Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions
Abstract
1. Introduction
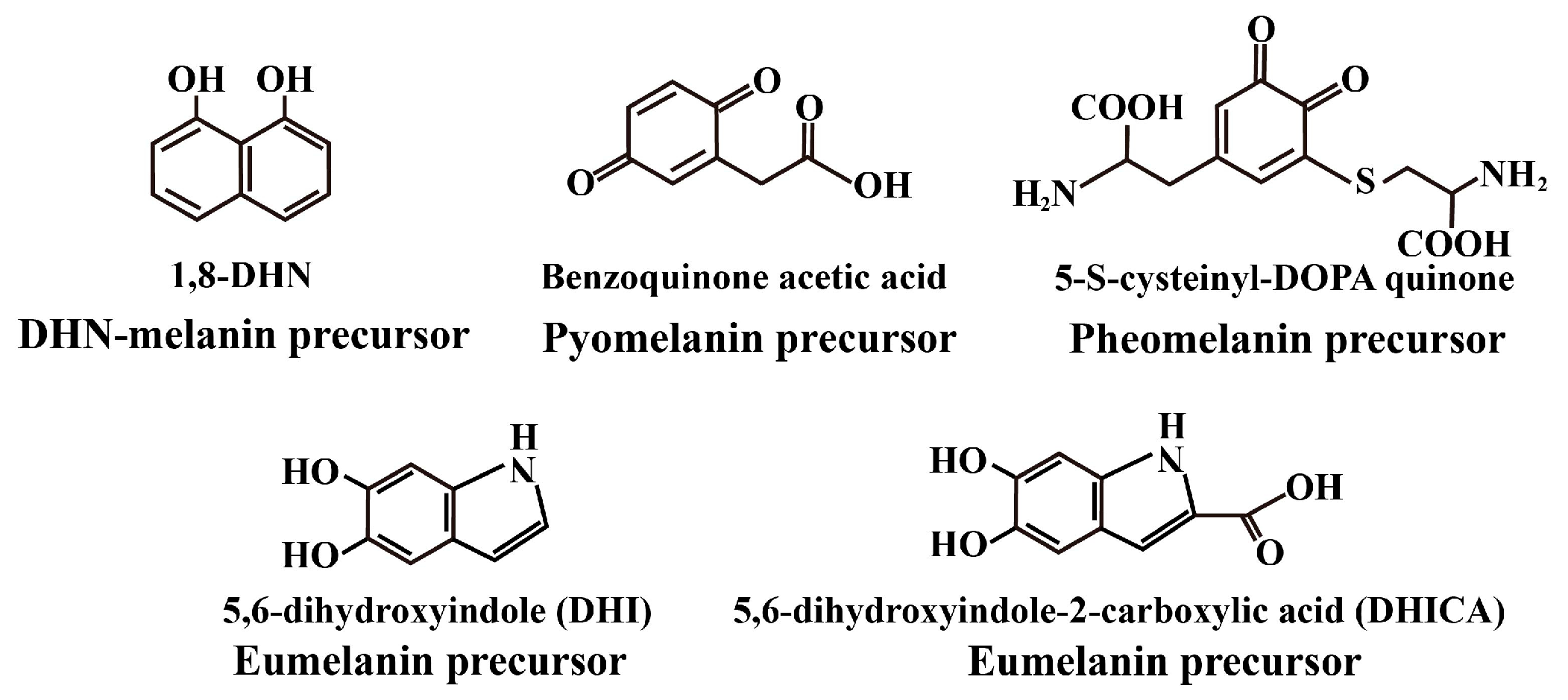
2. Classification and Biosynthetic Pathways of Fungal Melanin
3. The Relationship Between Melanin and Pathogenicity in Plant Pathogenic Fungi
3.1. Melanin Participates in Pathogenicity by Influencing Appressorial Integrity and Turgor Pressure Formation
3.2. Melanin Influences the Formation of Virulence Factors
3.3. Melanin Participates in Pathogen Overwintering
3.4. Melanin Is Not Involved in Pathogenicity in Specific Fungal Taxa
4. The Relationship Between Melanin and Stress Resistance in Plant Pathogenic Fungi
4.1. Resistance to UV Radiation
4.2. Heat Resistance
4.3. Heavy Metal Adsorption
5. Mechanisms of Action of Melanin Inhibitors Against Plant Pathogenic Fungi
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nicolaus, R.A. Melanins; Hermann Press: Paris, France, 1968. [Google Scholar]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [PubMed]
- Negro, J.J.; Finlayson, C.; Galván, I. Melanins in fossil animals: Is it possible to infer life history traits from the coloration of extinct species? Int. J. Mol. Sci. 2018, 19, 230. [Google Scholar]
- McNamara, M.E.; Rossi, V.; Slater, T.S.; Rogers, C.S.; Ducrest, A.-L.; Dubey, S.; Roulin, A. Decoding the evolution of melanin in vertebrates. Trends Ecol. Evol. 2021, 36, 430–443. [Google Scholar]
- Hu, S.S.; Dai, Y.Y.; Bai, S.C.; Zhao, B.H.; Wu, X.S.; Chen, Y. GNAI2 promotes proliferation and decreases apoptosis in rabbit melanocytes. Genes 2021, 12, 1130. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar]
- Singla, S.S.; Htut, K.Z.; Zhu, R.Y.; Davis, A.; Ma, J.Y.; Ni, Q.Z.; Burkart, M.D.; Maurer, C.; Miyoshi, T.; Dhinojwala, A. Isolation and characterization of allomelanin from pathogenic black knot fungus-a sustainable source of melanin. ACS Omega 2021, 6, 35514–35522. [Google Scholar]
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257. [Google Scholar] [PubMed]
- Nosanchuk, J.D.; Rosas, A.L.; Casadevall, A. The antibody response to fungal melanin in mice. J. Immunol. 1998, 160, 6026–6031. [Google Scholar]
- Rahmani Eliato, T.; Smith, J.T.; Tian, Z.; Kim, E.-S.; Hwang, W.; Andam, C.P.; Kim, Y.J. Melanin pigments extracted from horsehair as antibacterial agents. J. Mater. Chem. B 2021, 9, 1536–1545. [Google Scholar]
- Montefiori, D.C.; Zhou, J.Y. Selective antiviral activity of synthetic soluble L-tyrosine and L-dopa melanins against human immunodeficiency virus in vitro. Antivir. Res. 1991, 15, 11–25. [Google Scholar]
- ElObeid, A.S.; Kamal-Eldin, A.; Abdelhalim, M.A.K.; Haseeb, A.M. Pharmacological properties of melanin and its function in health. Basic Clin. Pharmacol. Toxicol. 2017, 120, 515–522. [Google Scholar] [PubMed]
- Steiner, U.; Oerke, E.-C. A melanin-deficient isolate of Venturia inaequalis reveals various roles of melanin in pathogen life cycle and fitness. J. Fungi 2022, 9, 35. [Google Scholar]
- Howard, R.J.; Valent, B. Breaking and entering: Host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar]
- Ni, L.N. Isolation and identification of a high-melanin-producing bacterium. Microbiol. China 2004, 01, 55–59. [Google Scholar]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar]
- Palonen, E.K.; Raina, S.; Brandt, A.; Meriluoto, J.; Keshavarz, T.; Soini, J.T. Melanisation of Aspergillus terreus-is butyrolactone I involved in the regulation of both DOPA and DHN types of pigments in submerged culture? Microorganisms 2017, 5, 22. [Google Scholar] [PubMed]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar]
- Wheeler, M.H. Comparisons of fungal melanin biosynthesis in ascomycetous, imperfect and basidiomycetous fungi. Trans. Br. Mycol. Soc. 1983, 81, 29–36. [Google Scholar]
- Wang, Y.; Aisen, P.; Casadevall, A. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 1996, 64, 2420–2424. [Google Scholar]
- Eisenman, H.C.; Frases, S.; Nicola, A.M.; Rodrigues, M.L.; Casadevall, A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology 2009, 155, 3860–3867. [Google Scholar]
- Zaidi, K.D.; Ali, A.S.; Ali, S.A. Purification and characterization of melanogenic enzyme tyrosinase from button mushroom. Enzyme Res. 2014, 2014, 120739. [Google Scholar] [PubMed]
- Olaizola, C.; Abramowski, Z.; Ayala, E.J. Photoprotective effect of fungal melanins against UVB in human skin cells. Micol. Apl. Int. 2013. [Google Scholar]
- Lu, M.; Yu, M.; Shi, T.; Ma, J.; Fu, X.; Meng, X.; Shi, L. Optimization of ultrasound-assisted extraction of melanin and its hypoglycemic activities from Sporisorium reilianum. J. Food Process Pres. 2020, 44, e14707. [Google Scholar]
- Ozturk, I.K.; Chettri, P.; Dupont, P.Y.; Barnes, I.; McDougal, R.L.; Moore, G.G.; Sim, A.; Bradshaw, R.E. Evolution of polyketide synthesis in a Dothideomycete forest pathogen. Fungal Genet. Biol. 2017, 106, 42–50. [Google Scholar]
- Bell, A.A.; Puhalla, J.E.; Tolmsoff, W.J.; Stipanovic, R.D. Use of mutants to establish (+)—Scytalone as an intermediate in melanin biosynthesis by Verticillium dahliae. Can. J. Microbiol. 1976, 22, 787–799. [Google Scholar] [PubMed]
- Zhao, S.J.; Yin, R.Y.; Zhang, M.W.; Zhai, Z.Q.; Shen, Z.; Mou, Y.; Xu, D.; Zhou, L.G.; Lai, D.W. Efficient gene editing in the slow-growing, non-sporulating, melanized, endophytic fungus Berkleasmium sp. Dzf12 using a CRISPR/Cas9 system. World J. Microbiol. Biotechnol. 2024, 40, 176. [Google Scholar]
- Schumacher, J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)—Encoding genes. Mol. Microbiol. 2016, 99, 729–748. [Google Scholar]
- Qin, Y.P.; Xia, Y.X. Melanin in fungi: Advances in structure, biosynthesis, regulation, and metabolic engineering. Microb. Cell Fact. 2024, 23, 334. [Google Scholar]
- Zeng, F.L.; Meng, Y.N.; Hao, Z.M.; Li, P.; Zhai, W.B.; Shen, S.S.; Cao, Z.Y.; Dong, J.G. Setosphaeria turcica ATR turns off appressorium-mediated maize infection and triggers melanin-involved self-protection in response to genotoxic stress. Mol. Plant Pathol. 2020, 3, 401–414. [Google Scholar]
- Chen, X.; Zhu, C.; Na, Y.; Ren, D.; Zhang, C.; He, Y.; Wang, Y.; Xiang, S.; Ren, W.; Jiang, Y.; et al. Compartmentalization of melanin biosynthetic enzymes contributes to self-defense against intermediate compound scytalone in Botrytis cinerea. mBio 2021, 12, e00007-21. [Google Scholar]
- Liu, N.; Cao, Z.Y.; Cao, K.K.; Ma, S.X.; Gong, X.D.; Jia, H.; Dai, D.Q.; Dong, J.G. Identification of laccase-like multicopper oxidases from the pathogenic fungus Setosphaeria turcica and their expression pattern during growth and infection. Eur. J. Plant Pathol. 2019, 153. [Google Scholar]
- Zhang, Z.X.; Jia, H.; Liu, N.; Li, H.X.; Meng, Q.J.; Wu, N.; Cao, Z.Y.; Dong, J.G. The zinc finger protein StMR1 affects the pathogenicity and melanin synthesis of Setosphaeria turcica and directly regulates the expression of DHN melanin synthesis pathway genes. Mol. Microbiol. 2022, 117, 261–273. [Google Scholar] [PubMed]
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal melanin: What do we know about structure? Front. Microbiol. 2015, 6, 1463. [Google Scholar]
- Money, N.P.; Caesar-TonThat, T.C.; Frederick, B.; Henson, J.M. Melanin synthesis is associated with changes in hyphopodial turgor, permeability, and wall rigidity in gaeumannomyces graminis var. graminis. Fungal Genet. Biol. 1998, 24, 240–251. [Google Scholar]
- Jacobson, E.S. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 4, 708–717. [Google Scholar]
- Zhang, P.; Zhou, S.; Wang, G.; An, Z.Q.; Liu, X.Z.; Li, K.; Yin, W.B. Two transcription factors cooperatively regulate DHN melanin biosynthesis and development in Pestalotiopsis Fici. Mol. Microbiol. 2019, 112, 649–666. [Google Scholar]
- Alviano, D.S.; Franzen, A.J.; Travassos, L.R.; Holandino, C.; Rozental, S.; Ejzemberg, R.; Alviano, C.S.; Rodrigues, M.L. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 2004, 72, 229–237. [Google Scholar]
- Nosanchuk, J.D.; Casadevall, A. Cellular charge of Cryptococcus neoformans: Contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 1997, 65, 1836–1841. [Google Scholar]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.P. Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar]
- Heinekamp, T.; Thywißen, A.; Macheleidt, J.; Keller, S.; Valiante, V.; Brakhage, A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2013, 3, 440. [Google Scholar]
- Sichel, G.; Corsaro, C.; Scalia, M.; Di Bilio, A.J.; Bonomo, R.P. In vitro scavenger activity of some favonoids and melanins against O2−dot. Free Radic. Biol. Med. 1991, 11, 1–8. [Google Scholar] [PubMed]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [PubMed]
- Ryder, L.S.; Talbot, N.J. Regulation of appressorium development in pathogenic fungi. Curr. Opin. Plant Biol. 2015, 26, 8–13. [Google Scholar]
- Quime, B.G.; Ryder, L.S.; Talbot, N.J. Live cell imaging of plant infection provides new insight into the biology of pathogenesis by the rice blast fungus Magnaporthe oryzae. J. Microsc. 2025, 297, 274–288. [Google Scholar]
- Dagdas, Y.F.; Yoshino, K.; Dagdas, G.; Ryder, L.S.; Bielska, E.; Steinberg, G.; Talbot, N.J. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 2012, 336, 1590–1595. [Google Scholar]
- Ryder, L.S.; Dagdas, Y.F.; Kershaw, M.J.; Venkataraman, C.; Madzvamuse, A.; Yan, X.; Cruz-Mireles, N.; Soanes, D.M.; Oses-Ruiz, M.; Styles, V.; et al. A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature 2019, 574, 423–427. [Google Scholar]
- Chumley, F.G.; Valent, B. Genetic analysis of melanin-deficient, nonpathogenic mutants of Magnaporthe grisea. Mol. Plant Microbe Interact 1990, 3, 135. [Google Scholar]
- Bearer, E.L.; Satpute-Krishnan, P. The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: Tracks, motors, and polymerization machines. Curr. Drug Targets Infect. Disord. 2002, 2, 247–264. [Google Scholar]
- Sinha, J.; Singh, Y.; Verma, P.K. Cytoskeleton remodeling: A central player in plant-fungus interactions. J. Exp. Bot. 2024, 75, 3269–3286. [Google Scholar]
- He, M.; Su, J.; Xu, Y.P.; Chen, J.Y.; Chern, M.S.; Lei, M.L.; Qi, T.; Wang, Z.K.; Ryder, L.S.; Tang, B.Z.; et al. Discovery of broad-spectrum fungicides that block septin-dependent infection processes of pathogenic fungi. Nat. Microbiol. 2020, 5, 1565–1575. [Google Scholar]
- Huang, P.Y.; Cao, H.J.; Li, Y.; Zhu, S.Y.; Wang, J.; Wang, Q.; Liu, X.H.; Lin, F.C.; Lu, J.P. Melanin promotes spore production in the rice blast fungus Magnaporthe oryzae. Front. Microbiol. 2022, 13, 843838. [Google Scholar]
- Tsuji, G.; Kenmochi, Y.; Takano, Y.; Sweigard, J.; Farrall, L.; Furusawa, I.; Horino, O.; Kubo, Y. Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol. Microbiol. 2000, 38, 940–954. [Google Scholar]
- Cho, Y.; Srivastava, A.; Ohm, R.A.; Lawrence, C.B.; Wang, K.-H.; Grigoriev, I.V.; Marahatta, S.P. Transcription factor Amr1 induces melanin biosynthesis and suppresses virulence in Alternaria brassicicola. PLoS Pathog. 2012, 8, e1002974. [Google Scholar]
- Wang, X.L.; Lu, D.X.; Tian, C.M. Analysis of melanin biosynthesis in the plant pathogenic fungus Colletotrichum gloeosporioides. Fungal Biol. 2021, 125, 679–692. [Google Scholar] [PubMed]
- Qin, X.Y.; Tian, C.M.; Meng, F.L. Comparative transcriptome analysis reveals the effect of the DHN melanin biosynthesis pathway on the appressorium turgor pressure of the poplar anthracnose-causing fungus Colletotrichum gloeosporioides. Int. J. Mol. Sci. 2023, 24, 7411. [Google Scholar]
- Wang, T.; Ren, D.D.; Guo, H.; Chen, X.; Zhu, P.K.; Nie, H.Z.; Xu, L. CgSCD1 is essential for melanin biosynthesis and pathogenicity of Colletotrichum gloeosporioides. Pathogens. 2020, 9, 141. [Google Scholar]
- Meng, Y.N.; Zeng, F.L.; Hu, J.J.; Li, P.; Xiao, S.L.; Zhou, L.H.; Gong, J.G.; Liu, Y.W.; Hao, Z.M.; Cao, Z.Y.; et al. Novel factors contributing to fungal pathogenicity at early stages of Setosphaeria turcica infection. Mol. Plant Pathol. 2022, 23, 32–44. [Google Scholar]
- Guo, X.Y.; Liu, N.; Liu, B.H.; Zhou, L.H.; Cao, Z.Y.; Han, J.M.; Dong, J.G. Melanin, DNA replication, and autophagy affect appressorium development in Setosphaeria turcica by regulating glycerol accumulation and metabolism. J. Integr. Agric. 2022, 21, 762–773. [Google Scholar]
- Ma, S.X.; Cao, K.K.; Liu, N.; Meng, C.; Cao, Z.Y.; Dai, D.Q.; Jia, H.; Zang, J.P.; Li, Z.Y.; Hao, Z.M.; et al. The StLAC2 gene is required for cell wall integrity, DHN-melanin synthesis and the pathogenicity of Setosphaeria turcica. Fungal Biol. 2017, 121, 589–601. [Google Scholar]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar]
- Ludwig, N.; Löhrer, M.; Hempel, M.; Mathea, S.; Schliebner, I.; Menzel, M.; Kiesow, A.; Schaffrath, U.; Deising, H.B.; Horbach, R. Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 2014, 27, 315–327. [Google Scholar] [PubMed]
- Li, R.; Li, Y.C.; Xu, W.Y.; Liu, W.J.; Xu, X.B.; Bi, Y.; Prusky, D. Aabrm1-mediated melanin synthesis is essential to growth and development, stress adaption, and pathogenicity in Alternaria alternata. Front. Microbiol. 2024, 14, 1327765. [Google Scholar]
- Gao, J.X.; Yu, C.J.; Wang, M.; Sun, J.N.; Li, Y.Q.; Chen, J. Involvement of a velvet protein ClVelB in the regulation of vegetative differentiation, oxidative stress response, secondary metabolism, and virulence in Curvularia lunata. Sci. Rep. 2017, 7, 46054. [Google Scholar]
- Sun, W.Q.; Zhao, L.H.; Zhou, J.L.; Feng, H.J.; Zhang, Y.L.; Feng, Z.L.; Zhu, H.Q.; Wei, F. VdP5CDH is involved in melanin formation, stress resistance and play a regulatory role in virulence of Verticillium dahliae. Front. Microbiol. 2024, 15, 1429755. [Google Scholar]
- Fan, R.; Klosterman, S.J.; Wang, C.H.; Subbarao, K.V.; Xu, X.M.; Shang, W.J.; Hu, X.P. Vayg1 is required for microsclerotium formation and melanin production in Verticillium dahliae. Fungal Genet. Biol. 2017, 98, 1–11. [Google Scholar]
- Li, H.; Wang, D.; Zhang, D.D.; Geng, Q.; Li, J.J.; Sheng, R.C.; Xue, H.S.; Zhu, H.; Kong, Z.Q.; Dai, X.F.; et al. A polyketide synthase from Verticillium dahliae modulates melanin biosynthesis and hyphal growth to promote virulence. BMC Biol. 2022, 20, 125. [Google Scholar]
- Li, J.J.; Zhou, L.; Yin, C.M.; Zhang, D.D.; Klosterman, S.J.; Wang, B.L.; Song, J.; Wang, D.; Hu, X.P.; Subbarao, K.V.; et al. The Verticillium dahliae Sho1-MAPK pathway regulates melanin biosynthesis and is required for cotton infection. Environ. Microbiol. 2019, 21, 4852–4874. [Google Scholar]
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe Jr, G.S.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803. [Google Scholar]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar]
- Li, Y.H.; Li, J.M.; Cai, X.X.; Gao, M.Y.; Diao, H.L.; Xiang, H.M.; Zhou, W.W.; Ma, R.Y. Microsclerotia formation of the biocontrol fungus Cordyceps javanica IF-1106 and evaluation of its stress tolerance and pathogenicity. Front. Microbiol. 2025, 16, 1583850. [Google Scholar]
- Zhang, C.H.; He, Y.F.; Zhu, P.K.; Chen, L.; Wang, Y.W.; Ni, B.; Xu, L. Loss of bcbrn1 and bcpks13 in Botrytis cinerea not only blocks melanization but also increases vegetative growth and virulence. Mol. Plant Microbe Interact. 2015, 28, 1091–1101. [Google Scholar] [PubMed]
- Liang, Y.; Xiong, W.; Steinkellner, S.; Feng, J. Deficiency of the melanin biosynthesis genes SCD1 and THR1 affects sclerotial development and vegetative growth, but not pathogenicity, in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2018, 19, 1444–1453. [Google Scholar] [PubMed]
- Derbyshire, M.C.; Gohari, A.M.; Mehrabi, R.; Kilaru, S.; Steinberg, G.; Ali, S.; Bailey, A.; Hammond-Kosack, K.; Kema, G.H.J.; Rudd, J.J. Phosphopantetheinyl transferase (Ppt)-mediated biosynthesis of lysine, but not siderophores or DHN melanin, is required for virulence of Zymoseptoria tritici on wheat. Sci. Rep. 2018, 8, 17069. [Google Scholar]
- Campana, R.; Fanelli, F.; Sisti, M. Role of melanin in the black yeast fungi Aureobasidium pullulans and Zalaria obscura in promoting tolerance to environmental stresses and to antimicrobial compounds. Fungal Biol. 2022, 126, 817–825. [Google Scholar]
- Zhou, Y.L.; Panhale, A.; Shvedunova, M.; Balan, M.; Gomez-Auli, A.; Holz, H.; Seyfferth, J.; Helmstädter, M.; Kayser, S.; Zhao, Y.L.; et al. RNA damage compartmentalization by DHX9 stress granules. Cell 2024, 187, 1701–1718. [Google Scholar]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [PubMed]
- Wang, Y.L.; Hu, X.P.; Fang, Y.L.; Anchieta, A.; Goldman, P.H.; Hernandez, G.; Klosterman, S.J. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae. Microbiology 2018, 164, 685–696. [Google Scholar]
- Yu, X.; Huo, L.; Liu, H.; Chen, L.F.; Wang, Y.; Zhu, X.D. Melanin is required for the formation of the multi-cellular conidia in the endophytic fungus Pestalotiopsis microspora. Microbiol. Res. 2015, 179, 1–11. [Google Scholar]
- Fan, R.; Wang, C.S.; Zhao, R.; Pan, Y.H.; Long, Y.H.; Zhao, Z.B. Research on 1,8-dihydroxynaphthalene melanin in plant pathogenic fungi. Microbiology. 2020, 47, 3671–3677. [Google Scholar]
- Yang, F.; Cheng, L.T.; Du, Y.L.; Xia, L.G.; Long, C.A. Functional identification of the DHN melanin synthesis gene cluster and its role in UV-C tolerance in citrus postharvest pathogenic fungus Penicillium digitatum. Fungal Biol. 2022, 126, 566–575. [Google Scholar]
- Kwon-Chung, K.J.; Polacheck, I.; Popkin, T.J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982, 150, 1414–1421. [Google Scholar] [PubMed]
- Casadevall, A.; Cordero, R.J.B.; Bryan, R.; Nosanchuk, J.; Dadachova, E. Melanin, radiation, and energy transduction in fungi. Microbiol. Spectr. 2017, 5, 10.1128. [Google Scholar]
- Mironenko, N.V.; Alekhina, I.A.; Zhdanova, N.N.; Bulat, S.A. Intraspecific variation in gamma-radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: A case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol. Environ. Saf. 2000, 45, 177–187. [Google Scholar]
- Suthar, M.; Dufossé, L.; Singh, S.K. The enigmatic world of fungal melanin: A comprehensive review. J. Fungi 2023, 9, 891. [Google Scholar]
- Peng, Z.; Luo, S.; Zhang, Z.J. Biosynthetic melanin with excellent performance can be used for heavy metal adsorption. J. Clean. Prod. 2023, 385, 135655. [Google Scholar]
- Blanchette, M.D.A. Metal ion adsorption by pseudosclerotial plates of Phellinus eirii. Mycologia 1996, 88, 98. [Google Scholar]
- Tran-Ly, A.N.; Ribera, J.; Schwarze, F.W.; Brunelli, M.; Fortunato, G. Fungal melanin-based electrospun membranes for heavy metal detoxification of water. Sustain. Mater. Technol. 2020, 23, e00146. [Google Scholar]
- Ushakova, N.; Dontsov, A.; Sakina, N.; Bastrakov, A.; Ostrovsky, M. Antioxidative properties of melanins and ommochromes from black soldier fly Hermetia illucens. Biomolecules 2019, 9, 408. [Google Scholar] [CrossRef]
- Hamada, T.; Asanagi, M.; Satozawa, T.; Araki, N.; Banba, S.; Higashimura, N.; Akase, T.; Hirase, K. Action mechanism of the novel rice blast fungicide tolprocarb distinct from that of conventional melanin biosynthesis inhibitors. J. Pestic. Sci. 2014, 39, 152–158. [Google Scholar]
- Banba, S.; Hamada, T.; Araki, N.; Ebihara, K. Synthesis and activities of tolprocarb derivatives against Pyricularia oryzae: Relationships among the activities for polyketide synthase, melanin biosynthesis, and rice blast. J. Pestic. Sci. 2017, 42, 25–31. [Google Scholar]
- Thompson, J.E.; Basarab, G.S.; Andersson, A.; Lindqvist, Y.; Jordan, D.B. Trihydroxynaphthalene reductase from Magnaporthe grisea: Realization of an active center inhibitor and elucidation of the kinetic mechanism. Biochemistry 1997, 36, 1852–1860. [Google Scholar] [PubMed]
- Mattoon, E.R.; Cordero, R.J.B.; Casadevall, A. Fungal melanins and applications in healthcare, bioremediation and industry. J. Fungi 2021, 7, 488. [Google Scholar]
- Chaudhary, D.; Chong, F.C.; Neupane, T.; Choi, J.; Jee, J.G. New inhibitors of laccase and tyrosinase by examination of cross-inhibition between copper-containing enzymes. Int. J. Mol. Sci. 2021, 22, 13661. [Google Scholar] [PubMed]
- Lee, M.K.; Ryu, H.; Jeong, H.H.; Lee, B. Brassinin abundant in Brassicaceae suppresses melanogenesis through dual mechanisms of tyrosinase inhibition. Foods 2022, 12, 121. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Liu, N.; Zhang, L.; Li, P.; Meng, Y.; Yuan, W.; Li, H.; Tantai, D.; Qu, Q.; Cao, Z.; et al. Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions. Plants 2025, 14, 2121. https://doi.org/10.3390/plants14142121
Jia H, Liu N, Zhang L, Li P, Meng Y, Yuan W, Li H, Tantai D, Qu Q, Cao Z, et al. Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions. Plants. 2025; 14(14):2121. https://doi.org/10.3390/plants14142121
Chicago/Turabian StyleJia, Hui, Ning Liu, Lu Zhang, Pan Li, Yanan Meng, Wei Yuan, Haixiao Li, Dezeng Tantai, Qing Qu, Zhiyan Cao, and et al. 2025. "Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions" Plants 14, no. 14: 2121. https://doi.org/10.3390/plants14142121
APA StyleJia, H., Liu, N., Zhang, L., Li, P., Meng, Y., Yuan, W., Li, H., Tantai, D., Qu, Q., Cao, Z., & Dong, J. (2025). Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions. Plants, 14(14), 2121. https://doi.org/10.3390/plants14142121





