Hydrogen Peroxide and Vitexin in the Signaling and Defense Responses of Passiflora incarnata Under Drought Stress
Abstract
1. Introduction
2. Results
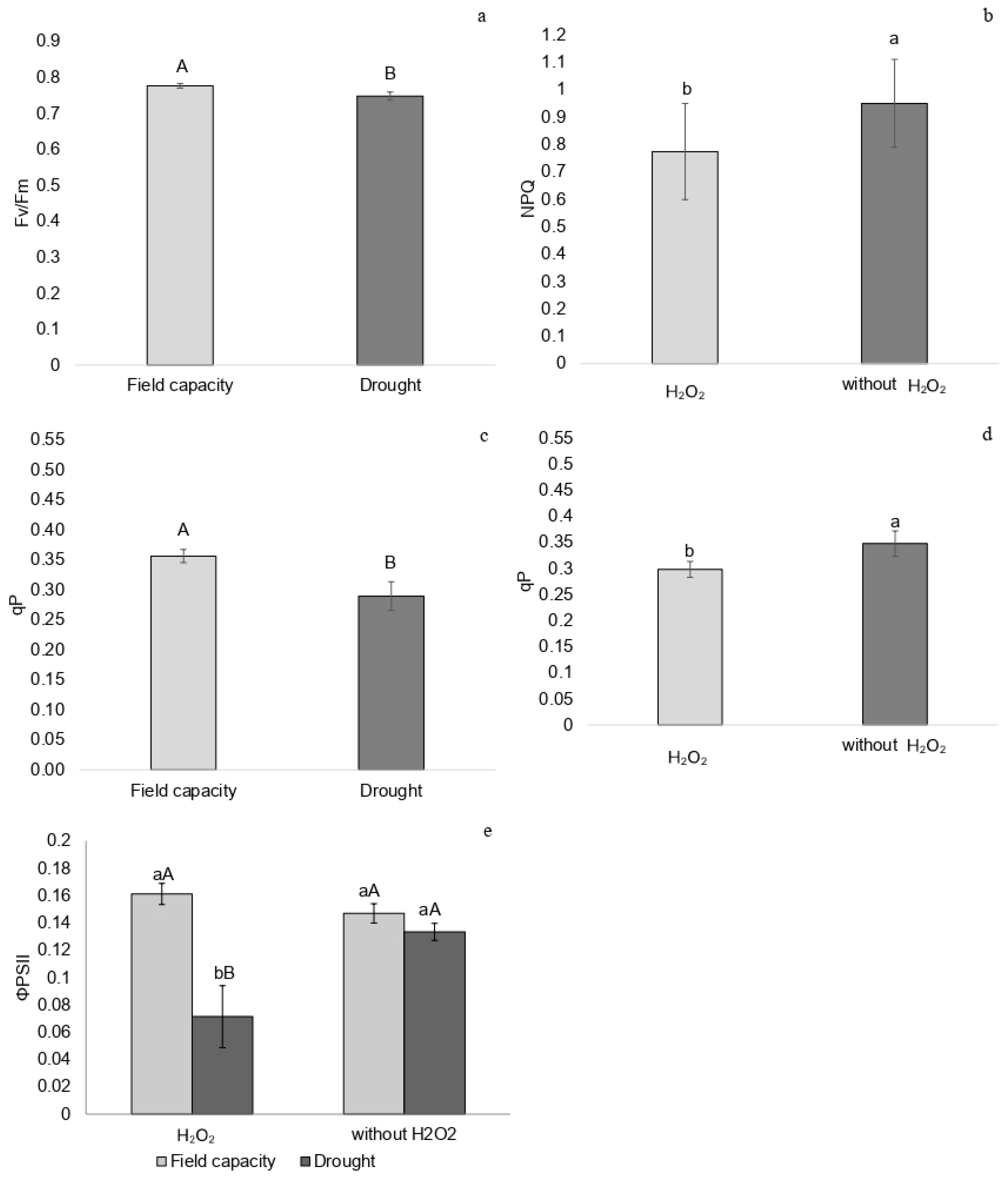
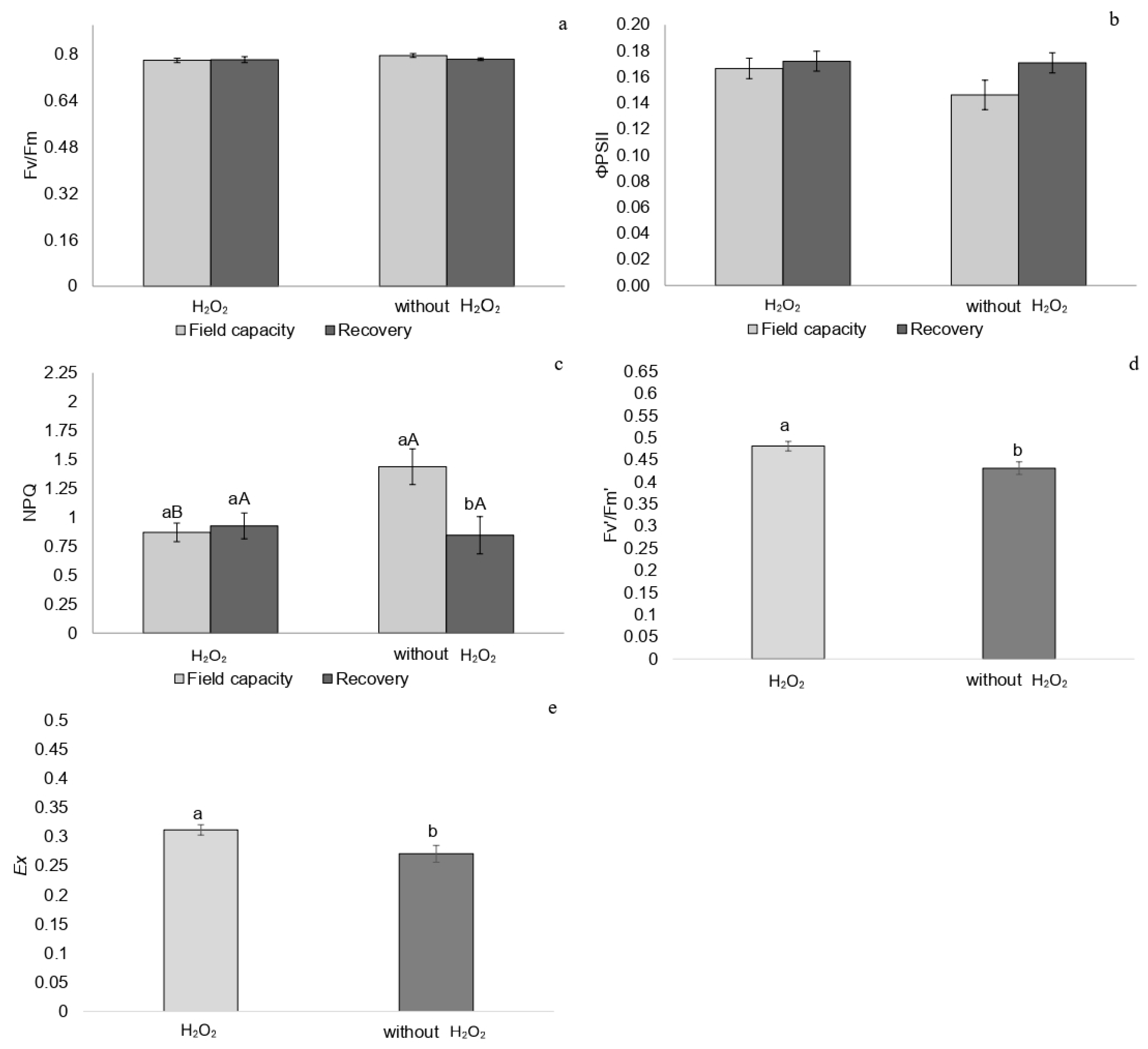
2.1. Chlorophyll a Fluorescence
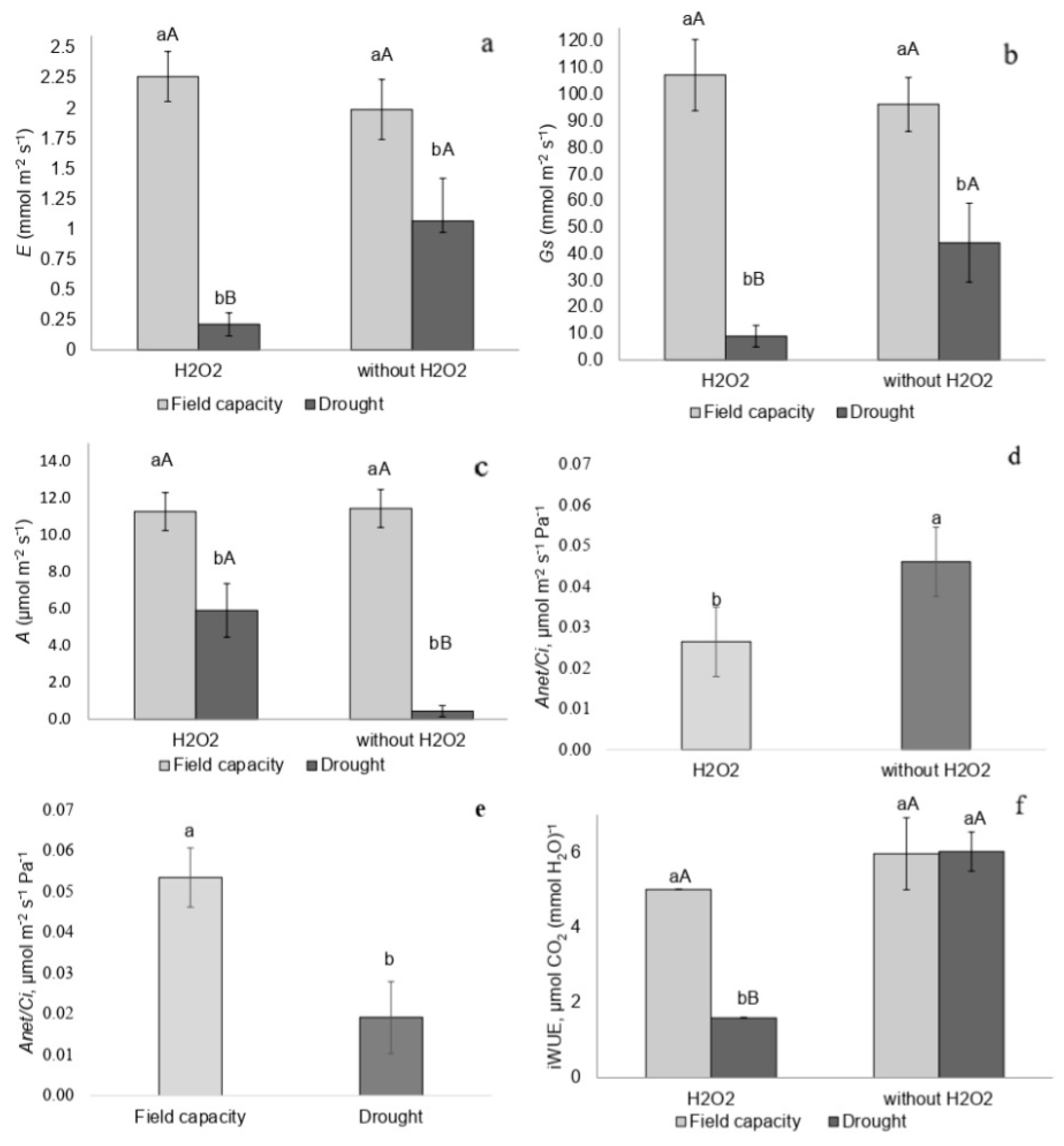
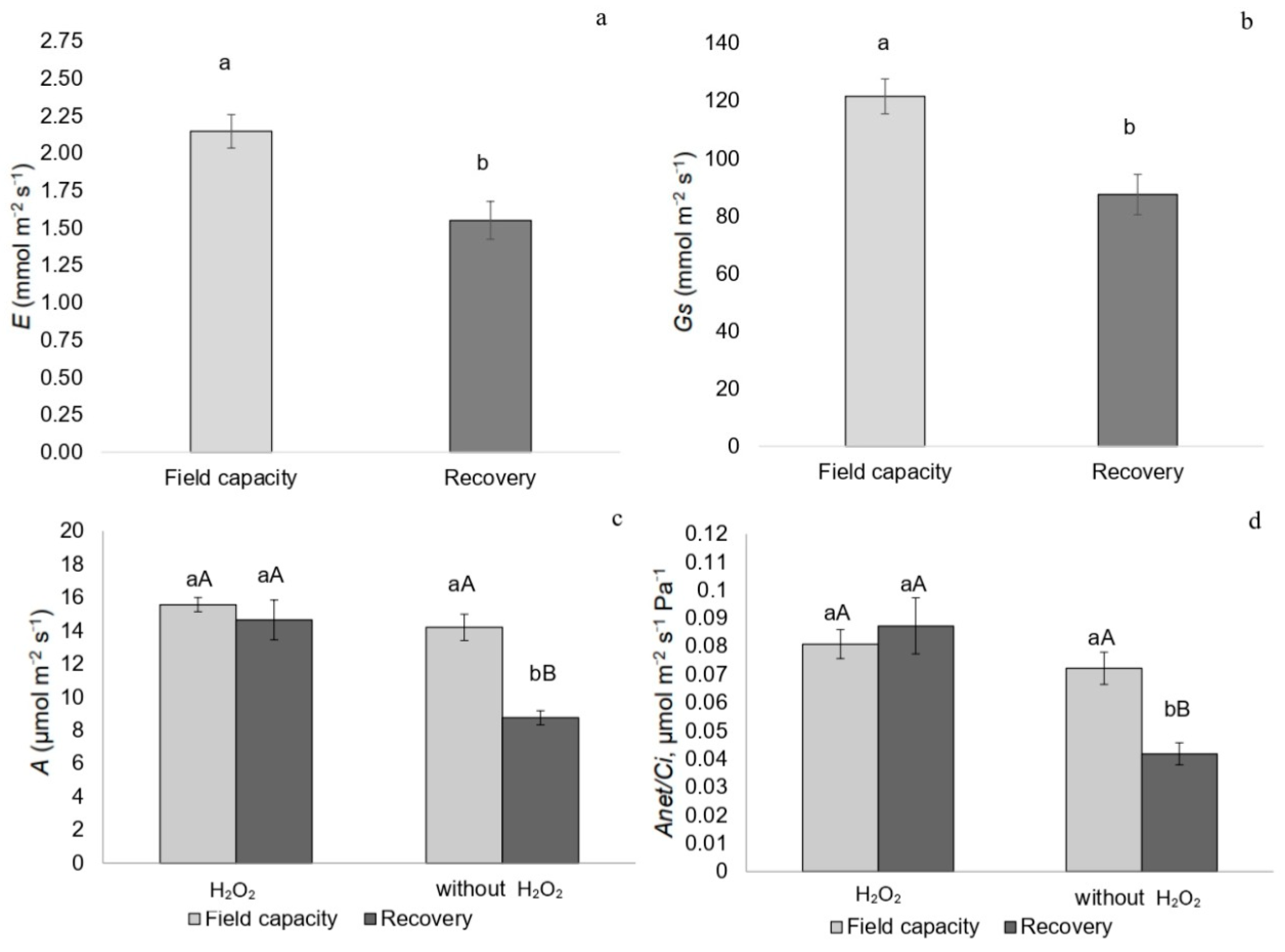
2.2. Gas Exchange
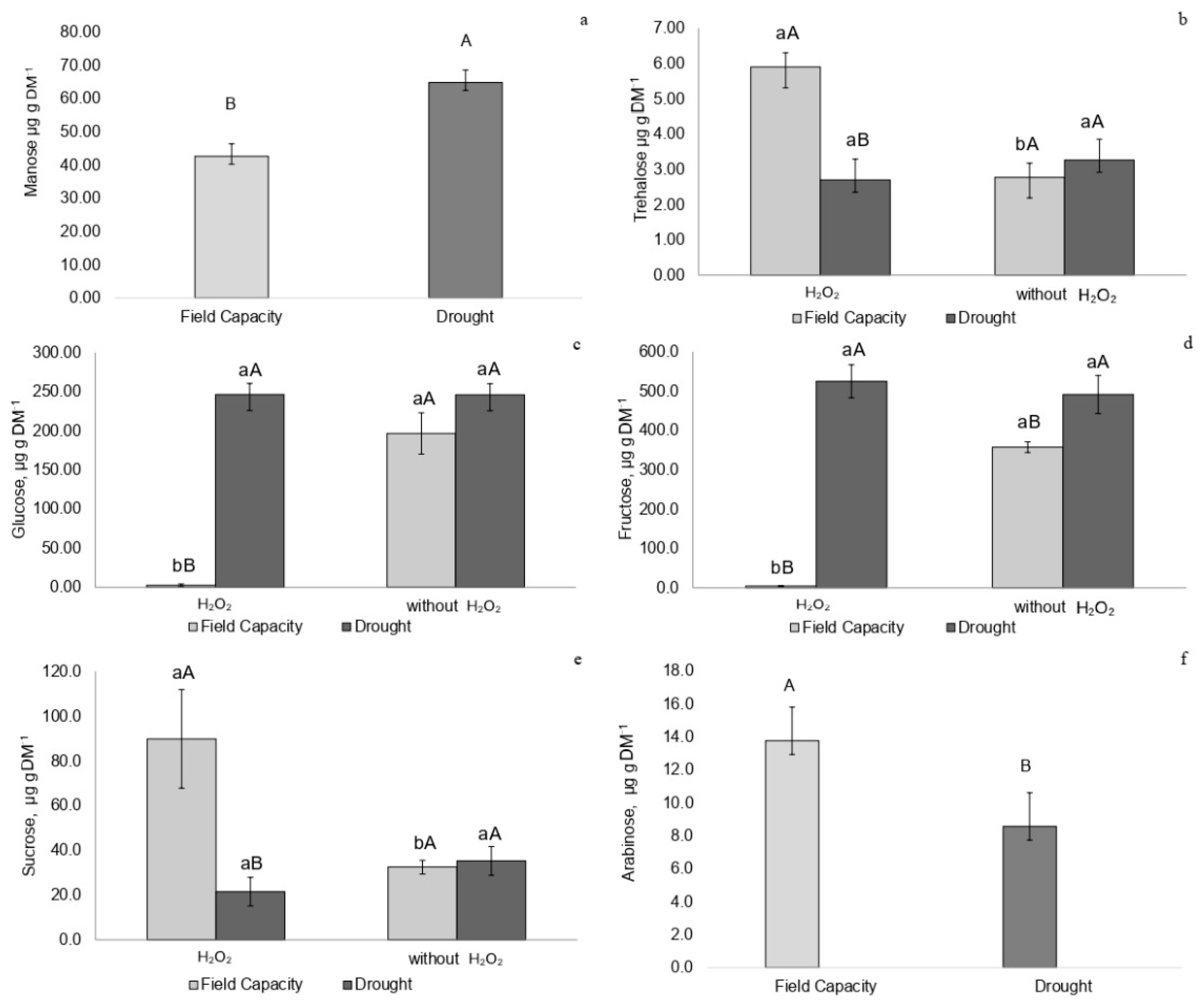
2.3. Carbohydrates Profile
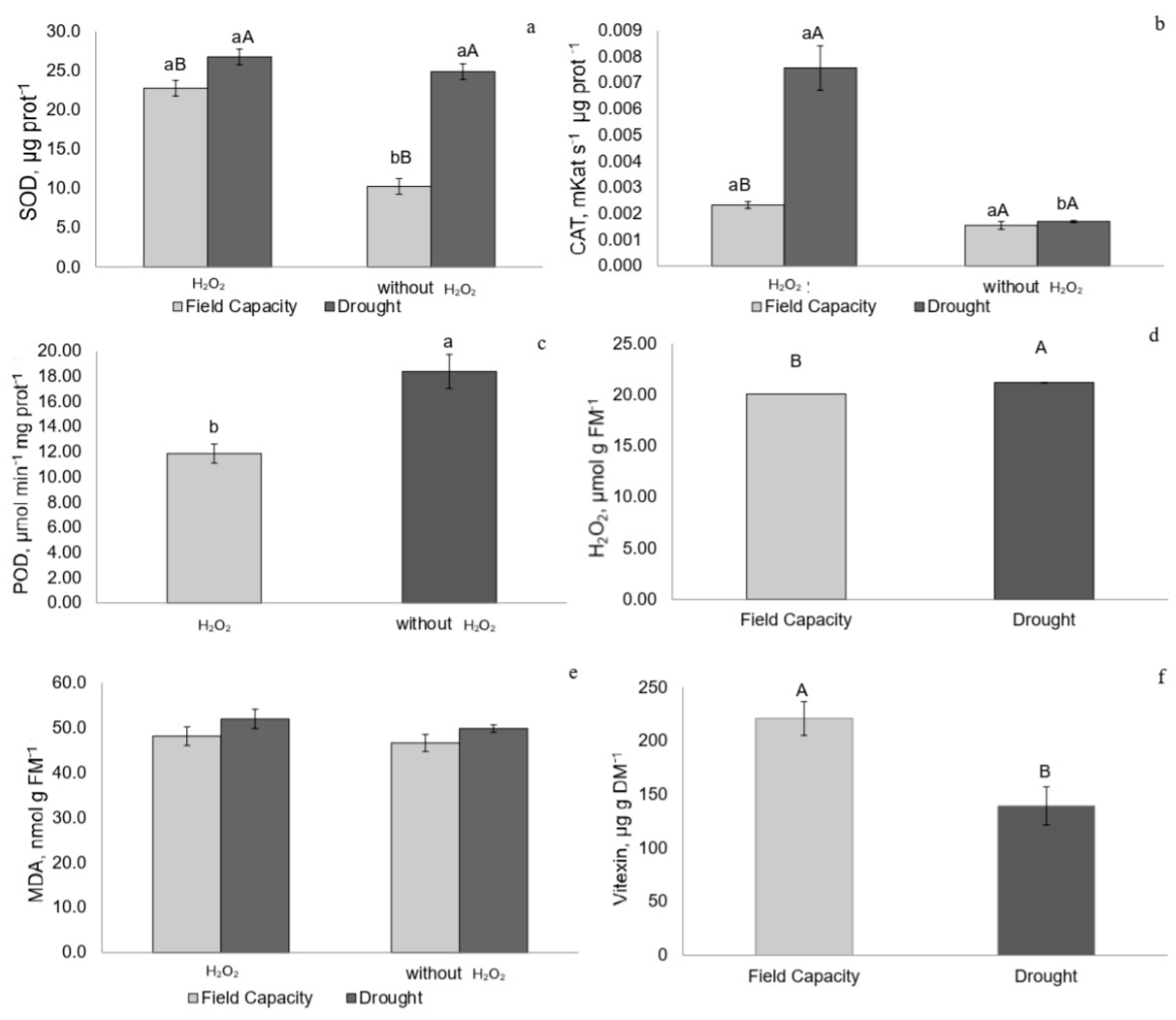
2.4. Enzymatic Activity of Superoxide Dismutase, Catalase, and Peroxidase
2.5. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Concentrations
2.6. Vitexin Concentration
2.7. Pearson Correlation Coefficients
3. Discussion
3.1. Photochemical Performance and Stress Intensity
3.2. Gas Exchange and Chlorophyll a Fluorescence
3.3. Sugar Metabolism and Osmotic Adjustment
3.4. Antioxidant Responses and H2O2 Signaling
3.5. Role of Vitexin in Membrane Protection
3.6. Post-Stress Recovery and Acclimation Mechanisms
4. Material and Methods
4.1. Growth Conditions, Experimental Design, and Treatments
4.2. Soil Water Potential
4.3. Chlorophyll a Fluorescence and Gas Exchange
4.4. Carbohydrate Extraction and Profiling
4.5. Lipid Peroxidation and Hydrogen Peroxide Content
4.6. Enzymatic Activity of Superoxide Dismutase (SOD, EC 1.15.1.1), Catalase (CAT, EC 1.11.1.6), and Peroxidase (POD, EC 1.11.1.7)
4.7. Vitexin
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Barzotto, G.R.; Cardoso, C.P.; Jorge, L.G.; Campos, F.G.; Boaro, C.S.F. Hydrogen Peroxide Signal Photosynthetic Acclimation of Solanum lycopersicum L. Cv Micro-Tom under Water Deficit. Sci. Rep. 2023, 13, 13059. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Wu, B.; Qi, F.; Liang, Y. Fuels for ROS Signaling in Plant Immunity. Trends Plant Sci. 2023, 28, 1124–1131. [Google Scholar] [CrossRef]
- Ang, M.C.Y.; Saju, J.M.; Porter, T.K.; Mohaideen, S.; Sarangapani, S.; Khong, D.T.; Wang, S.; Cui, J.; Loh, S.I.; Singh, G.P.; et al. Decoding Early Stress Signaling Waves in Living Plants Using Nanosensor Multiplexing. Nat. Commun. 2024, 15, 2943. [Google Scholar] [CrossRef] [PubMed]
- Zentgraf, U.; Andrade-Galan, A.G.; Bieker, S. Specificity of H2O2 Signaling in Leaf Senescence: Is the Ratio of H2O2 Contents in Different Cellular Compartments Sensed in Arabidopsis Plants? Cell Mol. Biol. Lett. 2022, 27, 4. [Google Scholar] [CrossRef]
- do Bonfim, R.A.A.; Cairo, P.A.R.; Barbosa, M.P.; da Silva, L.D.; Sá, M.C.; Almeida, M.F.; de Oliveira, L.S.; da Paz Brito, S.; Gomes, F.P. Effects of Plant Growth Regulators on Mitigating Water Deficit Stress in Young Yellow Passion Fruit Plants. Acta Physiol. Plant 2024, 46, 70. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Liu, S.; Peng, X. Exogenous Application of Hydrogen Peroxide Alleviates Drought Stress in Cucumber Seedlings. S. Afr. J. Bot. 2016, 106, 23–28. [Google Scholar] [CrossRef]
- Suksom, W.; Wannachai, W.; Boonchiangma, S.; Chanthai, S.; Srijaranai, S. Ion Chromatographic Analysis of Monosaccharides and Disaccharides in Raw Sugar. Chromatographia 2015, 78, 873–879. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Campos, F.G.; Vieira, M.A.R.; Amaro, A.C.E.; DelaCruz-Chacón, I.; Marques, M.O.M.; Ferreira, G.; Boaro, C.S.F. Nitrogen in the Defense System of Annona emarginata (Schltdl.) H. Rainer. PLoS ONE 2019, 14, e0217930. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross Talk between H2O2 and Interacting Signal Molecules under Plant Stress Response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef]
- Nadarajah, K.K. Ros Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Barzotto, G.R.; Cardoso, C.P.; Jorge, L.G.; Campos, F.G.; Boaro, C.S.F. Foliar H2O2 Application Improve the Photochemical and Osmotic Adjustment of Tomato Plants Subjected to Drought. Agriculture 2024, 14, 1572. [Google Scholar] [CrossRef]
- García-Castro, A.; Volder, A.; Restrepo-Diaz, H.; Starman, T.W.; Lombardini, L. Evaluation of Different Drought Stress Regimens on Growth, Leaf Gas Exchange Properties, and Carboxylation Activity in Purple Passionflower Plants. J. Am. Soc. Hortic. Sci. 2017, 142, 57–64. [Google Scholar] [CrossRef]
- Guo, J.; Luo, M.; Yan, J.; Zhang, M.; Tang, W.; Chen, K.; Wang, Y.; Wang, Q.; Guo, C.; Chen, M.; et al. External Application of Vitexin Enhances Drought Resistance by Promoting the Synthesis of Flavonoids and Other Hormones and Stabilizing the Cell Membrane in Wheat (Triticum aestivum L.). Plant Growth Regul. 2025, 105, 29–39. [Google Scholar] [CrossRef]
- Palatnik, J.F.; Valle, E.M.; Carrillo, N. Oxidative Stress Causes Ferredoxin-NADP+ Reductase Solubilization from the Thylakoid Membranes in Methyl Viologen-Treated Plants. Plant Physiol. 1997, 115, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-Light vs. Low-Light: Effect of Light Acclimation on Photosystem II Composition and Organization in Arabidopsis thaliana. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 411–419. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.-P. Hydrogen Peroxide Is Involved in Abscisic Acid-Induced Stomatal Closure in Vicia Faba 1. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Hydrogen Peroxide and Abscisic Acid Mediate Salicylic Acid-Induced Freezing Tolerance in Wheat. Front. Plant Sci. 2018, 9, 397026. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Živanović, B.; Komić, S.M.; Tosti, T.; Vidović, M.; Prokić, L.; Jovanović, S.V. Leaf Soluble Sugars and Free Amino Acids as Important Components of Abscisic Acid—Mediated Drought Response in Tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Trouverie, J.; Thévenot, C.; Rocher, J.; Sotta, B.; Prioul, J. The Role of Abscisic Acid in the Response of a Specific Vacuolar Invertase to Water Stress in the Adult Maize Leaf. J. Exp. Bot. 2003, 54, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, R.; Li, X.; Dong, D.; Wang, S. Sugar Metabolism and Transport in Response to Drought–Rehydration in Poa pratensis. Agronomy 2025, 15, 320. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zeng, W.H.; Li, Z.; Peng, Y. Mannose Regulates Water Balance, Leaf Senescence, and Genes Related to Stress Tolerance in White Clover under Osmotic Stress. Biol. Plant 2020, 64, 406–416. [Google Scholar] [CrossRef]
- Kozuleva, M.; Goss, T.; Twachtmann, M.; Rudi, K.; Trapka, J.; Selinski, J.; Ivanov, B.; Garapati, P.; Steinhoff, H.-J.; Hase, T.; et al. Ferredoxin:NADP(H) Oxidoreductase Abundance and Location Influences Redox Poise and Stress Tolerance. Plant Physiol. 2016, 172, 1480–1493. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Interactive Role of Epibrassinolide and Hydrogen Peroxide in Regulating Stomatal Physiology, Root Morphology, Photosynthetic and Growth Traits in Solanum lycopersicum L. under Nickel Stress. Environ. Exp. Bot. 2019, 162, 479–495. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen Peroxide Alleviates Nickel-Inhibited Photosynthetic Responses through Increase in Use-Efficiency of Nitrogen and Sulfur, and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Yooying, R. Enhancement of Solubility, Thermal Stability and Bioaccessibility of Vitexin Using Phosphatidylcholine-Based Phytosome. NFS J. 2023, 31, 28–38. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the Effects of Vitexin in Oxidative Stress-Related Diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Praveena, R.; Sadasivam, K.; Kumaresan, R.; Deepha, V.; Sivakumar, R. Experimental and DFT Studies on the Antioxidant Activity of a C-Glycoside from Rhynchosia Capitata. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 442–452. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams III, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using Chlorophyll Fluorescence to Assess the Fraction of Absorbed Light Allocated to Thermal Dissipation of Excess Excitation. Physiol. Plant 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Reis, A.D.P.; Carvalho, R.F.; Costa, I.B.; Girio, R.J.S.; Gualberto, R.; Spers, R.C.; Gaion, L.A. Hydrogen Peroxide Is Involved in Drought Stress Long-Distance Signaling Controlling Early Stomatal Closure in Tomato Plants. Braz. J. Biol. 2022, 82, e267343. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.V.; de Cantuário, F.S.; de Paiva Bento-da-Silva, E.P.; Mesquita, M.; de Moraes, M.G. Water Deficit Affects Leaf Non-Structural Carbohydrates and Biomass Partitioning in Chickpea1. Pesqui. Agropecu. Trop. 2024, 54, 2024. [Google Scholar] [CrossRef]
- Honório, A.B.M.; De-la-Cruz-Chacón, I.; da Silva, G.C.; Mimi, C.O.; Campos, F.G.; da Silva, M.R.; Boaro, C.S.F.; Ferreira, G. Differential Tolerance of Primary Metabolism of Annona Emarginata (Schltdl.) H. Rainer to Water Stress Modulates Alkaloid Production. Horticulturae 2024, 10, 220. [Google Scholar] [CrossRef]
- Cerný, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B.; Talent, B. Molecular Sciences Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Lussier, F.X.; Colatriano, D.; Wiltshire, Z.; Page, J.E.; Martin, V.J.J. Engineering Microbes for Plant Polyketide Biosynthesis. Comput. Struct. Biotechnol. J. 2012, 3, e201210020. [Google Scholar] [CrossRef]
- Deng, Y.; Li, C.; Li, H.; Lu, S. Identification and Characterization of Flavonoid Biosynthetic Enzyme Genes in Salvia Miltiorrhiza (Lamiaceae). Molecules 2018, 23, 1467. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in Soluble Carbohydrates during Storage of Caesalpinia Echinata LAM. (Brazilwood) Seeds, an Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Pagassini, J.A.V.; de Godoy, L.J.G.; Campos, F.G.; Barzotto, G.R.; Vieira, M.A.R.; Boaro, C.S.F. Silicon and Mechanical Damage Increase Polyphenols and Vitexin in Passiflora incarnata L. Sci. Rep. 2021, 11, 22064. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.P.P.; Cambraia, J.; Sant, A.R.; Mosquim, R.; Moreira, M.A. Aluminum Effects on Lipid Peroxidation and on the Activities of Enzymes of Oxidative Metabolism in Sorghum 1. Braz. J. Plant Physiol. 1999, 11, 137–143. [Google Scholar]
- Teisseire, H.; Guy, V. Copper-Induced Changes in Antioxidant Enzymes Activities in Fronds of Duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Wosch, L.; dos Santos, K.C.; Imig, D.C.; Santos, C.A.M. Comparative Study of Passiflora Taxa Leaves: II. A Chromatographic Profile. Rev. Bras. Farmacogn. 2017, 27, 40–49. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.G.; Barzotto, G.R.; Melo-Figueiredo, I.; Pagassini, J.A.V.; Boaro, C.S.F. Hydrogen Peroxide and Vitexin in the Signaling and Defense Responses of Passiflora incarnata Under Drought Stress. Plants 2025, 14, 2078. https://doi.org/10.3390/plants14132078
Campos FG, Barzotto GR, Melo-Figueiredo I, Pagassini JAV, Boaro CSF. Hydrogen Peroxide and Vitexin in the Signaling and Defense Responses of Passiflora incarnata Under Drought Stress. Plants. 2025; 14(13):2078. https://doi.org/10.3390/plants14132078
Chicago/Turabian StyleCampos, Felipe G., Gustavo R. Barzotto, Isabela Melo-Figueiredo, Jonas A. V. Pagassini, and Carmen S. F. Boaro. 2025. "Hydrogen Peroxide and Vitexin in the Signaling and Defense Responses of Passiflora incarnata Under Drought Stress" Plants 14, no. 13: 2078. https://doi.org/10.3390/plants14132078
APA StyleCampos, F. G., Barzotto, G. R., Melo-Figueiredo, I., Pagassini, J. A. V., & Boaro, C. S. F. (2025). Hydrogen Peroxide and Vitexin in the Signaling and Defense Responses of Passiflora incarnata Under Drought Stress. Plants, 14(13), 2078. https://doi.org/10.3390/plants14132078







