Combined Metabolomics and Network Pharmacology to Reveal Anti-Diabetic Mechanisms and Potential Pharmacological Components of Synsepalum dulcificum
Abstract
1. Introduction
2. Results
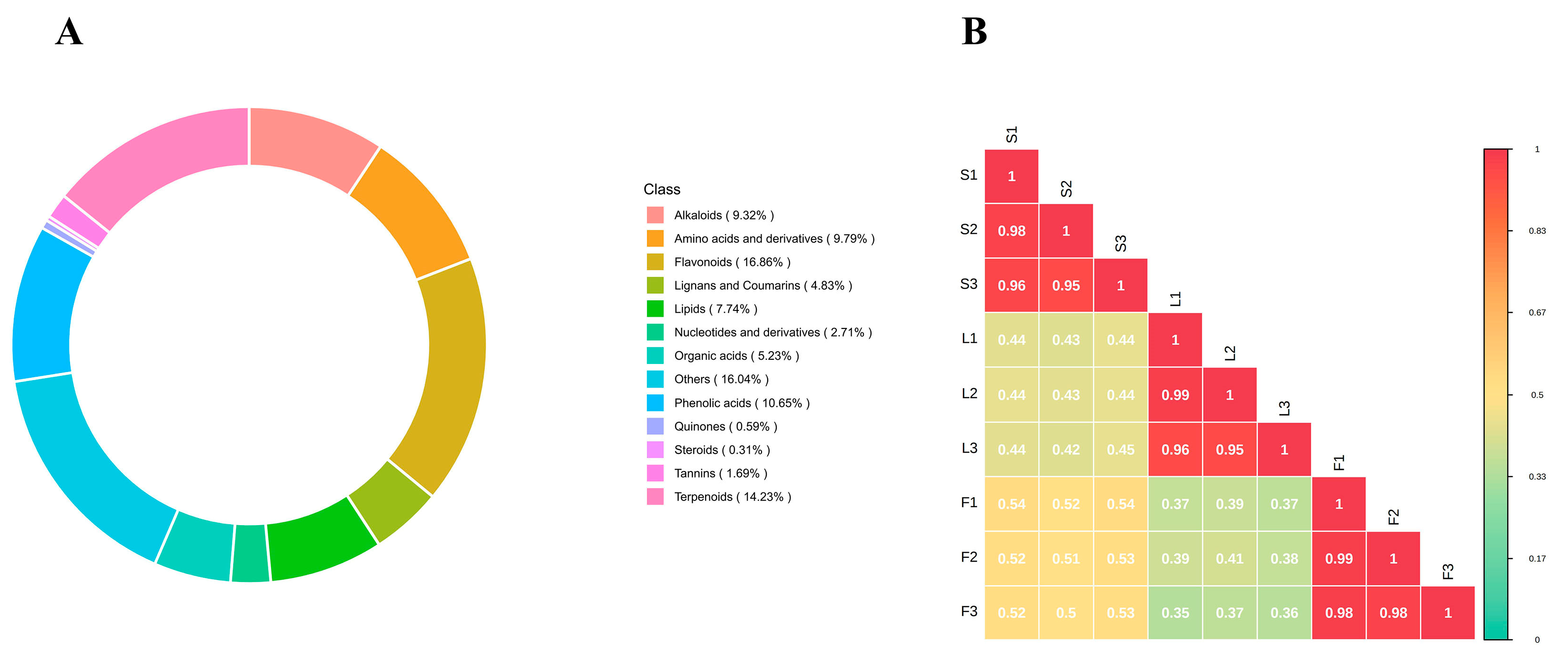
2.1. Qualitative Analysis of Secondary Metabolites in Different Parts of SD
2.2. Multivariate Statistical Analysis of Secondary Metabolites
2.3. Differences in Metabolic Products of SD in Different Tissue Locations
2.4. Screening, Functional Annotation, and Enrichment Analysis of Differential Metabolites in Three Tissues of SD
2.5. KEGG Annotation and Enrichment Analysis of Differential Metabolites
2.6. Network Pharmacology Analysis
2.6.1. Screening of DM-Related and SD-Related Targets
2.6.2. PPI Analysis of Core Targets
2.6.3. GO and KEGG Pathway Enrichment Analyses
2.6.4. Construction of the “C-T-P” Network Diagram
2.7. Molecular Docking Analysis
2.8. The Inhibitory Effect of Potential Bioactive Components from SD on α-Glucosidase
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Widely-Targeted Metabolomic Analysis of Secondary Metabolites
5.2.1. Sample Preparation and Extraction
5.2.2. LC-MS/MS Conditions
5.2.3. Qualitative and Quantitative Analysis of Secondary Metabolites
5.3. Network Pharmacology
5.3.1. Screening for Potential Active Ingredients
5.3.2. Prediction of Potential Active Ingredients and Diabetes-Related Targets
5.3.3. Construction of Drug–Compound–Target Networks and Pathway Analysis
5.3.4. The Construction of Protein–Protein Interaction (PPI) Network and Component–Target–Disease (C–T–P) Network
5.3.5. Molecular Docking Verification
5.4. In Vitro Inhibitory Activity Against α-Glucosidase
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DeFronzo, R.A. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications—A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Cardiovascular Safety of Antihyperglycemic Agents: “Do Good or Do No Harm”. Drugs 2018, 78, 1567–1592. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentao, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2017, 22, 56. [Google Scholar] [CrossRef]
- Tchokponhoué, D.A.; Achigan-Dako, E.G.; N’Danikou, S.; Nyadanu, D.; Kahane, R.; Odindo, A.O.; Sibiya, J. Comparative analysis of management practices and end-users’ desired breeding traits in the miracle plant [(Schumach & Thonn.) Daniell] across ecological zones and sociolinguistic groups in West Africa. J. Ethnobiol. Ethnomed. 2021, 17, 41. [Google Scholar] [CrossRef]
- He, Z.X.; Tan, J.S.; Abbasiliasi, S.; Lai, O.M.; Tam, Y.J.; Ariff, A.B. Phytochemicals, nutritionals and antioxidant properties of miracle fruit Synsepalum dulcificum. Ind. Crops Prod. 2016, 86, 87–94. [Google Scholar] [CrossRef]
- Jia, W.E.; Velaga, A.; Guad, R.M.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Choy, K.W.; Wu, Y.S. Deciphering as an Arising Phytotherapy Agent: Background, Phytochemical and Pharmacological Properties with Associated Molecular Mechanisms. Sains Malays. 2022, 51, 199–208. [Google Scholar] [CrossRef]
- Wagner, S.; de Cedrón, M.G.; del Hierro, J.N.; Martín-Hernández, D.; Siles, M.D.; Santoyo, S.; Jaime, L.; Martín, D.; Fornari, T.; de Molina, A.R. Biological Activities of Miracle Berry Supercritical Extracts as Metabolic Regulators in Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 6957. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Hong, Z.L.; Chen, H.A.; Chang, Y.C.; Yang, W.L.; Chang, H.C.; Mai, C.T.; Chen, C.Y. Bioconstituents from stems of Daniell (Sapotaceae) inhibit human melanoma proliferation, reduce mushroom tyrosinase activity and have antioxidant properties. J. Taiwan Inst. Chem. Eng. 2011, 42, 204–211. [Google Scholar] [CrossRef]
- Obafemi, T.O.; Akinmoladun, A.C.; Olaleye, M.T.; Agboade, S.O.; Onasanya, A.A. Antidiabetic potential of methanolic and flavonoid-rich leaf extracts of Synsepalum dulcificum in type 2 diabetic rats. J. Ayurveda Integr. Med. 2017, 8, 238–246. [Google Scholar] [CrossRef]
- Huang, W.H.; Chung, H.Y.; Xuan, W.S.; Wang, G.C.; Li, Y.L. The cholesterol-lowering activity of miracle fruit (Synsepalum dulcificum). J. Food Biochem. 2020, 44, 13185. [Google Scholar] [CrossRef]
- Yu, W.J.; Li, X.H.; Sun, Q.F.; Yi, S.X.; Zhang, G.W.; Chen, L.L.; Li, Z.Z.; Li, J.R.; Luo, L.P. Metabolomics and network pharmacology reveal the mechanism of Castanopsis honey against Streptococcus pyogenes. Food Chem. 2024, 441, 138388. [Google Scholar] [CrossRef]
- Zhao, B.J.; Zhang, Q.; He, Y.Q.; Cao, W.F.; Song, W.; Liang, X.C. Targeted metabolomics reveals the aberrant energy status in diabetic peripheral neuropathy and the neuroprotective mechanism of traditional Chinese medicine JinMaiTong. J. Pharm. Anal. 2024, 14, 225–243, Corrigendum in J. Pharm. Anal. 2024, 14, 100980. [Google Scholar] [CrossRef]
- Han, Y.; Guo, S.J.; Li, Y.P.; Li, J.N.; Zhu, L.F.; Liu, Y.W.; Lv, Y.F.; Yu, D.; Zheng, L.; Huang, C.H.; et al. Berberine ameliorate inflammation and apoptosis via modulating PI3K/AKT/NFκB and MAPK pathway on dry eye. Phytomedicine 2023, 121, 155081. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, B.; Chen, L.; Jiang, H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J. Transl. Med. 2023, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Wang, W.; Cao, Y.G.; Liang, D.J.; Zhang, W.L.; Zhang, Z.C.; Jiang, H.C.; Guo, M.Y.; Zhang, N.S. Inhibitory effects of astragalin on lipopolysaccharide-induced inflammatory response in mouse mammary epithelial cells. J. Surg. Res. 2014, 192, 573–581. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.H. Inhibitory Effect of Astragalin on Expression of Lipopolysaccharide-induced Inflammatory Mediators through NF-κB in Macrophages. Arch. Pharm. Res. 2011, 34, 2101–2107. [Google Scholar] [CrossRef]
- Liang, H.; Guan, M.; Li, T.; Li, S.; Ma, C.; Li, C. Critical review on biological effect and mechanisms of diterpenoids in Rosmarinus officinalis. Food Med. Homol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Zhu, G.T.; Wang, S.C.; Huang, Z.J.; Zhang, S.B.; Liao, Q.G.; Zhang, C.Z.; Lin, T.; Qin, M.; Peng, M.; Yang, C.K.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261.e12. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.F.; Liu, L.; Nie, Q.; Huang, M.J.; Luo, C.L.; Sun, Y.D.; Ma, Y.Y.; Yu, J.X.; Du, F.Q. HPLC-based metabolomics of revealing its antioxidant ability. Front. Plant Sci. 2023, 14, 1060242. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, J.J.; Xiao, F.Y.; Wu, Y.; Zhang, Z.T.; Shi, J.H.; Qian, J.; Wu, X.D.; Yan, F.H. Sinensetin protects against periodontitis through binding to Bach1 enhancing its ubiquitination degradation and improving oxidative stress. Int. J. Oral. Sci. 2024, 16, 38. [Google Scholar] [CrossRef]
- Chen, Y.J.; Shi, R.C.; Xiang, Y.C.; Fan, L.; Tang, H.; He, G.; Zhou, M.; Feng, X.Z.; Tan, J.D.; Huang, P.; et al. Malate initiates a proton-sensing pathway essential for pH regulation of inflammation. Signal Transduct. Target. Ther. 2024, 9, 367. [Google Scholar] [CrossRef]
- Beesley, A.; Beyer, S.F.; Wanders, V.; Levecque, S.; Bredenbruch, S.; Habash, S.S.; Schleker, A.S.S.; Gätgens, J.; Oldiges, M.; Schultheiss, H.; et al. Engineered coumarin accumulation reduces mycotoxin-induced oxidative stress and disease susceptibility. Plant Biotechnol. J. 2023, 21, 2490–2506. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.D.; Wang, H.M. Chemical Constituents from the Roots Of. Chem. Nat. Compd. 2010, 46, 448–449. [Google Scholar] [CrossRef]
- Cheng, M.J.; Hong, Z.L.; Chen, C.Y. Secondary Metabolites from the Stems of Synsepalum dulcificum. Chem. Nat. Compd. 2012, 48, 108–109. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Cornelio, K.; Bauca, T.; Chua, S.; Shen, C.C. Chemical Constituents of the Leaves, Stems, and Fruits of Synsepalum dulcificum. Chem. Nat. Compd. 2015, 51, 588–589. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lo, W.L.; Huang, L.Y.; Wang, C.J.; Chen, C.Y. Isolation of a 2-oxetanone from the fruits of Synsepalum dulcificum. Nat. Prod. Res. 2010, 24, 1850–1853. [Google Scholar] [CrossRef]
- Wang, J.F.; Shao, S.C.; Huang, R.; Wu, S.H. Chemical constituents and their antioxidant activities from the leaves of Synsepalum dulcificum. Nat. Prod. Res. 2021, 35, 6141–6146. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Food. 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Yan, L.; Vaghari-Tabari, M.; Malakoti, F.; Moein, S.; Qujeq, D.; Yousefi, B.; Asemi, Z. Quercetin: An effective polyphenol in alleviating diabetes and diabetic complications. Crit. Rev. Food Sci. 2023, 63, 9163–9186. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Chen, D.H.; Zhang, S.N.; Li, C.F.; Fang, Q.Y.; Chen, D.H.; Liu, J.B.; Jiang, X.; Zhang, Y.J.; Shen, W.J.; et al. Isoquercitrin Alleviates Diabetic Nephropathy by Inhibiting STAT3 Phosphorylation and Dimerization. Adv. Sci. 2025, 20, 2414587. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Kassim, N.K.; Soh, N.; Othman, Z.; Ismail, T. Antibacterial Property of Synsepalum dulcificum Leaves Aqueous Extract against Oral Pathogens and its Chemical Compounds. Arch. Orofac. Sci. 2020, 15, 55–64. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, J.F.; Huang, R.; Xu, T.C.; Liu, S.S.; Yang, Q.R.; Wu, S.H. An antioxidant lignan glycoside and triterpenoid saponins from the leaves of Synsepalum dulcificum. Phytochem. Lett. 2023, 53, 42–46. [Google Scholar] [CrossRef]
- Wu, Y.W.; Wu, C.Y.; Lin, F.; Wu, J.Y. Exercise training benefits pancreatic islet by modulating the insulin-like growth factor 1/phosphatidylinositol 3-kinase/protein kinase B pathway. World J. Diabetes 2025, 16, 101447. [Google Scholar] [CrossRef]
- Xiao, H.M.; Sun, X.H.; Lin, Z.Y.; Yang, Y.; Zhang, M.; Xu, Z.C.; Liu, P.Q.; Liu, Z.Q.; Huang, H.Q. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef]
- Xu, M.X.; Liu, X.X.; Zhang, H.L.; Xu, H.Y.; Ma, X.Y.; Yang, Y.P.; Duan, C.Q.; Tang, S.S.; Liu, Y.Q.; Li, C.; et al. A novel synthesised STAT3 inhibitor exerts potent anti-tumour activity by inducing lysosome-dependent cell death. Br. J. Pharmacol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhan, W.J.; Liang, M.J.; Huang, Y.F.; Liu, Y.; Wang, L.X.; Bei, W.J.; Guo, J. Ginsenoside Rb1 ameliorates the abnormal hepatic glucose metabolism by activating STAT3 in T2DM mice. J. Funct. Food. 2023, 104, 105534. [Google Scholar] [CrossRef]
- Inoue, H.; Ogawa, W.; Ozaki, M.; Haga, S.; Matsumoto, M.; Furukawa, K.; Hashimoto, N.; Kido, Y.; Mori, T.; Sakaue, H.; et al. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat. Med. 2004, 10, 168–174. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.Y.; Liang, K.N.; Bi, R.; Du, Y.A. Mesenchymal Stem Cells (MSCs): A Novel Therapy for Type 2 Diabetes. Stem Cells Int. 2022, 2022, 8637493. [Google Scholar] [CrossRef]
- Sementino, E.; Hassan, D.; Bellacosa, A.; Testa, J.R. AKT and the Hallmarks of Cancer. Cancer Res. 2024, 84, 4126–4139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Liu, Y.P.; Long, A.X.; Liu, W.; Sun, S.Y.; Lu, S.B.; Wu, X.X.; Jia, X.D.; Jose, P.A.; et al. Intestinal Gastrin/CCKBR Axis Protects against Type 2 Diabetes by Reducing Intestinal Glucose Absorption through the PI3K/Akt/eIF4B Signaling Pathway. Adv. Sci. 2025, 12, 2410032. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Yuan, Z.B.; Rao, Y.J. Current advances in the biotechnological synthesis of betulinic acid: New findings and practical applications. Syst. Microbiol. Biomanufacturing 2023, 3, 179–192. [Google Scholar] [CrossRef]
- Sutariya, B.; Taneja, N.; Saraf, M. Betulinic acid, isolated from the leaves of Syzygium cumin (L.) Skeels, ameliorates the proteinuria in experimental membranous nephropathy through regulating Nrf2/NF-κB pathways. Chem.-Biol. Interact. 2017, 274, 124–137. [Google Scholar] [CrossRef]
- Brusotti, G.; Montanari, R.; Capelli, D.; Cattaneo, G.; Laghezza, A.; Tortorella, P.; Loiodice, F.; Peiretti, F.; Bonardo, B.; Paiardini, A.; et al. Betulinic acid is a PPAR. antagonist that improves glucose uptake, promotes osteogenesis and inhibits adipogenesis. Sci Rep-Uk 2017, 7, 14. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, J.E.; Han, J.-S. Betulinic Acid Stimulates Glucose Uptake through the Activation of PI3K and AMPK in 3T3-L1 Adipocytes. J. Life Sci. 2022, 32, 762–770. [Google Scholar] [CrossRef]
- Kim, S.J.; Quan, H.Y.; Jeong, K.J.; Kim, D.Y.; Kim, G.W.; Jo, H.K.; Chung, S.H. Beneficial Effect of Betulinic Acid on Hyperglycemia via Suppression of Hepatic Glucose Production. J. Agric. Food Chem. 2014, 62, 434–442. [Google Scholar] [CrossRef]
- Dai, Y.L.; Sun, L.J.; Tan, Y.; Xu, W.Y.; Liu, S.; Zhou, J.; Hu, Y.L.; Lin, J.Y.; Yao, X.; Mi, P.B.; et al. Recent progress in the development of ursolic acid derivatives as anti-diabetes and anti-cardiovascular agents. Chem. Biol. Drug Des. 2023, 102, 1643–1657. [Google Scholar] [CrossRef]
- Wang, T.Z.; Zuo, G.W.; Yao, L.; Yuan, C.L.; Li, H.F.; Lai, Y.; Chen, Z.W.; Zhang, J.; Jin, Y.Q.; Yamahara, J.; et al. Ursolic acid ameliorates adipose tissue insulin resistance in aged rats via activating the Akt-glucose transporter 4 signaling pathway and inhibiting inflammation. Exp. Ther. Med. 2021, 22, 1466. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography-Mass Spectrometry, XCMS, and Chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, K.; Xu, Y.; Zhang, P.; Zhang, H.; Pan, S. Integrated metabolomics and network pharmacology to reveal antioxidant mechanisms and potential pharmacological ingredients of citrus herbs. Food Res. Int. 2023, 174, 113514. [Google Scholar] [CrossRef]
- Ru, J.L.; Li, P.; Wang, J.N.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminformatics 2014, 6, 13. [Google Scholar] [CrossRef]
- Liu, K.Y.; Wu, Y.C.; Liu, I.M.; Yu, W.C.; Cheng, J.T. Release of acetylcholine by syringin, an active principle of Eleutherococcus senticosus, to raise insulin secretion in Wistar rats. Neurosci. Lett. 2008, 434, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.S.; Liu, I.M.; Cheng, J.T.; Lin, C.L.; Hsu, F.L. Hypoglycemic effect of syringin from in streptozotocin-induced diabetic rats. Planta Med. 2008, 74, 109–113. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors 2007, 31, 201–210. [Google Scholar] [CrossRef]
- Wei, R.; Qin, X.M.; Li, Z.Y. Comparison of the inedible parts of white and green asparagus based on metabolomics and network pharmacology. Food Funct. 2023, 14, 7478–7488. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.M.; Su, Y.F.; Zheng, J.R.; Li, H.; Han, Z.C.; Kong, Y.Z.; Liu, H.; Zhang, Z.; Sai, C.M. Anti-cervical cancer mechanism of bioactive compounds from Alangium platanifolium based on the ‘compound-target-disease’ network. Heliyon 2023, 9, e20747. [Google Scholar] [CrossRef]
- Dong, Q.; Ren, G.X.; Li, Y.Z.; Hao, D.J. Network pharmacology analysis and experimental validation to explore the mechanism of kaempferol in the treatment of osteoporosis. Sci. Rep. 2024, 14, 7088. [Google Scholar] [CrossRef]
- Salahuddin, M.A.H.; Ismail, A.; Kassim, N.K.; Hamid, M.; Ali, M.S.M. Phenolic profiling and evaluation of antioxidant, α-glucosidase and α-amylase inhibitory activities of (Roxb) Leenh fruit extracts. Food Chem. 2020, 331, 127240. [Google Scholar] [CrossRef] [PubMed]














| Different Parts of S. dulcificum | Stems | Leaves | Fruits |
|---|---|---|---|
| IC50 (mg/mL) | 1.52 ± 0.34 | 0.90 ± 0.16 | 9.73 ± 0.70 |
| Component | IC50 ± SD (mM) |
|---|---|
| Quercetin | 1.26 ± 0.13 |
| Betulinic acid | 0.07 ± 0.02 |
| Squalene | 55.08 ± 1.80 |
| Syringin | 5.40 ± 1.20 |
| Stigmasterol | 2.11 ± 0.24 |
| Ursolic acid | 0.26 ± 0.04 |
| Corosolic acid | 2.12 ± 0.44 |
| Methyl gallate | 4.18 ± 1.09 |
| Acarbose * | 0.09 ± 0.02 |
| Dataset ID | Platforms | Sample Number | Control Sample Number | Disease Sample Number | Organism | Experiment Type | Attribute |
|---|---|---|---|---|---|---|---|
| GSE29221 | GPL6947 | 24 | 12 | 12 | Homo sapiens | Array | Test |
| GSE21340 | GPL80 | 20 | 10 | 10 | Homo sapiens | Array | Test |
| GSE55650 | GPL570 | 23 | 11 | 12 | Homo sapiens | Array | Test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, S.; Ding, R.; Wu, S. Combined Metabolomics and Network Pharmacology to Reveal Anti-Diabetic Mechanisms and Potential Pharmacological Components of Synsepalum dulcificum. Plants 2025, 14, 2132. https://doi.org/10.3390/plants14142132
Huang Y, Wang S, Ding R, Wu S. Combined Metabolomics and Network Pharmacology to Reveal Anti-Diabetic Mechanisms and Potential Pharmacological Components of Synsepalum dulcificum. Plants. 2025; 14(14):2132. https://doi.org/10.3390/plants14142132
Chicago/Turabian StyleHuang, Yong, Shiyu Wang, Rong Ding, and Shaohua Wu. 2025. "Combined Metabolomics and Network Pharmacology to Reveal Anti-Diabetic Mechanisms and Potential Pharmacological Components of Synsepalum dulcificum" Plants 14, no. 14: 2132. https://doi.org/10.3390/plants14142132
APA StyleHuang, Y., Wang, S., Ding, R., & Wu, S. (2025). Combined Metabolomics and Network Pharmacology to Reveal Anti-Diabetic Mechanisms and Potential Pharmacological Components of Synsepalum dulcificum. Plants, 14(14), 2132. https://doi.org/10.3390/plants14142132






