |
| MAS | marker-assisted selection |
| MTA | marker–trait association |
| ND | no data available |
| PHS | pre-harvest sprouting |
| QTL(s) | quantitative trait locus/loci |
| SDG | Sustainable Development Goal |
| SNP | single-nucleotide polymorphism |
| SSR | simple sequence repeat |
| SU | Stellenbosch University |
| TIA | Technology Innovation Agency |
| USDA | United States Department of Agriculture |
References
- Groos, C.; Gay, G.; Perretant, M.R.; Gervais, L.; Bernard, M.; Dedryver, F.; Charmet, G. Study of the relationship between pre-harvest sprouting and grain colour by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor. Appl. Genet. 2002, 104, 39–47. [Google Scholar] [CrossRef]
- Rodriguez, M.V.; Barrero, J.M.; Corbineau, F.; Gubler, R.L.F. Dormancy in cereals (not too much, not so little): About the mechanisms behind this trait. Seed Sci. Res. 2015, 25, 99–119. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Liu, G.; Mia, M.S.; Siddique, K.H.M.; Yan, G. Phenotypic and genotypic characterization of near-isogenic lines targeting a major 4BL QTL responsible for pre-harvest sprouting in wheat. BMC Plant Biol. 2019, 19, 348. [Google Scholar] [CrossRef]
- Yücel, C.; Baloch, F.S.; Hatipoǧlu, R.; Özkan, H. Genetic analysis of preharvest sprouting tolerance in bread wheat (Triticum aestivum L. emend. Thell.). Turk. J. Agric. For. 2011, 35, 9–22. [Google Scholar] [CrossRef]
- Tai, L.; Wang, H.-J.; Xu, X.-J.; Sun, W.-H.; Ju, L.; Liu, W.-T.; Li, W.-Q.; Sun, J.; Chen, K.-M. Pre-harvest sprouting in cereals: Genetic and biochemical mechanisms. J. Exp. Bot. 2021, 72, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Barrero, J.M.; Cavanagh, C.; Verbyla, K.L.; Tibbits, J.F.G.; Verbyla, A.P.; Huang, B.E.; Rosewarne, G.M.; Stephen, S.; Wang, P.; Whan, A.; et al. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biol. 2015, 16, 93. [Google Scholar] [CrossRef]
- Derera, N.F.; Bhatt, G.M.; McMaster, G.J. On the problem of pre-harvest sprouting of wheat. Euphytica 1977, 26, 299–308. [Google Scholar] [CrossRef]
- Torada, A.; Koike, M.; Ikeguchi, S.; Tsutsui, I. Mapping of a major locus controlling seed dormancy using backcrossed progenies in wheat (Triticum aestivum L.). Genome 2008, 51, 426–432. [Google Scholar] [CrossRef]
- Lang, J.; Fu, Y.; Zhou, Y.; Cheng, M.; Deng, M.; Li, M.; Zhu, T.; Yang, J.; Guo, X.; Gui, L.; et al. Myb10-D confers PHS-3D resistance to pre-harvest sprouting by regulating NCED in ABA biosynthesis pathway of wheat. New Phytol. 2021, 230, 1940–1952. [Google Scholar] [CrossRef]
- Lin, M.; Liu, S.; Zhang, G.; Bai, G. Effects of TaPHS1 and TaMKK3-A genes on wheat pre-harvest sprouting resistance. Agronomy 2018, 8, 210. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, W. Wheat alpha amylase. JSM Biotechnol. BioMed. Eng. 2018, 5, 1086. [Google Scholar]
- Simsek, S.; Ohm, J.-B.; Lu, H.; Rugg, M.; Berzonsky, W.; Alamri, M.S.; Mergoum, M. Effect of pre-harvest sprouting on physicochemical properties of starch in wheat. Foods 2014, 3, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Patwa, N.; Penning, B.W. Environmental impact on cereal crop grain damage from pre-harvest sprouting and late maturity alpha-amylase. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 23–41. [Google Scholar] [CrossRef]
- Li, C.; Nonogaki, H.; Barrero, J. Seed dormancy, germination and pre-harvest sprouting. Front. Plant Sci. 2018, 9, 1783. [Google Scholar] [CrossRef]
- Bainotti, C.; Cuniberti, M.; Masiero, B.; Donaire, G.; Gomez, D.; Reartes, F.; Salines, J.; Formica, M.; Fraschina, J.; Nisi, J.; et al. Characterization of wheat cultivars for pre-harvest sprouting. Agriscientia 2009, 26, 29–33. [Google Scholar] [CrossRef]
- Andreoli, C.; Bassoi-Ml, C.; Brunetta, D. Genetic control of seed dormancy and preharvest sprouting in wheat. Sci. Agricola 2006, 63, 564–566. [Google Scholar] [CrossRef]
- Ali, A.; Cao, J.; Jiang, H.; Chang, C.; Zhang, H.-P.; Sheikh, S.W.; Shah, L.; Ma, C. Unraveling molecular and genetic studies of wheat (Triticum aestivum L.) resistance against factors causing pre-harvest sprouting. Agronomy 2019, 9, 117. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Genetics of yield, abiotic stress tolerance and biofortification in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 1569–1602. [Google Scholar] [CrossRef]
- Martinez, S.A.; Godoy, J.; Huang, M.; Zhang, Z.; Carter, A.H.; Campbell, K.A.G.; Steber, C.M. Genome-wide association mapping for tolerance to preharvest sprouting and low falling numbers in wheat. Front. Plant Sci. 2018, 9, 141. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Kumar, T.S.; Zoclanclunon, Y.A.B.; Muthuramalingam, P.; Shilpha, J.; Satish, L.; Ramesh, M. Seed dormancy and pre-harvest sprouting in rice—An updated overview. Int. J. Mol. Sci. 2021, 22, 11804. [Google Scholar] [CrossRef]
- Vetch, J.M.; Stougaard, R.N.; Martin, J.M.; Giroux, M. Allelic impacts of TaPHS1, TaMKK3, and Vp1B3 on preharvest sprouting of northern great plains winter wheats. Crop Sci. 2019, 59, 140–149. [Google Scholar] [CrossRef]
- Vetch, J.M.; Stougaard, R.N.; Martin, J.M.; Giroux, M.J. Review: Revealing the genetic mechanisms of pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.). Plant Sci. 2019, 281, 180–185. [Google Scholar] [CrossRef]
- Singh, A.K.; Knox, R.E.; Clarke, J.M.; Clarke, F.R.; Singh, A.; DePauw, R.M.; Cuthbert, R.D. Genetics of pre-harvest sprouting resistance in a cross of Canadian adapted durum wheat genotypes. Mol. Breed. 2014, 33, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Barnard, A.; Smith, M.F. The effect of rainfall and temperature on the preharvest sprouting tolerance of winter wheat in the dryland production areas of the Free State Province. Field Crops Res. 2009, 112–113, 158–164. [Google Scholar] [CrossRef]
- Jiang, G.L.; Xiao, S. Factorial cross analysis of pre-harvest sprouting resistance in white wheat. Field Crops Res. 2005, 91, 63–69. [Google Scholar] [CrossRef]
- Morgan, G. Pre-Harvest Sprouting in Wheat (E-336 1/05). Texas A&M Agrilife Extension. Available online: https://taylor.agrilife.org/files/2015/01/E336-Pre-Harvest-Sprouting-in-Wheat.pdf (accessed on 26 November 2024).
- Nakamura, S.; Abe, F.; Kawahigashi, H.; Nakazono, K.; Tagiri, A.; Matsumoto, T.; Utsugi, S.; Ogawa, T.; Handa, H.; Ishida, H. A wheat homolog of MOTHER of FT and TFL1 acts in the regulation of germination. Plant Cell 2011, 23, 3215–3229. [Google Scholar] [CrossRef] [PubMed]
- Torada, A.; Ikeguchi, S.; Koike, M. Mapping and validation of PCR-based markers associated with a major QTL for seed dormancy in wheat. Euphytica 2005, 143, 251–255. [Google Scholar] [CrossRef]
- Das, A.; Kim, D.W.; Khadka, P.; Rakwal, R.; Rohila, J.S. Unraveling key metabolomic alterations in wheat embryos derived from freshly harvested and water-imbibed seeds of two wheat cultivars with contrasting dormancy status. Front. Plant Sci. 2017, 8, 1203. [Google Scholar] [CrossRef]
- Fakthongphan, J.; Graybosch, R.A.; Baenziger, P.S. Combining ability for tolerance to pre-harvest sprouting in common wheat (Triticum aestivum L.). Crop Sci. 2016, 56, 1025–1035. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, H.; Cheng, M.-P.; Dankwa, K.O.; Chen, Z.-X.; Li, Z.-Y.; Gao, S.; Liu, Y.-X.; Jiang, Q.-T.; Lan, X.-J.; et al. Genome-wide association study for pre-harvest sprouting resistance in a large germplasm collection of Chinese wheat landraces. Front. Plant Sci. 2017, 8, 401. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Kobayashi, D.; Jikumaru, Y.; Takebayashi, Y.; Nambara, E.; Seo, M.; Kamiya, Y.; Kushiro, T.; Kawakami, N. Highly sprouting-tolerant wheat grain exhibits extreme dormancy and cold imbibition-resistant accumulation of abscisic acid. Plant Cell Physiol. 2016, 57, 715–732. [Google Scholar] [CrossRef]
- Nonogaki, M.; Sall, K.; Nambara, E.; Nonogaki, H. Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds. Plant J. 2014, 78, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Cabral, A.L.; Jordan, M.C.; McCartney, C.A.; You, F.M.; Humphreys, D.G.; MacLachlan, R.; Pozniak, C.J. Identification of candidate genes, regions and markers for pre-harvest sprouting resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 340. [Google Scholar] [CrossRef]
- Rakoczy-Trojanowska, M.; Krajewski, P.; Bocianowski, J.; Schollenberger, M.; Wakulinski, W.; Milczarski, P.; Masojc, P.; Targonska-Karasek, M.; Banaszak, Z.; Banaszak, Z.; et al. Identification of single nucleotide polymorphism associated with brown rust resistance, a-amylase activity and pre-harvest sprouting in rye (Secale cereale L.). Plant Mol. Biol. Rep. 2017, 35, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Sydenham, S.L.; Barnard, A. Targeted Haplotype Comparisons between South African Wheat Cultivars Appear Predictive of Pre-harvest Sprouting Tolerance. Front. Plant Sci. 2018, 9, 63. [Google Scholar] [CrossRef]
- Wahl, T.I.; O’Rourke, A.D. The economics of sprout damage in wheat. Agribusiness 1994, 10, 27–41. [Google Scholar] [CrossRef]
- Black, M.; Beweley, J.D.; Halmer, P. Science, technology and uses. In The Encyclopedia of Seeds: Science, Technology and Uses; CABI Publishing: Wallingford, UK, 2006; pp. 528–530. [Google Scholar]
- Thomason, W.; Hughes, K.R.; Griffey, C.A.; Parrish, J.; Barbeau, W.E.; Walsh, O. Understanding Pre-Harvest Sprouting of Wheat. pp. 1–4. Available online: http://www.ext.vt.edu (accessed on 26 November 2024).
- Perkins, S.E.; Alexander, L.V.; Nairn, J.R. Increasing frequency, intensity and duration of observed global heatwaves and warm spells. Geophys. Res. Lett. 2012, 39, L20714. [Google Scholar] [CrossRef]
- Shorinola, O.; Bird, N.; Simmonds, J.; Berry, S.; Henriksson, T.; Jack, P.; Werner, P.; Gerjets, T.; Scholefield, D.; Balcarkova, B.; et al. The wheat Phs-A1 pre-harvest sprouting resistance locus delays the rate of seed dormancy loss and maps 0.3 cM distal to the PM19 genes in UK germplasm. J. Exp. Bot. 2016, 67, 4169–4178. [Google Scholar] [CrossRef]
- Maity, A.; Pramanik, P. Climate change and seed quality: An alarming issue in crop husbandry. Curr. Sci. 2013, 105, 1336–1338. Available online: https://www.researchgate.net/publication/258453138 (accessed on 12 June 2019).
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Barnard, A. Genetic diversity of South African winter wheat cultivars in relation to preharvest sprouting and falling number. Euphytica 2001, 119, 107–110. [Google Scholar] [CrossRef]
- Barnard, A.; Purchase, J.; Smith, M.; van Lill, D. Determination of the preharvest sprouting resistance of South African winter wheat (Triticum aestivum L.) cultivars. S. Afr. J. Plant Soil 1997, 14, 4–8. [Google Scholar] [CrossRef]
- Barnard, A.; Bona, L. Sprout damage and falling number in South African and Hungarian wheats. Cereal Res. Commun. 2004, 32, 259–264. [Google Scholar] [CrossRef]
- Barnard, A.; Smith, M.F. Determination of the influence of climate on falling number of winter wheat in the dryland production areas of the Free State Province of South Africa. Euphytica 2012, 188, 15–24. [Google Scholar] [CrossRef]
- Barnard, A.; Grain, S.A. Preharvest Sprouting Research—20 Years Later. Available online: https://www.grainsa.co.za/preharvest-sprouting-research-20-years-later (accessed on 1 August 2024).
- Barnard, A.; Purchase, J.L. The relationship of preharvest sprouted seed and seed treatment to emergence and yield of winter wheat (Triticum aestivum L.) in the Eastern Free State. Seed Sci. Technol. 1998, 26, 711–718. [Google Scholar]
- Guides, C.C. South Africa—Agricultural Sector. Available online: https://www.trade.gov/country-commercial-guides/south-africa-agricultural-sector (accessed on 1 October 2024).
- DALRRD. Trends in the Agricultural Sector. Department of Agriculture, Land Reform & Rural Development Agriculture, Republic of South Africa; Department of Agriculture, Land Reform and Rural Development: Pretoria, South Africa, 30 June 2023. Available online: https://www.dalrrd.gov.za/images/Branches/Economica%20Development%20Trade%20and%20Marketing/Statistc%20and%20%20Economic%20Analysis/statistical-information/trends-in-the-agricultural-sector-2023.pdf (accessed on 1 October 2024).
- SAGL. South African Wheat Crop Quality Report 2023/2024 Season. 2024. Available online: https://sagl.co.za/wheat/reports/#https-sagl-co-za-wp-content-uploads-wheat-report-2023-2024_web-pdf/1/ (accessed on 10 April 2025).
- IPAD. South Africa Wheat Area, Yield and Production. Available online: https://ipad.fas.usda.gov/countrysummary/default.aspx?id=SF&crop=Wheat (accessed on 10 April 2025).
- Smit, H.A.; Tolmay, V.L.; Barnard, A.; Jordaan, J.P.; Koekemoer, F.P.; Otto, W.M.; Pretorius, Z.A.; Purchase, J.L.; Tolmay, J.P.C. An overview of the context and scope of wheat (Triticum aestivum) research in South Africa from 1983 to 2008. S. Afr. J. Plant Soil 2010, 27, 81–96. [Google Scholar] [CrossRef]
- Van Niekerk, H.A. Southern Africa wheat pool. In The World Wheat Book: The History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Lavoisier Publishing: Paris, France, 2001; pp. 923–936. [Google Scholar]
- Gazette, S.G. Government notice no. R 1547 of 29 November 2019: Regulations Relating to the Grading, Packing and Marking of Bread Wheat Intended for Sale in the Republic of South Africa. Department of Agriculture, Land Reform and Rural Development, R 1547. 2019. Available online: https://sagl.co.za/wp-content/uploads/Page-17-2.pdf (accessed on 16 March 2024).
- Grain, A.-S. Production Guidelines for Small Grains. Available online: https://www.arc.agric.za/arc-sgi/Pages/SGI%20Pages%20Archive/Small-Grain-New-Homepage.aspx (accessed on 2 October 2024).
- Dube, E.; Khumalo, T. Wheat cultivar evaluation: Enhancing the sustainability of the wheat industry. Wheat Focus Magazine, 2 June 2023; p. 18. [Google Scholar]
- Biddulph, T.; Plummer, J.; Setter, T.; Mares, D. Influence of high temperature and terminal moisture stress on dormancy in wheat (Triticum aestivum L.). Field Crops Res. 2007, 103, 139–153. [Google Scholar] [CrossRef]
- Van Eeden, E.; Labuschagne, M.T. Sprouting tolerance and falling number in South African hybrid bread wheat cultivars and their parent lines. J. Cereal Sci. 2012, 56, 754–759. [Google Scholar] [CrossRef]
- Barnard, A.; Van Deventer, C.S.; Maartens, H. Genetic variability of preharvest sprouting—The South African situation. Euphytica 2005, 143, 291–296. [Google Scholar] [CrossRef]
- Khumalo, T.P.; Hlongoane, T.; Barnard, A.; Tsilo, T.J. Genomic regions influencing preharvest sprouting tolerance in two doubled-haploid wheat populations (Triticum aestivum L.). Agronomy 2022, 12, 832. [Google Scholar] [CrossRef]
- Khumalo, T.P. Phenotypic and Molecular Analyses of Preharvest Sprouting and Yield Related Traits in Two Doubled-Haploid Populations of Wheat (Triticum aestivum L.). Ph.D. Thesis, Department of Life & Consumer Sciences, University of South Africa, Pretoria, South Africa, 2021. Available online: https://hdl.handle.net/10500/32333 (accessed on 1 June 2025).
- Hull, S.; Swanepoel, P.; Botes, W. Navigating the Threat of Pre-Harvest Sprouting of Wheat; SA Grain Magazine: Pretoria, South Africa, 2024; pp. 66–67. Available online: https://sagrainmag.co.za/2024/05/06/navigating-the-threat-of-pre-harvest-sprouting-of-wheat/ (accessed on 5 June 2024).
- Hull, S.I.; Swanepoel, P.A.; Botes, W.C. A critical review of the factors influencing pre-harvest sprouting of wheat. Agron. J. 2024, 116, 3354–3367. [Google Scholar] [CrossRef]
- Singh, C.; Kamble, U.; Gupta, V.; Singh, G.; Sheoran, S.; Gupta, A.; Tyagi, B.; Kumar, P.; Mishra, C.N.; Krishnappa, G.; et al. Pre-harvest sprouting in wheat: Current status and future prospects. J. Cereal Res. 2021, 13, 1–22. [Google Scholar] [CrossRef]
- King, R.W.; Richards, R.A. Water uptake in relation to pre-harvest sprouting in wheat: Ear characteristics. Aust. J. Agric. Res. 1984, 35, 327–336. [Google Scholar] [CrossRef]
- Dhariwal, R.; Hiebert, C.W.; Sorrells, M.E.; Spaner, D.; Graf, R.J.; Singh, J.; Randhawa, H.S. Mapping pre-harvest sprouting resistance loci in AAC Innova × AAC Tenacious spring wheat population. BMC Genom. 2021, 22, 900. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ayele, B.T. Functional genomics of seed dormancy in wheat: Advances and prospects. Front. Plant Sci. 2014, 5, 458. [Google Scholar] [CrossRef]
- Liu, S.; Sehgal, S.K.; Li, J.; Lin, M.; Trick, H.N.; Yu, J.; Gill, B.S.; Bai, G. Cloning and characterization of a critical regulator for preharvest sprouting in wheat. Genetics 2013, 195, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Mrva, K. Wheat grain preharvest sprouting and late maturity alpha-amylase. Planta 2014, 240, 1167–1178. [Google Scholar] [CrossRef]
- Munkvold, J.D.; Tanaka, J.; Benscher, D.; Sorrells, M.E. Mapping quantitative trait loci for preharvest sprouting resistance in white wheat. Theor. Appl. Genet. 2009, 119, 1223–1235. [Google Scholar] [CrossRef]
- Olaerts, H.; Courtin, C.M. Impact of preharvest sprouting on endogenous hydrolases and technological quality of wheat and bread: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 698–713. [Google Scholar] [CrossRef]
- Patwa, N.; Penning, B.W. Genetics of a diverse soft winter wheat population for pre-harvest sprouting, agronomic, and flour quality traits. Front. Plant Sci. 2023, 14, 1137808. [Google Scholar] [CrossRef]
- Rabieyan, E.; Bihamta, M.R.; Moghaddam, M.E.; Mohammadi, V.; Alipour, H. Genome-wide association mapping and genomic prediction for pre-harvest sprouting resistance, low α-amylase and seed color in Iranian bread wheat. BMC Plant Biol. 2022, 22, 300. [Google Scholar] [CrossRef]
- Yang, J.H.; Yu, Y.X.; Cheng, J.S.; Tan, X.L.; Shen, W.P. Study on the pre harvest sprouting tolerance in Triticum aestivum spp. Yunnanense King. J. Tritic. Crops 2011, 31, 747–752. [Google Scholar]
- Zhu, Y.; Wang, S.; Wei, W.; Xie, H.; Liu, K.; Zhang, C.; Wu, Z.; Jiang, H.; Cao, J.; Zhao, L.; et al. Genome-wide association study of pre-harvest sprouting tolerance using a 90K SNP array in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2019, 132, 2947–2963. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Imtiaz, M.; Ogbonnaya, F.C.; Oman, J.; Van Ginkel, M. Characterization of quantitative trait loci controlling genetic variation for preharvest sprouting in synthetic backcross-derived wheat lines. Genetics 2008, 178, 1725–1736. [Google Scholar] [CrossRef]
- Lohwasser, U.; Arif, M.A.R.; Börner, A. Discovery of loci determining pre-harvest sprouting and dormancy in wheat and barley applying segregation and association mapping. Biol. Plant 2013, 57, 663–674. [Google Scholar] [CrossRef]
- Kim, S.Y.; Warpeha, K.M.; Huber, S.C. The brassinosteroid receptor kinase, BRI1, plays a role in seed germination and the release of dormancy by cold stratification. J. Plant Physiol. 2019, 241, 153031. [Google Scholar] [CrossRef]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.-M. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef]
- Mares, D.J. Genetic studies of sprouting tolerance in red and white wheat. In Pre-Harvest Sprouting in Cereals; Walker-Simmons, M.K., Ried, J.L., Eds.; American Associaton of Cereal Chemists: Saint Paul, MN, USA, 1993; pp. 21–29. [Google Scholar]
- Biddulph, T.B.; Mares, D.J.; Plummer, J.A.; Setter, T.L. Drought and high temperature increases preharvest sprouting tolerance in a genotype without grain dormancy. Euphytica 2005, 143, 277–283. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef]
- Strand, E. Studies on seed dormancy in small grain species. I Barley II Wheat. Norway J. Agric. Sci. 1989, 3, 101–115. [Google Scholar]
- Tuttle, K.M.; Martinez, S.A.; Schramm, E.C.; Takebayashi, Y.; Seo, M.; Steber, C.M. Grain dormancy loss is associated with changes in ABA and GA sensitivity and hormone accumulation in bread wheat, Triticum aestivum (L.). Seed Sci. Res. 2015, 25, 179–193. [Google Scholar] [CrossRef]
- Gao, X.; Hu, C.H.; Li, H.Z.; Yao, Y.J.; Meng, M.; Dong, J.; Zhao, W.C.; Chen, Q.J.; Li, X.Y. Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum L.): A review. J. Anim. Plant Sci. 2013, 23, 556–565. [Google Scholar]
- Gale, M.D. The genetics of preharvest sprouting in cereals, particularly wheat. In Preharvest Field Spouting in Cereals, 1st ed.; Derera, N.F., Ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- King, R.W. Manipulation of grain dormancy in wheat. J. Exp. Bot. 1993, 44, 1059–1066. [Google Scholar] [CrossRef]
- Ji, T.; Penning, B.; Baik, B.K. Pre-harvest sprouting resistance of soft winter wheat varieties and associated grain characteristics. J. Cereal Sci. 2018, 83, 110–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, X.; Xia, X.; He, Z. Cloning of seed dormancy genes (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor. Appl. Genet. 2014, 127, 855–866. [Google Scholar] [CrossRef]
- Debeaujon, I.; Leon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, an longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Torada, A.; Amano, Y. Effect of seed coat color on seed dormancy in different environments. Euphytica 2002, 126, 99–105. [Google Scholar] [CrossRef]
- Guo, G.; Xu, S.; Chen, H.; Hao, Y.; Mao, H. QTL mapping for wheat seed dormancy in a Yangmai16/Zhongmai895 double haploid population. Plants 2023, 12, 759. [Google Scholar] [CrossRef]
- Zanetti, S.; Winzeler, M.; Keller, M.; Keller, B.; Messmer, M. Genetic analysis of pre-harvest sprouting resistance in a wheat × spelt cross. Crop Sci. 2000, 40, 1406–1417. [Google Scholar] [CrossRef]
- Kato, K.; Nakamura, W.; Tabiki, T.; Miura, H.; Sawada, S. Detection of loci controlling seed dormancy on group 4 chromosomes of wheat and comparative mapping with rice and barley genomes. Theor. Appl. Genet. 2001, 102, 980–985. [Google Scholar] [CrossRef]
- Kulwal, P.L.; Kumar, N.; Gaur, A.; Khurana, P.; Khurana, J.P.; Tyagi, A.K.; Balyan, H.S.; Gupta, P.K. Mapping of a major QTL for pre-harvest sprouting tolerance on chromosome 3A in bread wheat. Theor. Appl. Genet. 2005, 111, 1052–1059. [Google Scholar] [CrossRef]
- Mori, M.; Uchino, N.; Chono, M.; Kato, K.; Miura, H. Mapping QTLs for grain dormancy on wheat chromosome 3A and the group 4 chomosomes, and their combined effect. Theor. Appl. Genet. 2005, 110, 1315–1323. [Google Scholar] [CrossRef]
- Fofana, B.; Humphreys, D.G.; Rasul, G.; Cloutier, S.; Brule-Babel, A.; Woods, S.; Lukow, O.M.; Somers, D.J. Mapping quantitative trait loci controlling pre-harvest sprouting resistance in a red × white seeded spring wheat cross. Euphytica 2009, 165, 509–521. [Google Scholar] [CrossRef]
- Liu, S.; Cai, S.; Graybosch, R.; Chen, C.; Bai, G. Quantitative trait loci for resistance to pre-harvest sprouting in US hard white winter wheat Rio Blanco. Theor. Appl. Genet. 2008, 117, 691–699. [Google Scholar] [CrossRef]
- Ogbonnaya, F.C.; Imtiaz, M.; Ye, G.; Hearnden, P.R.; Hernandez, E.; Eastwood, R.F. Genetic and QTL analyses of seed dormancy and pre-harvest sprouting resistance in the wheat germplasm CN10955. Theor. Appl. Genet. 2008, 116, 891–902. [Google Scholar] [CrossRef]
- Ren, X.; Lan, X.; Liu, D.; Wang, J.; Zheng, Y. Mapping QTLs for pre-harvest sprouting tolerance on chromosome 2D in a synthetic hexaploid wheat × common wheat cross. J. Appl. Genet. 2008, 49, 333–341. [Google Scholar] [PubMed]
- Rasul, G.; Humphreys, D.G.; Brule-Babel, A.; McCartney, C.A.; Knox, R.E.; DePauw, R.M.; Somers, D.J. Mapping QTLs for pre-harvest sprouting traits in the spring wheat cross ‘RL4452/AC Domain’. Euphytica 2009, 168, 363–378. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J.; Singh, R.; Garg, T.; Chhuneja, P.; Balyan, H.S. QTL analysis for grain colour and pre-harvest sprouting in bread wheat. Plant Sci. 2009, 177, 114–122. [Google Scholar] [CrossRef]
- Miao, X.L.; Zhang, Y.J.; Xia, X.C.; He, Z.H.; Zhang, Y.; Yan, J. Mapping quantitative trait loci for pre-harvest sprouting resistance in white-grained winter wheat line CA 0431. Crop Pasture Sci. 2013, 64, 573–579. [Google Scholar] [CrossRef]
- Lin, M.; Cai, S.; Wang, S.; Liu, S.; Zhang, G.; Bai, G. Genotyping-by-sequencing (GBS) identified SNP tightly linked to QTL for pre-harvest sprouting resistance. Theor. Appl. Genet. 2015, 128, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Knox, R.E.; Clarke, F.R.; Pozniak, C.J.; DePauw, R.M.; Cuthbert, R.D.; Fox, S. Maximizing the identification of QTL for pre-harvest sprouting resistance using seed dormancy measures in a white-grained hexaploid wheat population. Euphytica 2015, 205, 287–309. [Google Scholar] [CrossRef]
- Shao, M.; Bai, G.; Rife, T.W.; Poland, J.; Lin, M.; Liu, S.; Chen, H.; Kumssa, T.; Fritz, A.; Trick, H.; et al. QTL mapping of pre-harvest sprouting resistance in a white wheat cultivar Danby. Theor. Appl. Genet. 2018, 131, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Wu, Q.; Chen, Y.; Zhang, P.; Zhang, Y.; Hu, W.; Wang, X.; Zhao, H.; Dong, L.; et al. Molecular characterization of a novel TaGL3-5A allele and its association with grain length in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2019, 132, 1799–1814. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.; Lu, J.; Si, H.; Ma, C. Genetic improvement of wheat with pre-harvest sprouting resistance in China. Genes 2023, 14, 837. [Google Scholar] [CrossRef]
- Gautam, T.; Kumar, K.; Agarwal, P.; Tyagi, S.; Jaiswal, V.; Gahlaut, V.; Kumar, S.; Prasad, P.; Chhuneja, P.; Singh, H.; et al. Development of white-grained PHS-tolerant wheats with high grain protein and leaf rust resistance. Mol. Breed. 2021, 41, 42. [Google Scholar] [CrossRef]
- Torada, A.; Koike, M.; Ogawa, T.; Takenouchi, Y.; Tadamura, K.; Wu, J.; Matsumoto, T.; Kawaura, K.; Ogihara, Y. A causal gene for seed dormancy on wheat chromosome 4A encodes a MAP kinase kinase. Curr. Biol. 2016, 26, 782–787. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.-P.; Zhao, Q.-X.; Feng, J.-M.; Si, H.-Q.; Lu, J.; Ma, C.-X. Rich allelic variations of Viviparous-1A and their associations with seed dormancy/pre-harvest sprouting of common wheat. Euphytica 2011, 179, 343–353. [Google Scholar] [CrossRef]
- Chang, C.; Feng, J.M.; Si, H.Q.; Yin, B.; Zhang, H.P.; Ma, C.X. Validating a novel allele of viviparous-1 (Vp-1Bf) associated with high seed dormancy of Chinese wheat landrace, Wanxianbaimaizi. Mol. Breed. 2010, 25, 517–525. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.P.; Feng, J.M.; Yin, B.; Si, H.Q.; Ma, C.X. Identifying alleles of Viviparous-1B associated with pre-harvest sprouting in micro-core collections of Chinese wheat germplasm. Mol. Breed. 2009, 25, 481–490. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.L.; Xia, L.Q.; Chen, X.M.; Xia, X.C.; Yu, Z.; He, Z.H.; Roder, M. Development and validation of a Viviparous-1 STS marker for pre-harvest sprouting tolerance in Chinese wheats. Theor. Appl. Genet. 2007, 115, 971–980. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.L.; Liu, S.X.; Sun, Y.Q.; Meng, J.Y.; Xia, L.Q. Characterization of the rich haplotypes of Viviparous-1A in Chinese wheats and development of a novel sequence-tagged site marker for pre-harvest sprouting resistance. Mol. Breed. 2014, 33, 75–88. [Google Scholar] [CrossRef]
- Ashikawa, I.; Abe, F.; Nakamura, S. Ectopic expression of wheat and barley DOG1-like genes promotes seed dormancy in Arabidopsis. Plant Sci. 2010, 179, 536–542. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, X.; Wang, S.; Zhu, M.; Carver, B.F.; Yan, L. TaMFT-A1 is associated with seed germination sensitive to temperature in winter wheat. PLoS ONE 2013, 8, e73330. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Min, X.; Shan, S.; Jiang, H.; Cao, J.; Li, L. Isolation and characterization of TaQsd1 genes for period of dormancy in common wheat (Triticum aestivum L.). Mol. Breed. 2019, 39, 150. [Google Scholar] [CrossRef]
- Lang, J.; Jiang, H.; Cheng, M.; Wang, M.; Gu, J.; Dong, H.; Li, M.; Guo, X.; Chen, Q.; Wang, J. Variation of TaMyb10 and their function on grain color and pre-harvest sprouting resistance of wheat. Plant J. 2024, 118, 1388–1399. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, L.-X.; Chen, X.-J.; Cao, J.-J.; Wu, Z.-Y.; Liu, K.; Zhang, C.; Wei, W.-X.; Xie, H.-Y.; Li, L.; et al. A novel 33-bp insertion in the promoter of TaMFT-3A is associated with pre-harvest sprouting resistance in common wheat. Mol. Breed. 2018, 38, 69. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X.; He, Z. The seed dormancy allele TaSdr-A1a associated with pre-harvest sprouting tolerance is mainly present in Chinese wheat landraces. Theor. Appl. Genet. 2017, 130, 81–89. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
- Sinha, D.; Maurya, A.K.; Abdi, G.; Majeed, M.; Agarwal, R.; Mukherjee, R.; Ganguly, S.; Aziz, R.; Bhatia, M.; Majgaonkar, A.; et al. Integrated genomic selection for accelerating breeding programs of climate-smart cereals. Genes 2023, 14, 1484. [Google Scholar] [CrossRef]
- Avana-Tientcheu, M.L.; Tiambo, C.K. Breeding and productivity in ending hunger and achieving food security and nutrition. In Zero Hunger. Encyclopedia of the UN Sustainable Development Goals; Filho, W.L., Azul, A., Brandli, L., Ozuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern plant breeding techniques in crop improvement and genetic diversity: From molecular markers and gene editing to artificial intelligence—A critical review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Zhang, H.P.; Chang, C.; Si, H.Q.; Lu, J.; Ma, C.X. Developing of molecular marker for pre-harvest sprouting resistance and its application in wheat MAS breeding. Sci. Technol. Rev. 2016, 34, 81–86. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S.; Sandhu, K.S.; Kumar, N.; Saripalli, G.; Prakash, R.; Nambardar, A.; Sharma, H.; Gautam, T.; Balyan, H.S.; et al. GWAS and genomic prediction for pre-harvest sprouting tolerance involving sprouting score and two other related traits in spring wheat. Mol. Breed. 2023, 43, 14. [Google Scholar] [CrossRef]
- Aziz, M.A.; Masmoudi, K. Molecular breakthroughs in modern plant breeding techniques. Hortic. Plant J. 2024, 11, 15–41. [Google Scholar] [CrossRef]
- Ćeran, M.; Miladinovic, D.; Dordevic, V.; Trkulja, D.; Radanovic, A.; Glogovac, S.; Kondic-Spika, A. Genomics-assisted speed breeding for crop improvement: Present and future. Front. Sustain. Food Syst. 2024, 8, 1383302. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Maharajan, T.; Ceasar, S.A. Application of CRISPR/Cas9 genome editing system to reduce the pre- and post-harvest yield losses in cereals. Open Biotechnol. J. 2022, 16, e2205190. [Google Scholar] [CrossRef]
- Abe, F.; Haque, E.; Hisano, H.; Tanaka, T.; Kamiya, Y.; Mikami, M.; Kawaura, K.; Endo, M.; Onishi, K.; Hayashi, T.; et al. Genome-edited triple-recessive mutation alters seed dormancy in wheat. Cell Rep. 2019, 28, 1362–1369. [Google Scholar] [CrossRef]
- Alamillo, J.M.; López, C.M.; Rivas, F.J.M.; Torralbo, F.; Bulut, M.; Alseekh, S. Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein and hairy roots: A perfect match for gene functional analysis and crop improvement. Curr. Opin. Biotechnol. 2023, 79, 102876. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J. Current insights into weak seed dormancy and pre-harvest sprouting in crop species. Plants 2024, 13, 2559. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Y.; Fan, Y.; Wang, Y.; Li, P.; Xiong, J.; He, Y.; Cheng, S.; Ye, X.; Wang, F.; et al. CRISPR/Cas9-mediated restoration of Tamyb10 to create pre-harvest sprouting-resistant red wheat. Plant Biotechnol. J. 2023, 21, 665–667. [Google Scholar] [CrossRef]
- DePauw, R.M.; Clarke, F.R.; Fofana, B.; Knox, R.; Humphreys, G.; Cloutier, S. RL4137 contributes preharvest sprouting resistance to Canadian wheats. Euphytica 2009, 168, 347–361. [Google Scholar] [CrossRef]
- DePauw, R.M.; Knox, R.E.; Singh, A.K.; Fox, S.L.; Humphreys, D.G.; Hucl, P. Developing standardized methods for breeding preharvest sprouting resistant wheat, challenges and successes in Canadian wheat. Euphytica 2012, 188, 7–14. [Google Scholar] [CrossRef]
- Morris, G.F.; DeMacon, V.L. Seed dormancy and tissue culture response in wheat. Crop Sci. 1994, 34, 1324–1329. [Google Scholar] [CrossRef]
- ProAgri, S.A. Wheat Production: The Use of Molecular Markers to Assist in Pre-Harvest Sprouting Research. Available online: https://www.proagri.co.za/en/wheat-production-use-molecular-markers-assist-pre-harvest-sprouting-research/ (accessed on 9 September 2021).
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.K.; Manmathan, H.K.; Anderson, V.A.; Poland, J.A.; Morris, C.F.; Haley, S. Improving genomic prediction for pre-harvest sprouting tolerance in wheat by weighting large-effect quantitative trait loci. Crop Sci. 2017, 57, 1315–1324. [Google Scholar] [CrossRef]
- Huang, T.; Qu, B.; Li, H.P.; Zuo, D.Y.; Zhao, Z.X.; Liao, Y.C. A maize viviparous 1 gene increases seed dormancy and preharvest sprouting tolerance in transgenic wheat. J. Cereal Sci. 2012, 55, 166–173. [Google Scholar] [CrossRef]
- Demirci, Y.; Zhang, B.; Unver, T. CRISPR/Cas9: An RNA-guided highly precise synthetic tool for plant genome editing. J. Cell Physiol. 2018, 233, 1844–1859. [Google Scholar] [CrossRef]
- Jasin, M.; Haber, J.E. The democratization of gene editing: Insights from site-specific cleavage and double-strand break repair. DNA Repair 2016, 44, 6–16. [Google Scholar] [CrossRef]
- Easterling, W.E.; Aggarwal, P.K.; Batima, P.; Brander, K.M.; Erda, L.; Howden, S.M.; Kirilenko, A.; Morton, J.; Soussana, J.-F.; Schmidhuber, J.; et al. Food, fibre and forest products. In Climate Change: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 273–313. [Google Scholar]
- Rosegrant, M.W.; Agcaoili, M. Global Food Demand, Supply, and Price Prospects to 2010; International Food Policy Research Institute: Washington, DC, USA, 2010. [Google Scholar]
- ARC-Small Grain. Report on the National Small Grain Cultivar Evaluation Programme Under Dryland Conditions in the Summer Rainfall Region for 2021 Season. 2021. Available online: https://www.arc.agric.za/arc-sgi/ (accessed on 30 March 2022).
- ARC-Small Grain. Report on the National Small Grain Cultivar Evaluation Programme Under Dryland Conditions in the Summer Rainfall Region for 2020 Season. 2020. Available online: https://www.arc.agric.za/arc-sgi/ (accessed on 30 March 2022).
- ARC-Small Grain. Report on the National Small Grain Cultivar Evaluation Programme Under Dryland Conditions in the Summer Rainfall Region for 2019 Season. 2019. Available online: https://www.arc.agric.za/arc-sgi/ (accessed on 30 March 2022).
- Singh, K.; Shukla, S.; Kadam, S.; Semwal, V.K.; Singh, N.K.; Khanna-Chopra, R. Genomic regions and underlying candidate genes associated with coleoptile length under deep sowing conditions in a wheat RIL population. J. Plant Biochem. Biotechnol. 2015, 24, 324–330. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar] [CrossRef]
- Liton, M.M.U.A.; McCartney, C.A.; Hiebert, C.W.; Kumar, S.; Jordan, M.C.; Ayele, B.T. Identification of loci for pre-harvest sprouting resistance in the highly dormant spring wheat RL4137. Theor. Appl. Genet. 2021, 134, 113–124. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, D.; Chen, X.; Li, Y.; Hu, M.; Sun, S.; Su, Q.; Su, Y.; Li, S. Identification of QTLs and a candidate gene for reducing pre-harvest sprouting in Aegilops tauschii–Triticum aestivum chromosome segment substitution lines. Int. J. Mol. Sci. 2021, 22, 3729. [Google Scholar] [CrossRef] [PubMed]
- Deng-Cai, L.; Xiu-Jin, L.; Zhi-Rong, W.; You-Liang, Z.; Yong-Hong, Z.; Jun-Liang, Y.; Chi, Y. Evaluation of Aegilops tauschii Cosson for preharvest sprouting tolerance. Genet. Resour. Crop Evol. 1998, 45, 495–498. [Google Scholar] [CrossRef]
- Yu, M.; Chen, G.; Zhang, L.; Liu, Y.; Liu, D.; Wang, J.; Pu, Z.; Zhang, L.; Lan, X.; Wei, Y.; et al. QTL mapping for important agronomic traits in synthetic hexaploid wheat derived from Aegiliops tauschii ssp. tauschii. J. Integr. Agric. 2014, 13, 1835–1844. [Google Scholar] [CrossRef]
- Xiao, S.H.; Zhang, X.Y.; Yan, C.S.; Lin, H. Germplasm improvement for preharvest sprouting resistance in Chinese white-grained wheat: An overview of the current strategy. Euphytica 2002, 126, 35–38. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.M.; He, Z.H.; Röder, M.; Xia, L.Q. Distribution of Vp-1 alleles in Chinese white-grained landraces, historical and current wheat cultivars. Cereal Res. Commun. 2009, 37, 169–177. [Google Scholar] [CrossRef]
- Bassoi, M.C.; Flintham, J. Relationship between grain colour and preharvest sprouting-resistance in wheat. Pesqui. Agropecu. Bras. 2005, 40, 981–988. [Google Scholar] [CrossRef]
- Flintham, J.E. Grain color and sprout-resistance in wheat. In Pre-Harvest Sprouting in Cereals, 6th ed.; Walker-Simmons, M.K., Ried, J.L., Eds.; American Association of Cereal Chemists: Saint Paul, MN, USA, 1993; pp. 30–36. [Google Scholar]
- Santos, L.; Pinto, R.J.B.; Franco, F.A.; Schuster, I. Inheritance and potential use of grain color in the identification of genotypes resistant to pre-harvest sprouting in wheat. Crop Breed. Appl. Biotechnol. 2010, 10, 218–224. [Google Scholar] [CrossRef]
- Nörnberg, R.; Gonzalez da Silva, J.A.; de Souza Luche, H.; Tessmann, E.; Kavalco, S.A.F.; Zimmer, C.M.; Baretta, D.; da Maia, L.C.; de Oliveira, A.C. Tolerance to preharvest sprouting and yield of wheat genotypes from different breeding programs. Pesqui. Agropecu. Bras. 2015, 50, 698–706. [Google Scholar] [CrossRef]
- Jiménez, N.; Mares, D.; Mrva, K.; Lizana, C.; Contreras, S.; Schwember, A.R. Susceptibility to preharvest sprouting of Chilean and Australian elite cultivars of common wheat. Crop Sci. 2017, 57, 462–474. [Google Scholar] [CrossRef]
- Rigatti, A.; Meira, D.; Olivoto, T.; Meier, C.; Nardino, M.; Lunkes, A.; Klein, L.A.; Fassini, F.; Moro, E.D.; Marchioro, V.S.; et al. Grain yield and its associations with pre-harvest sprouting in wheat. J. Agric. Sci. 2019, 11, 142. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Mccouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef]
- Flintham, J.E. Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci. Res. 2000, 10, 43–50. [Google Scholar] [CrossRef]
- Lukow, O.M.; Bushuk, W. Influence of germination on wheat quality. I. Functional (breadmaking) and biochemical properties. Cereal Chem. 1984, 61, 336–339. [Google Scholar]
- Chamberlain, N.; Collins, T.H.; McDermott, E.E. Alpha-amylase and bread properties. Int. J. Food Sci. Technol. 2007, 16, 127–152. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Appolonia, B.D. Sprouting in hard red spring wheat. Bak. Dig. 1979, 53, 17–19. [Google Scholar]
- Olaerts, H.; Vandekerckhove, L.; Courtin, C.M. A closer look at the bread making process and the quality of bread as a function of the degree of preharvest sprouting of wheat (Triticum aestivum). J. Cereal Sci. 2018, 80, 188–197. [Google Scholar] [CrossRef]
- Perten, H. Application of the falling number method for evaluating alpha-amylase activity. Cereal Chem. 1964, 41, 127–140. [Google Scholar]
- Felicio, J.C.; De Oliveira Camargo, C.E.; Germani, R.; De Freitas, J.G. Grain yield and sprouting process within the head of wheat genotypes. Pesqui. Agropecu. Bras. 2002, 37, 289–294. [Google Scholar] [CrossRef]
- Nornberg, R.; de Souza Luche, H.; Gonzalez da Silva, J.A.; Zimmer, C.M.; Cima, F.; Olivo, M.; de Oliveira, A.C. The challenge of finding high grain yield and pre-harvest sprouting tolerant genotypes in Brazilian wheat germplasm. Aust. J. Crop Sci. 2016, 10, 977–984. [Google Scholar] [CrossRef]
- Bunjkar, A.; Walia, P.; Sandal, S.S. Unlocking genetic diversity and germplasm characterization with molecular markers: Strategies for crop improvement. J. Adv. Biol. Biotechnol. 2024, 27, 160–173. [Google Scholar] [CrossRef]
- Bhandari, R.; Paudel, H.; Nyaupane, S.; Poudel, M.R. Climate resilient breeding for high yields and stable wheat (Triticum aestivum L.) lines under irrigated and abiotic stress environments. Plant Stress 2024, 11, 100352. [Google Scholar] [CrossRef]
- Acevedo, M.; Zurn, J.; Molero, G.; Singh, P.K.; He, X. The role of wheat in global food security. In Agricultural Development and Sustainable Intensification: Technology and Policy Challenges in the Face of Climate Change, 1st ed.; Nagothu, U.S., Ed.; Routledge: Oxfordshire, UK, 2018; p. 30. [Google Scholar]
- Dadrasi, A.; Chaichi, M.; Nehbandani, A.; Sheikhi, A.; Salmani, F.; Nemati, A. Addressing food insecurity: An exploration of wheat production expansion. PLoS ONE 2023, 18, e0290684. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. BioScience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. J. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ahammed, G.J.; Li, X.; Shi, K. Elevated CO2 improves photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front. Plant Sci. 2018, 26, 1739. [Google Scholar] [CrossRef]
- Nations, U. Sustainable Development Goals. Goal 2: Zero Hunger. Available online: https://www.un.org/sustainabledevelopment/hunger (accessed on 18 November 2024).
- Statistics, U.N. Sustainable Development Goals (SDGs). Available online: https://unstats.un.org/sdgs/report/2016/goal-02 (accessed on 18 November 2024).
- Ferrari, M.; Benvenuti, L.; Rossi, L.; De Santis, A.; Sette, S.; Martone, D.; Piccinelli, R.; Le Donne, C.; Leclercq, C.; Turrini, A. Could dietary goals and climate change mitigation be achieved through optimized diet? The experience of modeling the national food consumption data in Italy. Front. Nutr. 2020, 7, 48. [Google Scholar] [CrossRef]
- Sachs, J.; Lafortune, G.; Kroll, C.; Fuller, G.; Woelm, F. From Crisis to Sustainable Development: The SDGs as Roadmap to 2030 and Beyond; Sustainable Development Report: Cambridge, UK, 2022. [Google Scholar]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M. Path-coefficient and correlation analysis in Bambara groundnut (Vigna subterranea [L.] Verdc.) accessions over environments. Sci. Rep. 2022, 12, 245. [Google Scholar] [CrossRef]
- Lan, X.; Wei, Y.; Liu, D.; Yan, Z.; Zheng, Y. Inheritance of seed dormancy in Tibetan semi-wild wheat accession Q1028. J. Appl. Genet. 2005, 46, 133–138. [Google Scholar] [PubMed]
- Lan, X.J.; Zheng, Y.L.; Liu, D.C.; Wei, Y.M.; Yan, Z.H.; Zhou, Y.H. Tolerant mechanism and chromosome location of genes controlling sprouting tolerance in Aegilops tauschii Cosson. Agr. Sci. China 2002, 1, 265–268. [Google Scholar]
- Brown, L.K.; Wiersma, A.T.; Olson, E.L. Preharvest sprouting and α-amylase activity in soft winter wheat. J. Cereal Sci. 2018, 79, 311–318. [Google Scholar] [CrossRef]
- Conway, G. The Doubly Green Revolution: Food for All in the 21st Century; Pengiun books: London, UK, 1997. [Google Scholar]
- Reynolds, M.P.; Borlaug, N.E. Impacts of breeding on international collaborative wheat improvement. J. Agric. Sci. 2006, 144, 3–17. [Google Scholar] [CrossRef]
- Lantican, M.A.; Dubin, H.J.; Morris, M.L.; Heisey, P.W. Impacts of International Wheat Breeding Research in the Developing World, 1988–2002; CIMMYT: El Batán, Mexico, 2005; p. 54. [Google Scholar]
- Feng, Y.; Han, Y.; Han, B.; Zhao, Y.; Yang, Y.; Xing, Y. A 4 bp InDel in the promoter of wheat gene TaAFP-B affecting seed dormancy confirmed in transgenic rice. Front. Plant Sci. 2022, 13, 837805. [Google Scholar] [CrossRef]
- Hisano, H.; Hoffie, R.E.; Abe, F.; Munemori, H.; Matsuura, T.; Endo, M.; Mikami, M.; Nakamura, S.; Kumlehn, J.; Sato, K. Regulation of germination by targeted mutagenesis of grain dormancy genes in barley. Plant Biotechnol. J. 2022, 20, 37–46. [Google Scholar] [CrossRef]
- Jia, Y.; Barrero, J.M.; Wang, J.; Considine, M.J.; Nakamura, S.; Li, C. Editorial: Seed dormancy, germination, and pre-harvest sprouting, volume II. Front. Plant Sci. 2024, 15, 1399510. [Google Scholar] [CrossRef]
- Nakamura, S. Grain dormancy genes responsible for preventing pre-harvest sprouting in barley and wheat. Breed. Sci. 2018, 68, 295–304. [Google Scholar] [CrossRef]
- Nakamura, S.; Pourkheirandish, M.; Morishige, H.; Sameri, M.; Sato, K.; Komatsuda, T. Quantitative trait loci and maternal effects affecting the strong grain dormancy of wild barley (Hordeum vulgare ssp. spontaneum). Front. Plant Sci. 2017, 8, 1840. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, Y.; Chen, W.; Yang, J.; Cheng, M.; Watt, C.; Cheng, J.; Wang, Z.; Tan, Z.; Li, M. Characterization and expression quantitative trait loci analysis of TaABI4, a pre-harvest sprouting related gene in wheat. Seed Sci. Res. 2021, 31, 188–198. [Google Scholar] [CrossRef]

| Production Region | Pre-Harvest Sprouting Tolerance in the Early 1990s | Status “Then” | Pre-Harvest Sprouting Tolerance Now | Status “Now” |
|---|---|---|---|---|
| Summer Rainfall | 23% | Poor | >60% | Good–excellent |
| Irrigation | 42% | Moderate | 62% | Good |
| Winter rainfall | - | - | 70–80% | Excellent |
| Group | Scoring System | Characteristic(s) |
|---|---|---|
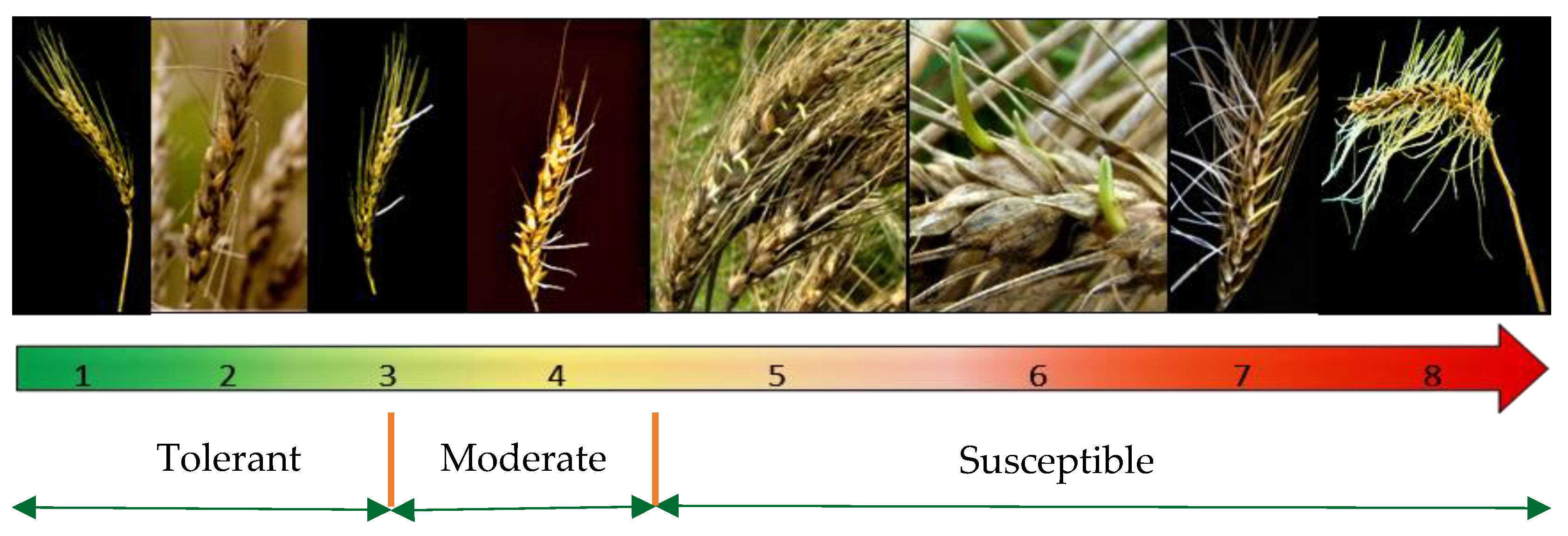
| Tolerant | 1–3 | Excellent response. None-to-minimal signs of sprouting, such as the emergence of few roots. |
| Moderate | 3.1–4.5 | Moderately tolerant or moderately susceptible response. Visible roots and signs of shoots. The reaction of these cultivars/genotypes is strongly influenced by environmental conditions rather than the genetic makeup, which makes them unstable across environments and years [24]. |
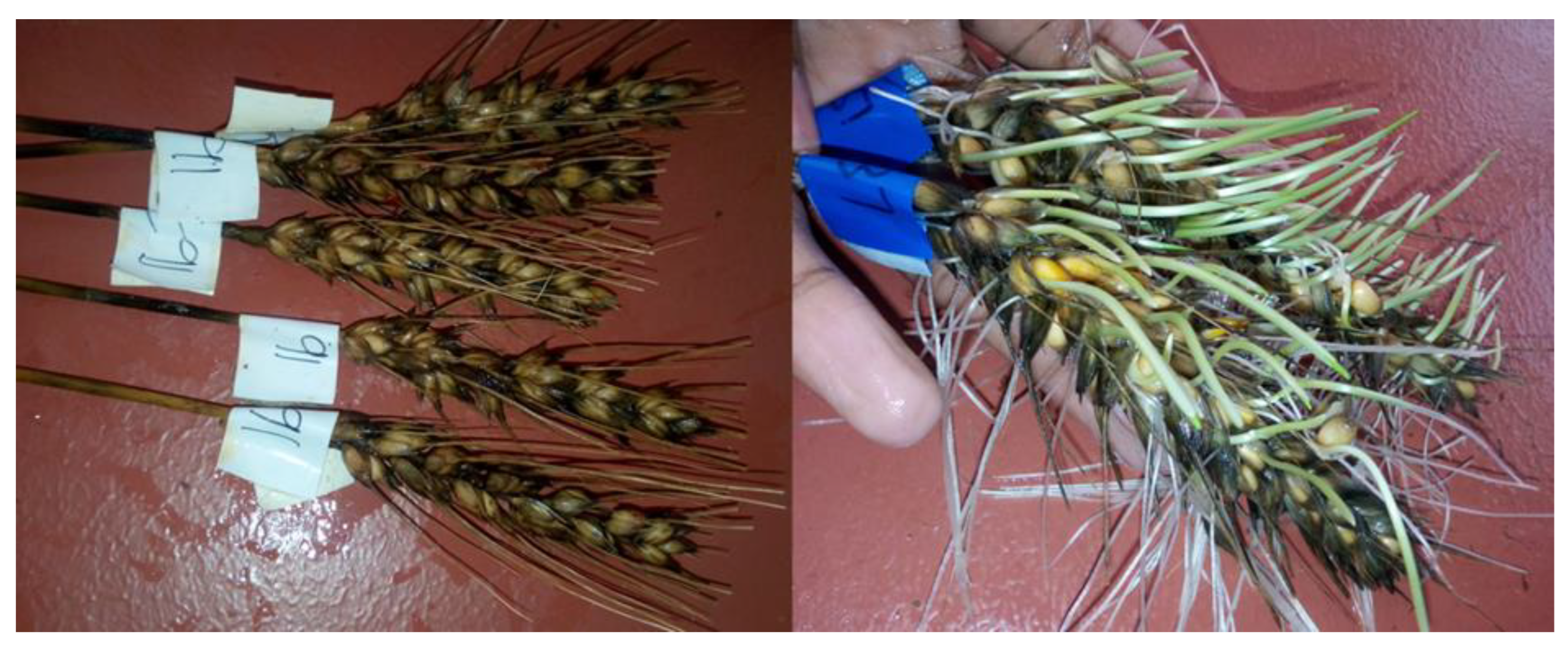
| Susceptible | >4.5 | Poor tolerance. Swollen, discolored, and heavily germinated kernels, fully grown roots and shoots (see Figure 1). |
| Genes | Chromosomes | Markers | Physical Site/Mb | Favorable Alleles | References |
|---|---|---|---|---|---|
| TaVp-1 | 3AL | Vp-1A | 659.6 | TaVp-1Ab, TaVp-1Ad | Chang et al., 2011 [115] |
| Vp1A3 | TaVp-1Agm | Yang et al., 2013 [119] | |||
| 3BL | Vp1-b2 | 693.3 | TaVp-1Bb, TaVp-1Bc, TaVp-1Bd, TaVp-1Be and TaVp-1Bf | Chang et al., 2009a; Chang et al., 2009b [116,117] | |
| Vp1B3 | TaVp-1Bb, TaVp-1Bc | Yang et al., 2007 [118] | |||
| TaDOG1L1 | 3AS | DOG1L1 | 534.5 | - | Ashikawa et al., 2010 [120] |
| TaMFT/TaPHS1 | MFT-A1 | TaMFT-A1b | King & Richards, 1984 [67] | ||
| MFT-A2 | TaMFT-3Aa | Jiang et al., 2018 [124] | |||
| MFT-3A | 7.3 | SNP-222(C) | Nakamura et al., 2011 [27] | ||
| TaPHS1-SNP1/TaPHS1-SNP1 | SNP646/666 (G/A) | Liu et al., 2013 [70] | |||
| TaSdr | 2A | Sdr2A | 158.5 | TaSdr-A1a | Zhang et al., 2017 [125] |
| Wild Relatives/Landraces/Lines Identified with Pre-Harvest Sprouting Tolerance | Country | References |
|---|---|---|
| Aegilops tauschii | China | Deng-Cai et al., 1998 [158] |
| Triticum Turgidum and Aegilops tauschii (DD) | China | Deng-Cai et al., 1998; Yu et al., [158,159] |
| Synthetic hexaploid wheat genotypes (SHW-L1) | China | Yang et al., 2014 [119] |
| Chinese Landraces viz., Xiaoyuhua, Yongchuanbaike, and Baiyuhua | China | Xiao et al., 2002 [160] |
| Hongheshangtou and Wanxianbaimaizi | China | Yang et al., 2009; Chang et al., 2010 [116,161] |
| RL4137 | Canada | Bassoi & Flintham 2005 [162] |
| BRS177 | Brazil | Flintham 1993 [163] |
| Frontana | Brazil | Santos et al., 2010; Nornberg et al., 2015 [164,165] |
| RL4137 | Canada | DePauw et al., 2009; DePauw et al., 2012 [139,140] |
| Konde, Kumpa, and Swindy cultivars | Australia | Jimenez et al., 2017 [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumalo-Mthembu, T.P.; Mmereki, P.; Mzimela, N.P.; Barnard, A.; Tsilo, T.J. Breeding Wheat (Triticum aestivum L.) for Pre-Harvest Sprouting Tolerance in South Africa: Current Status and Future Prospects. Plants 2025, 14, 2134. https://doi.org/10.3390/plants14142134
Khumalo-Mthembu TP, Mmereki P, Mzimela NP, Barnard A, Tsilo TJ. Breeding Wheat (Triticum aestivum L.) for Pre-Harvest Sprouting Tolerance in South Africa: Current Status and Future Prospects. Plants. 2025; 14(14):2134. https://doi.org/10.3390/plants14142134
Chicago/Turabian StyleKhumalo-Mthembu, Thobeka Philile, Palesa Mmereki, Nokulunga Prudence Mzimela, Annelie Barnard, and Toi John Tsilo. 2025. "Breeding Wheat (Triticum aestivum L.) for Pre-Harvest Sprouting Tolerance in South Africa: Current Status and Future Prospects" Plants 14, no. 14: 2134. https://doi.org/10.3390/plants14142134
APA StyleKhumalo-Mthembu, T. P., Mmereki, P., Mzimela, N. P., Barnard, A., & Tsilo, T. J. (2025). Breeding Wheat (Triticum aestivum L.) for Pre-Harvest Sprouting Tolerance in South Africa: Current Status and Future Prospects. Plants, 14(14), 2134. https://doi.org/10.3390/plants14142134







