Understanding Chilling Injury and Sugar Metabolism-Related Genes and Metabolites in ‘Red Haven’ Peaches
Abstract
1. Introduction
2. Results
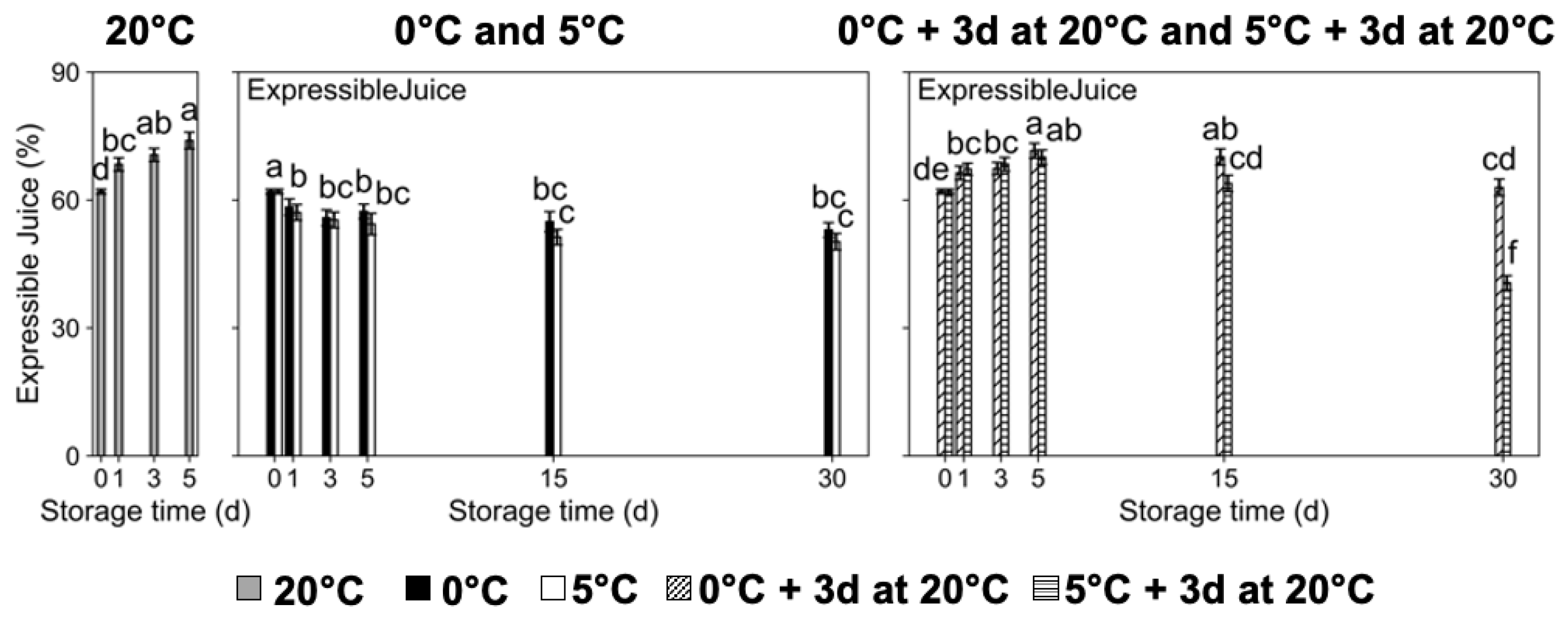
2.1. Chilling Injury Incidence
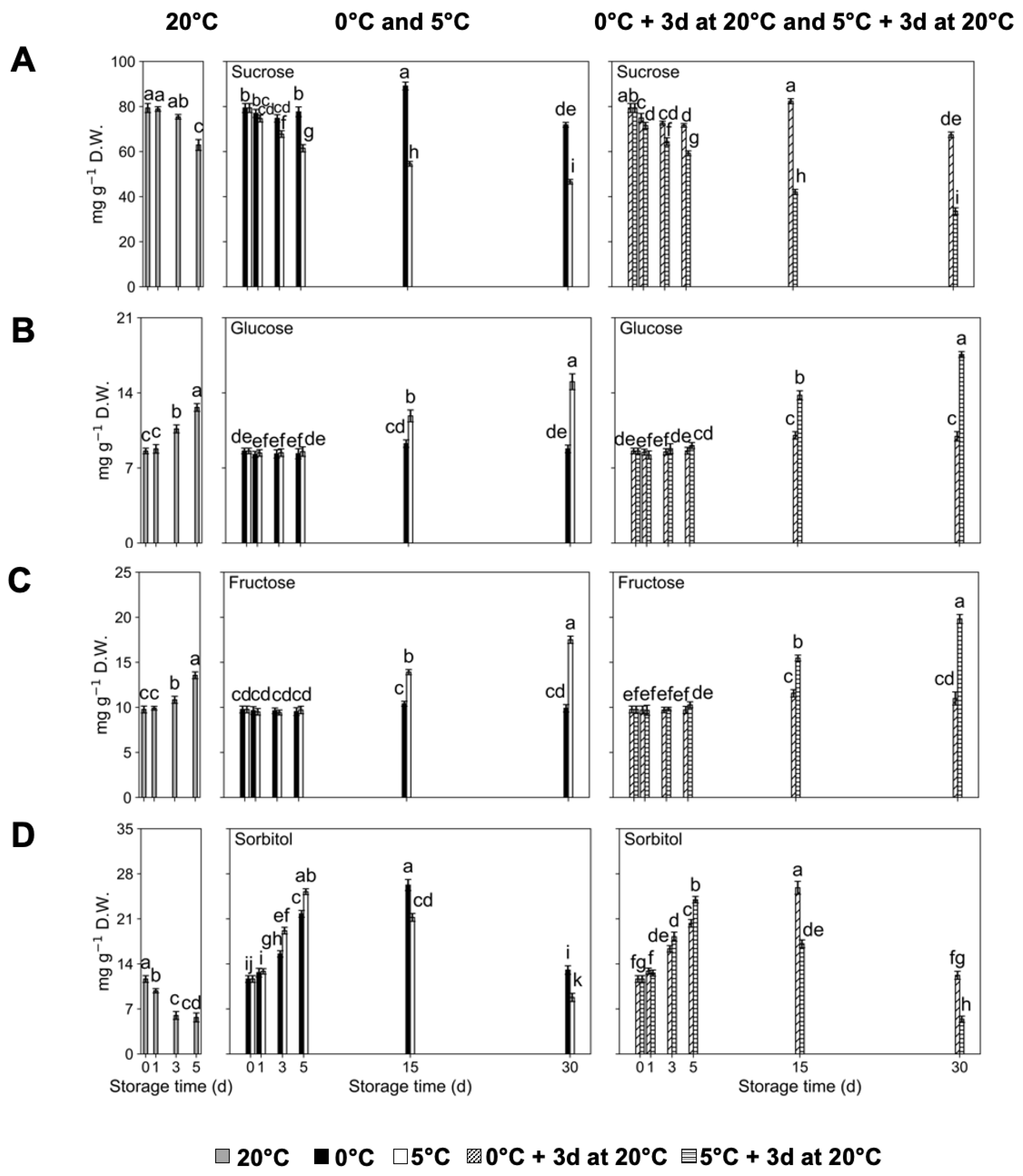
2.2. Sugar Concentration
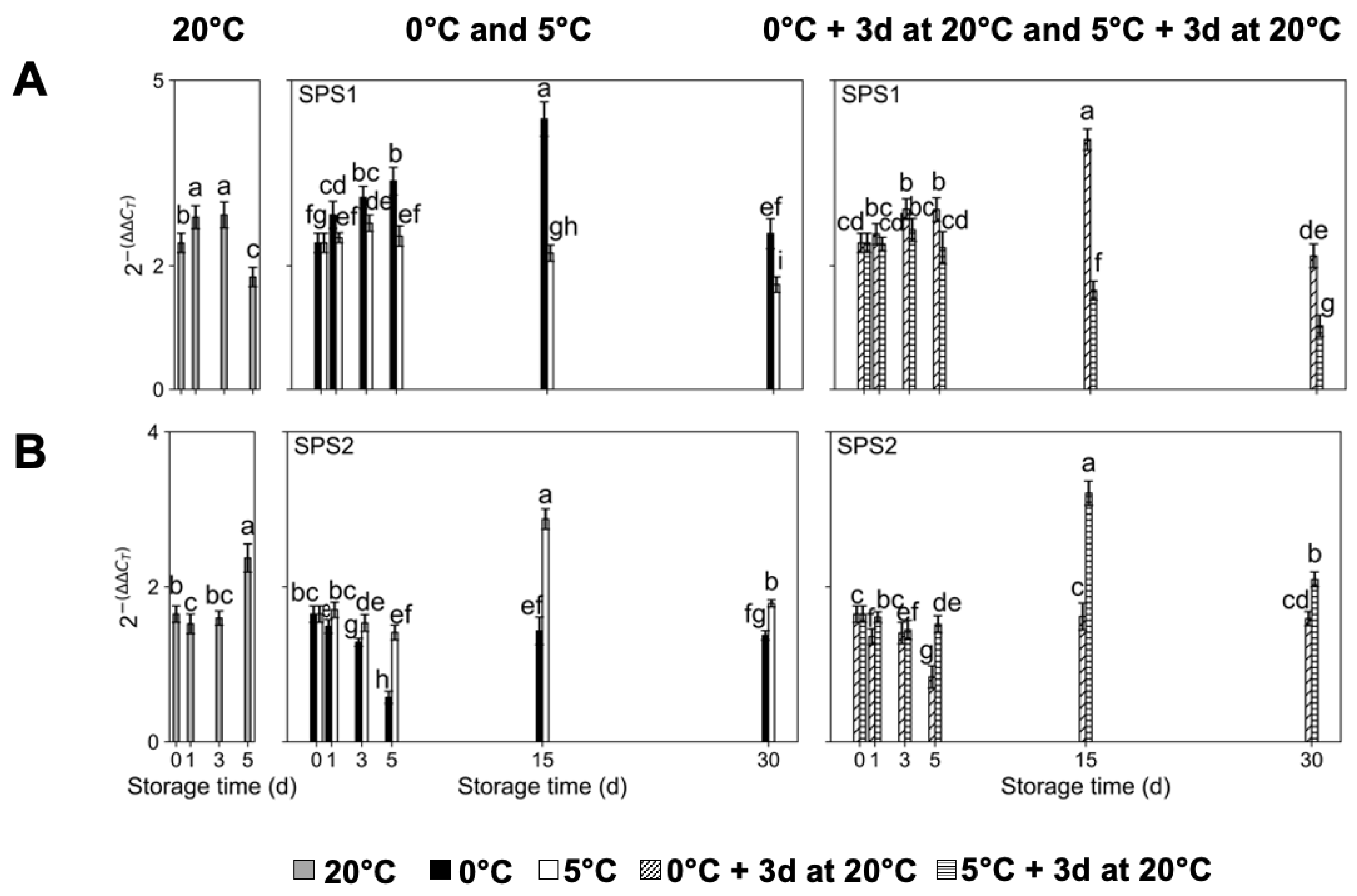
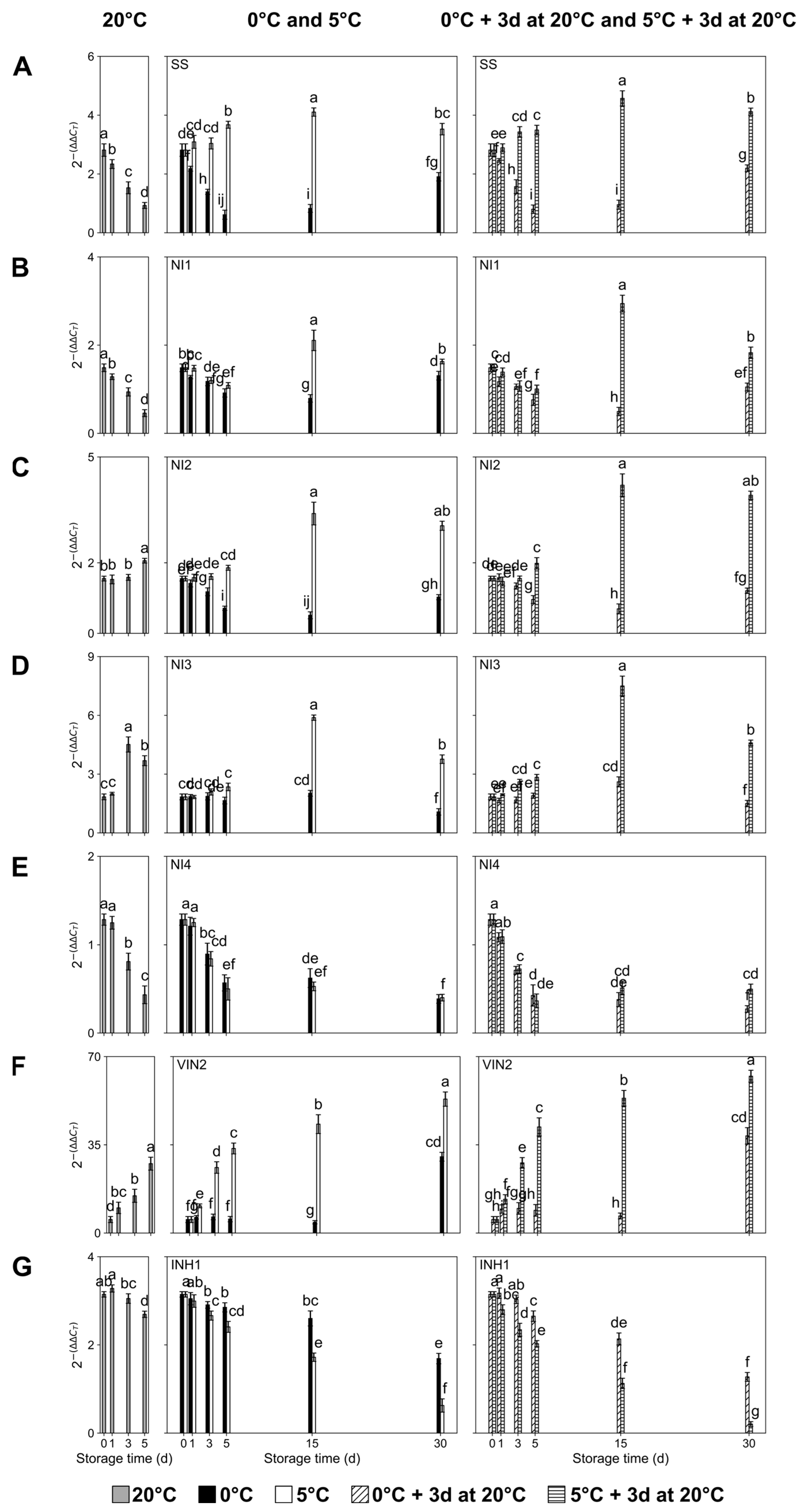
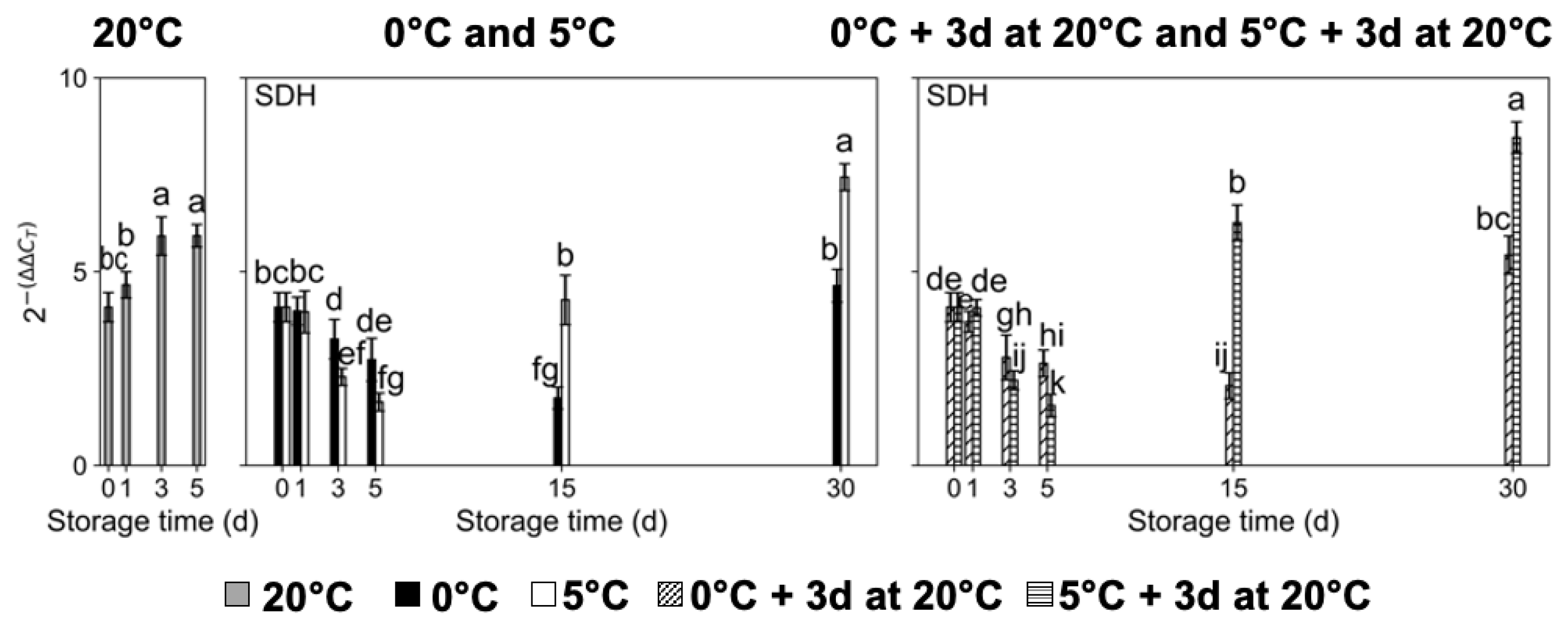
2.3. Transcript Accumulation of Sugar Metabolism-Related Genes
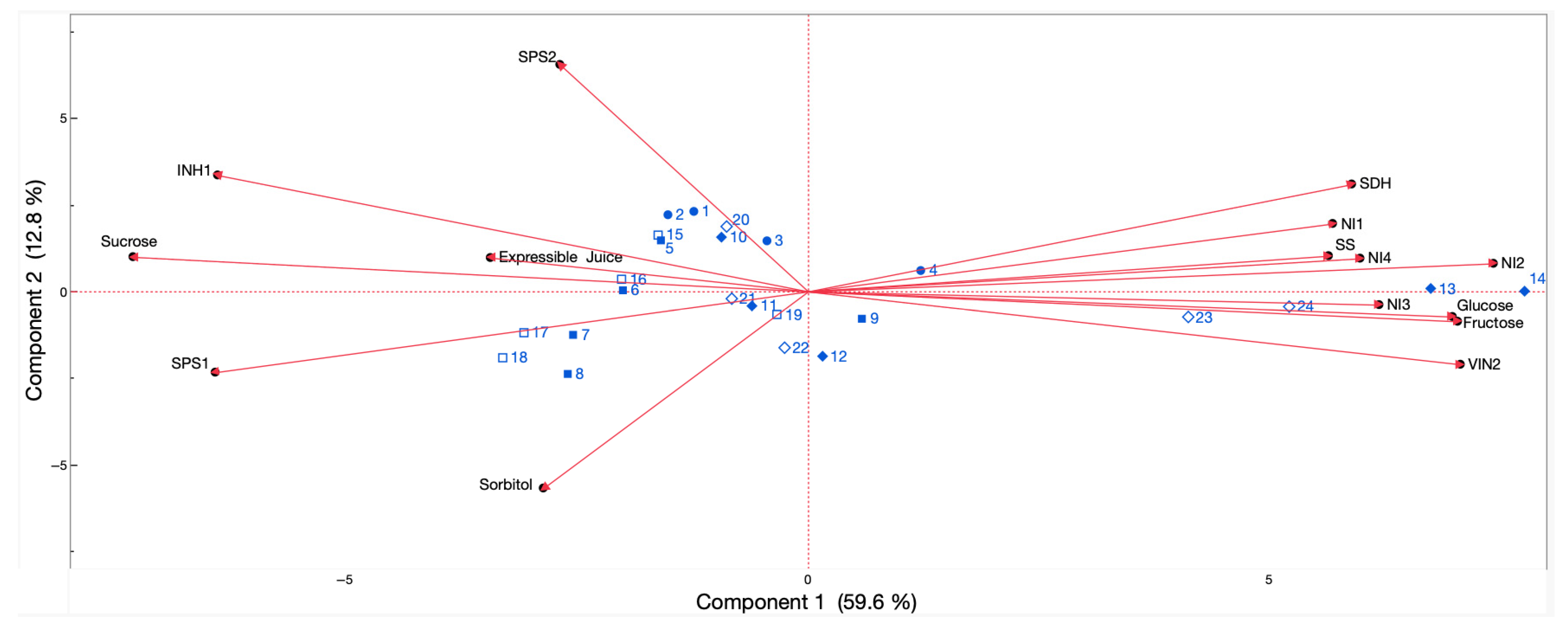
2.4. Associations Amongst Chilling Injury Incidence, Sugar Concentration, and Sugar Metabolism-Related Genes
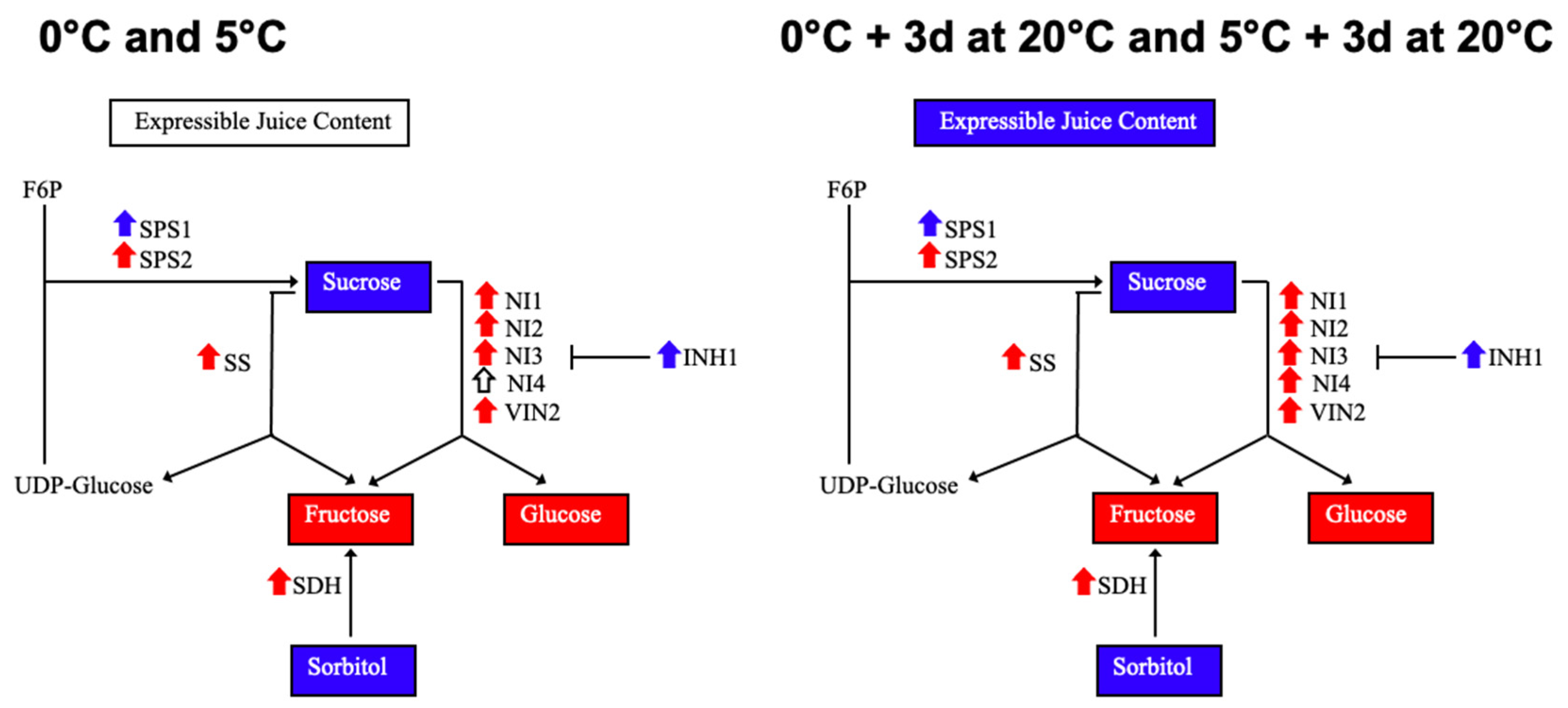
3. Discussion
4. Materials and Methods
4.1. Fruit Material
4.2. Postharvest Fruit Storage
4.3. Chilling Injury Determination
4.4. Sugar Quantification
4.5. Real-Time Quantitative RT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Tang, J.; Brummell, D.A.; Song, C.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Abscisic Acid Alleviates Chilling Injury in Cold-Stored Peach Fruit by Regulating the Metabolism of Sucrose. Sci. Hortic. 2022, 298, 111000. [Google Scholar] [CrossRef]
- Farcuh, M.; Hopfer, H. Aroma Volatiles as Predictors of Chilling Injury Development during Peach (Prunus persica (L.) Batsch) Cold Storage and Subsequent Shelf-Life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Yu, L.; Liu, H.; Shao, X.; Yu, F.; Wei, Y.; Ni, Z.; Xu, F.; Wang, H. Effects of Hot Air and Methyl Jasmonate Treatment on the Metabolism of Soluble Sugars in Peach Fruit during Cold Storage. Postharvest Biol. Technol. 2016, 113, 8–16. [Google Scholar] [CrossRef]
- Infante, R.; Farcuh, M.; Meneses, C. Monitoring the Sensorial Quality and Aroma through an Electronic Nose in Peaches during Cold Storage. J. Sci. Food Agric. 2008, 88, 2073–2078. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling Injury in Peach and Nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Mitchell, F.G.; Ju, Z. Susceptibility to Chilling Injury of Peach, Nectarine, and Plum Cultivars Grown in California. HortScience 1999, 34, 1116–1118. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Labavitch, J.M. Developing a Quantitative Method to Evaluate Peach (Prunus persica) Flesh Mealiness. Postharvest Biol. Technol. 2002, 25, 151–158. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Lurie, S.; Crisosto, C.H.; Labavitch, J.M. Cell Wall Metabolism during the Development of Chilling Injury in Cold-Stored Peach Fruit: Association of Mealiness with Arrested Disassembly of Cell Wall Pectins. J. Exp. Bot. 2004, 55, 2041–2052. [Google Scholar] [CrossRef]
- Lill, R.E.; Van Der Mespel, G.J. A Method for Measuring the Juice Content of Mealy Nectarines. Sci. Hortic. 1988, 36, 267–271. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The Genetics of Fruit Flavour Preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, Physicochemical and Volatile Compound Analysis of Short and Long Shelf-Life Melon (Cucumis melo L.) Genotypes at Harvest and after Postharvest Storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef] [PubMed]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M. V Carbon Metabolism of Peach Fruit after Harvest: Changes in Enzymes Involved in Organic Acid and Sugar Level Modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, H.; Baldazzi, V.; Van Leeuwen, C.; Bertin, N.; Gautier, H.; Wu, B.; Duchêne, E.; Gomès, E.; Delrot, S. Inter-Species Comparative Analysis of Components of Soluble Sugar Concentration in Fleshy Fruits. Front. Plant Sci. 2016, 7, 649. [Google Scholar] [CrossRef]
- Farcuh, M.; Li, B.; Rivero, R.M.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar Metabolism Reprogramming in a Non-Climacteric Bud Mutant of a Climacteric Plum Fruit during Development on the Tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar Metabolism in Relation to Chilling Tolerance of Loquat Fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Gong, Y.; Zhu, Y.; Wang, H.; Zhang, X.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The Metabolism of Soluble Carbohydrates Related to Chilling Injury in Peach Fruit Exposed to Cold Stress. Postharvest Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Yu, F.; Ni, Z.; Shao, X.; Yu, L.; Liu, H.; Xu, F.; Wang, H. Differences in Sucrose Metabolism in Peach Fruit Stored at Chilling Stress versus Nonchilling Stress Temperatures. HortScience 2015, 50, 1542–1548. [Google Scholar] [CrossRef]
- Lauxmann, M.A.; Borsani, J.; Osorio, S.; Lombardo, V.A.; Budde, C.O.; Bustamante, C.A.; Monti, L.L.; Andreo, C.S.; Fernie, A.R.; Drincovich, M.F.; et al. Deciphering the Metabolic Pathways Influencing Heat and Cold Responses during Post-Harvest Physiology of Peach Fruit. Plant Cell Environ. 2014, 37, 601–616. [Google Scholar] [CrossRef]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant Sugars Are Crucial Players in the Oxidative Challenge during Abiotic Stress: Extending the Traditional Concept. Plant. Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant Sugars: Homeostasis and Transport under Abiotic Stress in Plants. Physiol. Plant. 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Gautam, T.; Dutta, M.; Jaiswal, V.; Zinta, G.; Gahlaut, V.; Kumar, S. Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses. Cells 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene Regulation of Sugar Metabolism in Climacteric and Non-Climacteric Plums. Postharvest Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Eveland, A.L.; Jackson, D.P. Sugars, Signalling, and Plant Development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; Valluru, R. Sucrose, Sucrosyl Oligosaccharides, and Oxidative Stress: Scavenging and Salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Rudolph, A.S.; Wistrom, C.A.; Spargo, B.J.; Anchordoguy, T.J. Interactions of Sugars with Membranes. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, H.; Peng, Q.; Jiang, Q.; Wang, H.; Fang, T.; Liao, L.; Wang, L.; He, H.; Han, Y. Assessment of Sugar Components and Genes Involved in the Regulation of Sucrose Accumulation in Peach Fruit. J. Agric. Food Chem. 2016, 64, 6723–6729. [Google Scholar] [CrossRef]
- Abidi, W.; Cantín, C.M.; Jiménez, S.; Giménez, R.; Moreno, M.Á.; Gogorcena, Y. Influence of Antioxidant Compounds, Total Sugars and Genetic Background on the Chilling Injury Susceptibility of a Non-melting Peach (Prunus persica (L.) Batsch) Progeny. J. Sci. Food Agric. 2015, 95, 351–358. [Google Scholar] [CrossRef]
- Monti, L.L.; Bustamante, C.A.; Budde, C.O.; Gabilondo, J.; Müller, G.L.; Lara, M.V.; Drincovich, M.F. Metabolomic and Proteomic Profiling of Spring Lady Peach Fruit with Contrasting Woolliness Phenotype Reveals Carbon Oxidative Processes and Proteome Reconfiguration in Chilling-Injured Fruit. Postharvest Biol. Technol. 2019, 151, 142–151. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, J.; Jiang, W. Changes in Sugar Metabolism Caused by Exogenous Oxalic Acid Related to Chilling Tolerance of Apricot Fruit. Postharvest Biol. Technol. 2016, 114, 10–16. [Google Scholar] [CrossRef]
- Yamaki, S. Physiology and Metabolism of Fruit Development-Biochemistry of Sugar Metabolism and Compartmentation in Fruits. Postharvest Physiol. Fruits 1994, 398, 109–120. [Google Scholar] [CrossRef]
- Klann, E.M.; Chetelat, R.T.; Bennett, A.B. Expression of Acid Invertase Gene Controls Sugar Composition in Tomato (Lycopersicon) Fruit. Plant Physiol. 1993, 103, 863–870. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Wei, Y.; Shao, X. PpINH1, an Invertase Inhibitor, Interacts with Vacuolar Invertase PpVIN2 in Regulating the Chilling Tolerance of Peach Fruit. Hortic. Res. 2020, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.S.; Sulzenbacher, G.; Lafond, M.; Desseaux, V.; Reca, I.B.; Perrier, J.; Bellincampi, D.; Fourquet, P.; Lévêque, C.; Giardina, T. Functional Characterization of a Vacuolar Invertase from Solanum Lycopersicum: Post-Translational Regulation by N-Glycosylation and a Proteinaceous Inhibitor. Biochimie 2014, 101, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, A.H.; Jiang, J. Gene Expression Patterns of Invertase Gene Families and Modulation of the Inhibitor Gene in Tomato Sucrose Metabolism. Genet. Mol. Res 2013, 12, 3412–3420. [Google Scholar] [CrossRef]
- He, X.; Wei, Y.; Kou, J.; Xu, F.; Chen, Z.; Shao, X. PpVIN2, an Acid Invertase Gene Family Member, Is Sensitive to Chilling Temperature and Affects Sucrose Metabolism in Postharvest Peach Fruit. Plant Growth Regul. 2018, 86, 169–180. [Google Scholar] [CrossRef]
- Bianco, R.L.; Rieger, M. Roles of Sorbitol and Sucrose in Growth and Respiration of ‘Encore’ peaches at the Three Developmental Stages. J. Am. Soc. Hortic. Sci. 2002, 127, 297–302. [Google Scholar] [CrossRef]
- Dagar, A.; Weksler, A.; Friedman, H.; Ogundiwin, E.A.; Crisosto, C.H.; Ahmad, R.; Lurie, S. Comparing Ripening and Storage Characteristics of ‘Oded’ Peach and Its Nectarine Mutant ‘Yuval’ . Postharvest Biol. Technol. 2011, 60, 1–6. [Google Scholar] [CrossRef]
- Zhou, H.W.; Lurie, S.; Ben-Arie, R.; Dong, L.; Burd, S.; Weksler, A.; Lers, A. Intermittent Warming of Peaches Reduces Chilling Injury by Enhancing Ethylene Production and Enzymes Mediated by Ethylene. J. Hortic. Sci. Biotechnol. 2001, 76, 620–628. [Google Scholar]
- Brizzolara, S.; Hertog, M.; Tosetti, R.; Nicolai, B.; Tonutti, P. Metabolic Responses to Low Temperature of Three Peach Fruit Cultivars Differently Sensitive to Cold Storage. Front. Plant Sci. 2018, 9, 706. [Google Scholar] [CrossRef]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Jaunet, T.; Chi-Ham, C.; Cantu, D.; Bradford, K.J.; Van Deynze, A. Texture Diversity in Melon (Cucumis melo L.): Sensory and Physical Assessments. Postharvest Biol. Technol. 2020, 159, 111024. [Google Scholar] [CrossRef]
- Veerappan, K.; Natarajan, S.; Chung, H.; Park, J. Molecular Insights of Fruit Quality Traits in Peaches, Prunus Persica. Plants 2021, 10, 2191. [Google Scholar] [CrossRef]
- Obenland, D.M.; Crisosto, C.H.; Rose, J.K.C. Expansin Protein Levels Decline with the Development of Mealiness in Peaches. Postharvest Biol. Technol. 2003, 29, 11–18. [Google Scholar] [CrossRef]
- Fruk, G.; Cmelik, Z.; Jemric, T.; Hribar, J.; Vidrih, R. Pectin Role in Woolliness Development in Peaches and Nectarines: A Review. Sci. Hortic. 2014, 180, 1–5. [Google Scholar] [CrossRef]
- Ciacciulli, A.; Chiozzotto, R.; Attanasio, G.; Cirilli, M.; Bassi, D. Identification of a Melting Type Variant among Peach (P. persica L. Batsch) Fruit Textures by a Digital Penetrometer. J. Texture Stud. 2018, 49, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tonutti, P.; Bonghi, C.; Ramina, A. Fruit Firmness and Ethylene Biosynthesis in Three Cultivars of Peach (Prunus persica L. Batsch). J. Hortic. Sci. 1996, 71, 141–147. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-Freezing Temperature Storage Enhances Chilling Tolerance in Nectarine Fruit through Its Regulation of Soluble Sugars and Energy Metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic Acid Treatment Alleviates Chilling Injury in Peach Fruit by Promoting Sugar and Ethylene Metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Y.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. NMR Revealed That Trehalose Enhances Sucrose Accumulation and Alleviates Chilling Injury in Peach Fruit. Sci. Hortic. 2022, 303, 111190. [Google Scholar] [CrossRef]
- Fan, X.; Du, Z.; Cui, X.; Ji, W.; Ma, J.; Li, X.; Wang, X.; Zhao, H.; Liu, B.; Guo, F. Preharvest Methyl Salicylate Treatment Enhance the Chilling Tolerance and Improve the Postharvest Quality of Apricot during Low Temperature Storage. Postharvest Biol. Technol. 2021, 177, 111535. [Google Scholar] [CrossRef]
- Zhou, J.; Min, D.; Li, Z.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X. Effects of Chilling Acclimation and Methyl Jasmonate on Sugar Metabolism in Tomato Fruits during Cold Storage. Sci. Hortic. 2021, 289, 110495. [Google Scholar] [CrossRef]
- Castonguay, Y.; Nadeau, P.; Lechasseur, P.; Chouinard, L. Differential Accumulation of Carbohydrates in Alfalfa Cultivars of Contrasting Winterhardiness. Crop Sci. 1995, 35, 509–516. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Li, W.; He, B.-J.; Gao, Y.-H.; Lu, J.-X. Sucrose Metabolism in Grape (Vitis vinifera L.) Branches under Low Temperature during Overwintering Covered with Soil. Plant Growth Regul. 2014, 72, 229–238. [Google Scholar] [CrossRef]
- Holland, N.; Menezes, H.C.; Lafuente, M.T. Carbohydrates as Related to the Heat-Induced Chilling Tolerance and Respiratory Rate of ‘Fortune’Mandarin Fruit Harvested at Different Maturity Stages. Postharvest Biol. Technol. 2002, 25, 181–191. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- van den Bogaart, G.; Hermans, N.; Krasnikov, V.; de Vries, A.H.; Poolman, B. On the Decrease in Lateral Mobility of Phospholipids by Sugars. Biophys. J. 2007, 92, 1598–1605. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F.; et al. Metabolic Profiling during Peach Fruit Development and Ripening Reveals the Metabolic Networks That Underpin Each Developmental Stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, Y.; Cao, S.; Wang, H.; Song, Y. Soluble Sugar Content and Metabolism as Related to the Heat-Induced Chilling Tolerance of Loquat Fruit during Cold Storage. Food Bioprocess Technol. 2013, 6, 3490–3498. [Google Scholar] [CrossRef]
- Bogdanović, J.; Mojović, M.; Milosavić, N.; Mitrović, A.; Vučinić, Ž.; Spasojević, I. Role of Fructose in the Adaptation of Plants to Cold-Induced Oxidative Stress. Eur. Biophys. J. 2008, 37, 1241–1246. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Q.; Pan, Y.; Zhang, Z.; Yuan, R.; Nie, Y. Abnormal Behavior of Chilling Injury in Postharvest Papaya Fruit Is Associated with Sugar Metabolism. J. Food Sci. 2022, 87, 919–928. [Google Scholar] [CrossRef]
- Zhou, D.; Li, R.; Zhang, H.; Chen, S.; Tu, K. Hot Air and UV-C Treatments Promote Anthocyanin Accumulation in Peach Fruit through Their Regulations of Sugars and Organic Acids. Food Chem. 2020, 309, 125726. [Google Scholar] [CrossRef]
- Chen, X.; Song, B.; Liu, J.; Yang, J.; He, T.; Lin, Y.; Zhang, H.; Xie, C. Modulation of Gene Expression in Cold-Induced Sweetening Resistant Potato Species Solanum Berthaultii Exposed to Low Temperature. Mol. Genet. Genom. 2012, 287, 411–421. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Kumar, G.N.M.; Knowles, N.R. Maturation and Ripening of Fruit of Amelanchier Alnifolia Nutt. Are Accompanied by Increasing Oxidative Stress. Ann. Bot. 1998, 81, 203–211. [Google Scholar] [CrossRef]
- Jimenez, A.; Creissen, G.; Kular, B.; Firmin, J.; Robinson, S.; Verhoeyen, M.; Mullineaux, P. Changes in Oxidative Processes and Components of the Antioxidant System during Tomato Fruit Ripening. Planta 2002, 214, 751–758. [Google Scholar] [CrossRef]
- Airianah, O.B.; Vreeburg, R.A.M.; Fry, S.C. Pectic Polysaccharides Are Attacked by Hydroxyl Radicals in Ripening Fruit: Evidence from a Fluorescent Fingerprinting Method. Ann. Bot. 2016, 117, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Ishizawa, Y.; Sanada, T. Differences in Sugar Composition in Prunus Persica Fruit and the Classification by the Principal Component Analysis. J. Jpn. Soc. Hortic. Sci. 1990, 59, 307–312. [Google Scholar] [CrossRef]
- Gao, Z.; Maurousset, L.; Lemoine, R.; Yoo, S.-D.; Van Nocker, S.; Loescher, W. Cloning, Expression, and Characterization of Sorbitol Transporters from Developing Sour Cherry Fruit and Leaf Sink Tissues. Plant Physiol. 2003, 131, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Tajima, H.; Lerno, L.A.; Blumwald, E. Changes in Ethylene and Sugar Metabolism Regulate Flavonoid Composition in Climacteric and Non-Climacteric Plums during Postharvest Storage. Food Chem. Mol. Sci. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Zhou, H.; Su, M.; Du, J.; Zhang, X.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Crucial Roles of Sorbitol Metabolism and Energy Status in the Chilling Tolerance of Yellow Peach. Plant Physiol. Biochem. 2023, 204, 108092. [Google Scholar] [CrossRef]
- Sanches, A.G.; Pedrosa, V.M.D.; Checchio, M.V.; Fernandes, T.F.S.; Guevara, J.E.M.; Gratao, P.L.; de Almeida Teixeira, G.H. Polyols Can Alleviate Chilling Injury in ‘Palmer’Mangoes during Cold Storage. Food Control 2021, 129, 108248. [Google Scholar] [CrossRef]
- Sanches, A.G.; da Silva, M.B.; Wong, M.C.C.; de Oliveira, A.R.G.; Pedrosa, V.M.D.; Fernandes, T.F.S.; Gratão, P.L.; de Almeida Teixeira, G.H. Sorbitol Immersion Controls Chilling Injury in CA Stored ‘Palmer’Mangoes. Postharvest Biol. Technol. 2022, 185, 111800. [Google Scholar] [CrossRef]
- Bustamante, C.A.; Monti, L.L.; Gabilondo, J.; Scossa, F.; Valentini, G.; Budde, C.O.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Differential Metabolic Rearrangements after Cold Storage Are Correlated with Chilling Injury Resistance of Peach Fruits. Front. Plant Sci. 2016, 7, 1478. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Y.; Zhou, J.; Zhu, S. Effects of Exogenous Nitric Oxide on Contents of Soluble Sugars and Related Enzyme Activities in ‘Feicheng’Peach Fruit. J. Sci. Food Agric. 2011, 91, 1795–1800. [Google Scholar] [CrossRef]
- Brooks, S.J.; Moore, J.N.; Murphy, J.B. Quantitative and Qualitative Changes in Sugar Content of Peach Genotypes (Prunus persica (L.) Batsch). J. Am. Soc. Hortic. Sci. 1993, 118, 97–100. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.-Q. The Sucrose-Cleaving Enzymes of Plants Are Crucial for Development, Growth and Carbon Partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Itai, A.; Tanahashi, T. Inhibition of Sucrose Loss during Cold Storage in Japanese Pear (Pyrus pyrifolia Nakai) by 1-MCP. Postharvest Biol. Technol. 2008, 48, 355–363. [Google Scholar] [CrossRef]
- Cao, K.; Wei, Y.; Chen, Y.; Jiang, S.; Chen, X.; Wang, X.; Shao, X. PpCBF6 Is a Low-Temperature-Sensitive Transcription Factor That Binds the PpVIN2 Promoter in Peach Fruit and Regulates Sucrose Metabolism and Chilling Injury. Postharvest Biol. Technol. 2021, 181, 111681. [Google Scholar] [CrossRef]
- Vizzotto, G.; Pinton, R.; Varanini, Z.; Costa, G. Sucrose Accumulation in Developing Peach Fruit. Physiol. Plant. 1996, 96, 225–230. [Google Scholar] [CrossRef]
- Bianco, R.L.; Rieger, M.; Sung, S.-J.S. Activities of Sucrose and Sorbitol Metabolizing Enzymes in Vegetative Sinks of Peach and Correlation with Sink Growth Rate. J. Am. Soc. Hortic. Sci. 1999, 124, 381–388. [Google Scholar] [CrossRef]
- Zrenner, R.; Schüler, K.; Sonnewald, U. Soluble Acid Invertase Determines the Hexose-to-Sucrose Ratio in Cold-Stored Potato Tubers. Planta 1996, 198, 246–252. [Google Scholar] [CrossRef]
- Teo, G.; Suzuki, Y.; Uratsu, S.L.; Lampinen, B.; Ormonde, N.; Hu, W.K.; DeJong, T.M.; Dandekar, A.M. Silencing Leaf Sorbitol Synthesis Alters Long-Distance Partitioning and Apple Fruit Quality. Proc. Natl. Acad. Sci. USA 2006, 103, 18842–18847. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-Climacteric Ripening and Sorbitol Homeostasis in Plum Fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lei, C.; Tan, M.; Wang, J.; Li, C.; Zou, Y. Increased Soluble Sugar Accumulation in Postharvest Peaches in Response to Different Defense Priming Elicitors. Hortic. Environ. Biotechnol. 2023, 64, 115–131. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Sugars and Organic Acids in Japanese Plums (Prunus salicina Lindell) as Influenced by Maturation, Harvest Date, Storage Temperature and Period. Int. J. Food Sci. Technol. 2009, 44, 1973–1982. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A Simple and Efficient Method for Isolating RNA from Pine Trees. Plant Mol. Biol. Report. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, Z.; Wang, F.; Zhou, J.; Zhang, Z. Selection of Reliable Reference Genes for Gene Expression Studies in Peach Using Real-Time PCR. BMC Mol. Biol. 2009, 10, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farcuh, M. Understanding Chilling Injury and Sugar Metabolism-Related Genes and Metabolites in ‘Red Haven’ Peaches. Plants 2025, 14, 2133. https://doi.org/10.3390/plants14142133
Farcuh M. Understanding Chilling Injury and Sugar Metabolism-Related Genes and Metabolites in ‘Red Haven’ Peaches. Plants. 2025; 14(14):2133. https://doi.org/10.3390/plants14142133
Chicago/Turabian StyleFarcuh, Macarena. 2025. "Understanding Chilling Injury and Sugar Metabolism-Related Genes and Metabolites in ‘Red Haven’ Peaches" Plants 14, no. 14: 2133. https://doi.org/10.3390/plants14142133
APA StyleFarcuh, M. (2025). Understanding Chilling Injury and Sugar Metabolism-Related Genes and Metabolites in ‘Red Haven’ Peaches. Plants, 14(14), 2133. https://doi.org/10.3390/plants14142133




