Abstract
Strain 53B2 was isolated from a commercial maize (Zea mays L.) field located in the Yaqui Valley, Mexico. Its draft genome comprises 5,844,085 bp, with a G + C content of 37.5%, an N50 of 602,122 bp, an L50 of 4, and a total of 129 contigs. Genome-based taxonomic affiliation showed this strain belonged to Priestia megaterium. Genome annotation revealed 6394 coding DNA sequences (CDSs), organized into 332 subsystems. Among these, several CDSs were associated with traits relevant to plant growth promotion, including categories such as iron acquisition and metabolism (40 CDSs) and secondary metabolism (6 CDSs), among others. In vitro metabolic assays supported genomic predictions, confirming the strain’s ability to produce IAA, solubilize phosphate, and tolerate abiotic stress. Additionally, greenhouse trials demonstrated that inoculation with Priestia megaterium 53B2 significantly enhanced plant growth parameters (p ≤ 0.05) versus uninoculated control: stem height increased by 22.8%, root length by 35.7%, stem and root fresh weights by 39.6% and 66.1%, and stem and root dry weights by 33.7% and 44.7%, respectively. This first report on the beneficial potential of Priestia megaterium 53B2 highlights its potential as a sustainable bioinoculant for maize cultivation.
1. Introduction
Ensuring global food security is one of the greatest challenges of the 21st century. As the world population continues to grow and the effects of climate change intensify, the demand for agricultural production is expected to rise dramatically. Projections indicate that food output must increase by 70–100% by 2050 to sustain the global population [1]. In this context, cereals will remain fundamental to human nutrition, as they contribute significantly to daily caloric and protein intake [2]. Research in agriculture has changed over the decades. For example, since the 1950s, agricultural progress has been largely driven by the goal of boosting staple crop production in response to growing concerns about population growth and the capacity of food systems to meet rising demand [3]. Whole grains such as maize provide essential nutrients that contribute to addressing the global triple burden of malnutrition: undernutrition, micronutrient deficiencies, and overnutrition-related conditions, such as obesity and non-communicable diseases [4]. Maize is one of the world’s most widely cultivated crops, with global production averaging 1229.63 million tons annually [5]. Its adaptability to diverse agroecological environments (including a broad range of temperatures, altitudes, latitudes, and soil types) makes it a staple in many regions [2]. In 2023, maize production in Mexico surpassed 27.5 million metric tons, making a 3.74% increase compared to the previous year [6]. In this context, the Yaqui Valley—located in the State of Sonora, Mexico—is internationally recognized for its role in the scientific developments led by Dr. Norman E. Borlaug, which contributed to the Green Revolution between the 1960s and the 1980s [7]. Today, it remains one of the main farming regions of Mexico and one of the most intensively cultivated agricultural regions in the world, relying heavily on irrigation systems, chemical fertilizers, and improved crop varieties to produce key crops such as wheat, soybeans, maize, safflower, and sorghum [8,9]. In 2023 alone, Sonora contributed approximately 503,014 tons (1.76%) of maize to national production; however, decades of intensive cultivation have led to significant soil degradation, characterized by low organic matter content (<1.5%), high susceptibility to wind erosion due to the region’s arid climate (estimated 200 ton ha−1 year−1), and the development of saline or alkaline soil conditions. Additionally, the overuse of agrochemicals and intensive agronomic practices has reduced fertilizer efficiency, posing challenges to long-term soil health and crop productivity [10].
Maize, like many crops, is increasingly vulnerable to the combined pressures of climate change, facing both biotic stressors, such as pests and diseases, and abiotic stressors, including water scarcity, salinity, and extreme temperatures. These factors can severely impact plant physiology, leading to yield losses ranging from 50% to 82% [11]. Moreover, the adoption of high-input agricultural practices aimed at maximizing productivity has exacerbated environmental issues such as soil degradation, water shortages, eutrophication, and deforestation, while also posing potential risks to human health [12,13]. In response to the increasing challenges of sustainable global food production, especially under the pressure of climate change and population growth, there is a critical need to implement innovative and sustainable agricultural strategies. One such approach involves the use of beneficial microorganisms, particularly plant growth-promoting microorganisms (PGPMs), which play a vital role in maintaining the health and functionality of agroecosystems [14]. PGPMs support plant development by improving nutrient acquisition, mitigating environmental stress, and providing biocontrol against phytopathogens through multiple mechanisms [15]. Currently, they are widely applied as microbial inoculants, contributing directly to plant nutrition via biological nitrogen fixation, the mobilization of organic and inorganic phosphorus, and siderophore production. Additionally, they enhance growth by producing phytohormones such as indole-3-acetic acid (IAA), gibberellins, cytokinins, and the enzyme ACC deaminase, which helps modulate ethylene levels under stress conditions. Beyond these direct benefits, PGPMs are also effective in disease management; they suppress plant pathogens through competition for essential nutrients and space, induction of systemic resistance, and production of compounds like antibiotics, exopolysaccharides, hydrogen cyanide, and lytic enzymes [11,14,16]. Given their multifunctionality, the bioprospecting of new microbial strains with biotechnological applications in agriculture has become a priority, aiming to enhance productivity while minimizing agrochemical inputs and improving soil health [17,18].
In this sense, the genus Priestia belongs to the Bacillaceae family, characterized by Gram-positive, mostly rod-shaped bacteria. Recently reclassified, species within this genus have been previously reported as plant growth-promoting bacteria in maize, tomato, tea, wheat, rice, bean, and soybean, among others [12,19,20,21]. Thus, the present study offers a detailed genomic and functional characterization of strain 53B2, isolated from a maize (Zea mays L.) commercial field in the Yaqui Valley, Mexico, including genomic mining enabled the identification of genes associated with plant-growth promotion traits, which were subsequently validated through both metabolic assays and greenhouse trials.
2. Results
2.1. Morphological and Metabolic Characterization
Strain 53B2 was isolated as one of the most abundant bacterial strains associated with maize (Zea mays L.) in a commercial field located in the Yaqui Valley, Mexico. Strain 53B2 showed a circular, white, flat, opaque colony, and Gram-positive, rod-shaped cells with a size of 3.97 × 1.18 µm (Figure 1 and Figure 2). Endospore staining revealed the presence of green-stained endospores within pink/red vegetative cells of strain 53B2, confirming its ability to form endospores under nutrient-limited conditions. Microscopy images showed that the endospores were predominantly located in a central position within the cells (Figure 1).
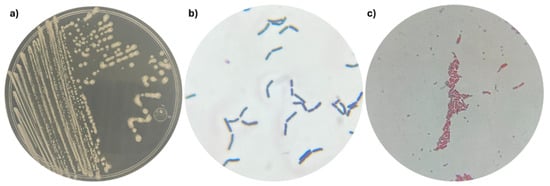
Figure 1.
Morphology of strain 53B2: (a) Macroscopic morphology of the colonies; (b) Microscopic morphology of Gram-positive cells; (c) Spores, endospores, and vegetative cells of strain 53B2. Spores and endospores were stained with malachite green stain and appear green/light blue; vegetative cells are stained with safranin and appear pink/red.
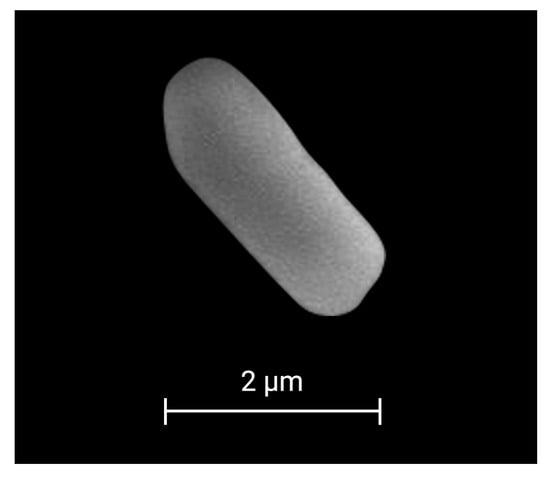
Figure 2.
Scanning electron microscopy image of a rod-shaped cell of strain 53B2.
2.2. Genomic Analysis
After DNA extraction and whole-genome sequencing, strain 53B2’s genomic DNA resulted in a total of 776,413 paired-end reads (2 × 250 bp). The genome assembly yielded 129 contigs (≥200 bp), totaling 5,844,085 base pairs, with a G + C content of 37.5%. The assembly quality metrics included an N50 value of 602,122 bp and an L50 of 4. No plasmid sequences were identified. This Whole Genome Shotgun project has been submitted to DDBJ/ENA/GenBank and is available under the accession number JBLZXA000000000. The version reference in this study corresponds to JBLZXA010000000. Analysis of the 16S rRNA gene revealed 99.92% sequence identity with Priestia megaterium NBRC 15308T, 99.76% with Priestia aryabhattai B8W22T, and 99.04% with Priestia flexa NBRC 15715T (Table 1). Phylogenetic reconstruction based on the 16S rRNA gene sequence, in combination with morphological features, confirmed that strain 53B2 belongs to the genus Priestia (Figure 3). In addition, OGRI analysis confirms the taxonomic identification of strain 53B2 as Priestia megaterium, due to ANI (96.23) and GGDC (76.58) values being higher than those established for species delimitation (ANI ≥ 95–96% and GGDC ≥ 70%) (Table 1).

Table 1.
16S rRNA-based similarity, ANIb, ANIm, OrthoANI, and Genome-to-Genome Distance Calculator (GGDC) values of closely related species strain 53B2.

Figure 3.
Phylogenetic relationships based on 16S rRNA gene sequences between strain 53B2 and closely related Priestia species: Priestia megaterium NBRC 15308T (JJMH01000057), P. aryabhattai B8W22T (EF114313), and P. flexa NBRC 15715T (BCVD01000224). Bacillus vallismortis DV1-F-3T (JH600237) was used as an outgroup. The phylogenetic tree was constructed using CLC Sequence Viewer version 8.0, employing the Jukes–Cantor distance model and the neighbor-joining method, with 1000 bootstrap replicates. The scale bar (0.035) represents the number of nucleotide substitutions per site.
2.3. Genome Annotation
Annotation of the Priestia megaterium 53B2 genome using the RAST platform revealed 166 RNA genes and 6394 coding DNA sequences (CDSs), organized into 332 functional subsystems (Figure 4). Among these, the most significant subsystems were amino acids and derivatives (383 CDS), carbohydrates (351 CDS), protein metabolism (233), cofactors, vitamins, prosthetic groups and pigments (168 CDS), and nucleosides and nucleotides (113 CDS). Additionally, multiple subsystems related to plant growth promotion functions were detected. These included (i) 40 CDS involved in iron acquisition and metabolism, notably 18 CDS related to siderophore biosynthesis (molecules that sequester iron and reduce its availability to plant pathogens); (ii) 6 CDS associated with secondary metabolite production, with 4 of them involved in plant hormone synthesis; and (iii) 20 CDS involved in phosphorus metabolism. The genome also harbored genes supporting environmental stress adaptation, with 55 CDS related to stress responses, including osmotic (17 CDS) and oxidative (19 CDS) stress pathways. Complementing these results, a circular chromosome map generated using Prokka annotated 6028 CDS, along with 144 tRNA genes and 1 tmRNA (Figure 5), further confirming the genome’s coding potential and functional diversity.
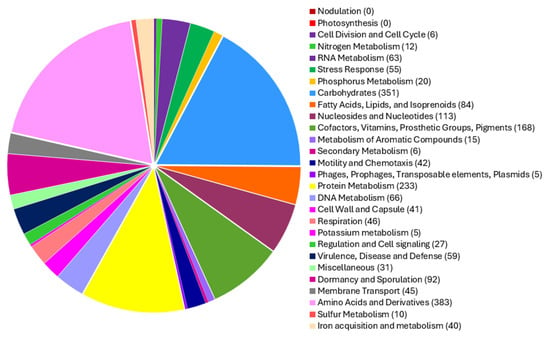
Figure 4.
Pie chart with the distribution of subsystem categories among the CDSs of Priestia megaterium 53B2, generated using the RAST server (version 2.0).
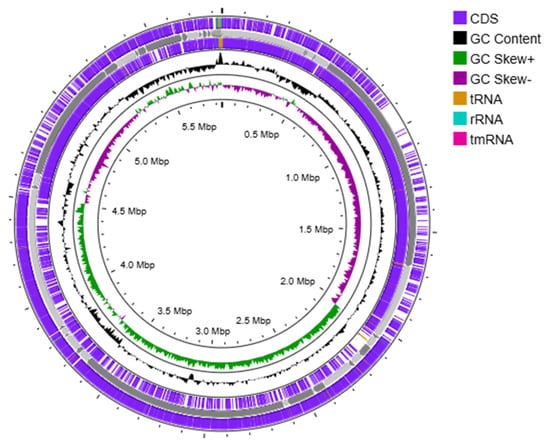
Figure 5.
Circular chromosome map of Priestia megaterium 53B2, including the distribution of CDSs, tRNAs, rRNAs, and GC content skew, created through genome annotation using Prokka (version 1.2.0).
2.4. Genome Mining
Genome analysis of Priestia megaterium 53B2 revealed that approximately 25% of its genetic content is related to plant colonization processes. Additionally, 22% was associated with stress management and biocontrol functions, 21% with competitive exclusion mechanisms, 13% with biofertilization capabilities, 11% with phytohormone production and plant signaling, 7% with bioremediation potential, and 1% with the stimulation of plant immune responses (Figure 6 and Table S1).
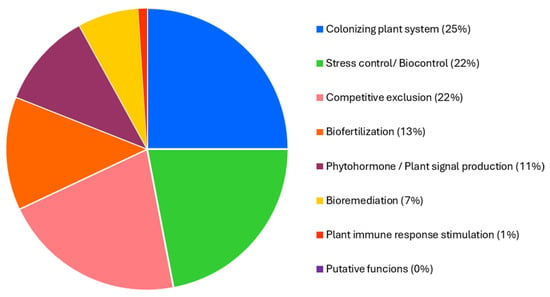
Figure 6.
PLaBAse analysis of the genome of Priestia megaterium 53B2 by blastp + hmmer.
Genome mining analysis performed using the AntiSMASH 7.0 web server identified three secondary metabolites: surfactin (13% similarity), carotenoid (50%), and the siderophore schizokinen (62%). However, none of these clusters showed a similarity above the 75% threshold typically used to consider a biosynthetic gene cluster (BGC) as potentially functional or well-characterized for biocontrol activity [22]. Therefore, no putative biocontrol-related BGCs were detected in strain 53B2, suggesting a limited capacity for secondary metabolite-mediated antagonism against phytopathogens.
2.5. Metabolic Characterization and Biocontrol Assay
Metabolic assays of Priestia megaterium 53B2 demonstrated its potential ability to promote the growth of plants. For example, strain 53B2 was able to synthesize indole-3-acetic acid (IAA) at a concentration of 2.29 µg/mL and a phosphate solubilization efficiency of 36.83 ± 11.06%; however, the ability to produce siderophores was not observed (Figure 7). The strain also exhibited resilience at various abiotic stress conditions, showing tolerance to salinity (34.24%), water deficit (99.23%), and elevated temperatures (99.22%). Moreover, biocontrol activity against Fusarium verticillioides, Curvularia sp. H3-5 and Fusarium sp. H2-3 was not observed.
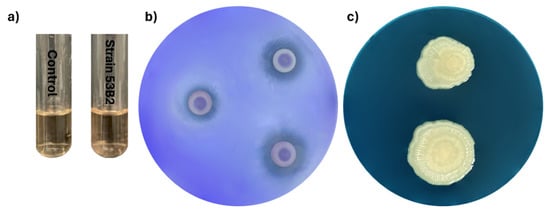
Figure 7.
Metabolic characterization of P. megaterium 53B2: (a) production of IAA using Salkowski reagent; (b) phosphate solubilization on Pikovskaya (PVK) agar; (c) siderophore production on Chrome Azurol S (CAS) agar.
2.6. Maize Growth Promotion Assay
The inoculation of Priestia megaterium 53B2 in maize led to statistically significant increases (p ≤ 0.05) in stem height (22.84%), root length (35.72%), stem and root weight (39.59% and 66.07%, respectively), and stem and root dry weight (33.66% and 44.73%, respectively), compared to the uninoculated control (Table 2).

Table 2.
Maize plants’ growth promotion by the inoculation of Priestia megaterium 53B2.
3. Discussion
Since the need for sustainable strategies in agriculture continues to grow, the search for alternative solutions to enhance crop productivity while minimizing environmental impact has become increasingly critical. In this context, bioprospecting for plant growth-promoting bacteria (PGPB) offers a promising strategy. These microorganisms employ a variety of mechanisms to enhance plant growth and protect plants from pathogens, both directly and indirectly [13].
With the advancement and widespread availability of sequencing technologies, the identification and reclassification of microbial taxa have accelerated significantly. A notable example is the recent taxonomic revision of the genus Priestia, a group of Gram-positive, primarily rod-shaped bacteria within the Bacillaceae family, recently reclassified by Gupta and colleagues [20]. In this sense, strain 53B2 was isolated from a commercial maize field and exhibited colony morphology typically associated with Priestia species: circular, white, flat, opaque colony, and Gram-positive, with rod-shaped cells (Figure 1 and Figure 2) [12,21,23,24,25,26]. For taxonomic affiliation, the 16S rRNA gene sequence was retrieved from the sequenced genome, as this gene contains both conserved and variable regions that are widely used to distinguish between bacterial genera [27,28]. Analysis of the 16S rRNA sequence (Table 1, Figure 3) provided strong evidence supporting the assignment of strain 53B2 to the genus Priestia. Moreover, OGRI analysis revealed that strain 53B2 belongs to the species Priestia megaterium (Table 1). Additionally, while plasmids are frequently observed among Priestia species, approximately 25% of known strains within this genus have been reported to lack plasmids [19], such as the case of Priestia megaterium 53B2. The ability of strain 53B2 to form endospores is consistent with the sporulation capacity commonly reported in Priestia megaterium strains [29,30]. Genome analysis of Priestia megaterium 53B2 revealed a comprehensive set of genes associated with sporulation, spo0A, spo0B, spo0E, spo0F, and spo0M, as well as the operons spoIIIA-spoIIID, spoIIA, spoIIAB, spoIIB-spoIVFB, along with coat protein genes, all linked to endospore development (Table S1). The presence of spo0A, the master regulator, and upstream phosphorelay genes (spo0B, spo0F) confirms the conserved signal transduction pathway that initiates under nutrient-limited conditions [31,32,33,34,35,36,37]. Gene spo0E, acting as a phosphatase, fine-tunes spo0A phosphorylation, integrating stress signals via σB, linking environmental stress responses directly to sporulation control [33,38,39,40,41]. The identification of genes encoding coat proteins (cotA, cotD, cotE, cotH, cotN, cotV, cotX, cotY, and cotZ) further supports the capacity of P. megaterium 53B2 to produce structurally resilient endospores [42,43,44]. Beyond sporulation, spo0A also plays a pivotal role in stress resilience, controlling genes involved in survival under temperature fluctuations and oxidative pressure, particularly in soil-dwelling Bacillus species [32,33,36,40,43,45]. Together, the presence of a complete sporulation gene toolkit along with coat proteins suggests that Priestia megaterium 53B2 is well-equipped for endospore formation and stress resistance, findings corroborated by the malachite green staining results showing central endospores (Figure 1). This genomic evidence further supports strain 53B2’s classification within the spore-forming genus Priestia and strengthens its ecological relevance for survival under harsh environmental conditions.
The plant-associated microbiota plays a vital role in improving nutrient availability by mobilizing otherwise inaccessible resources, such as inorganic phosphate and iron, through mechanisms like solubilization, mineralization, and the secretion of iron-chelating siderophores [46]. To determine the potential of Priestia megaterium 53B2 in enhancing corn production, its genome was annotated and mined, and the obtained information was correlated with metabolic and functional traits. Specifically, genome analysis revealed 18 CDSs related to siderophore biosynthesis, including Sdab, Sdad, SbsM, PchA, PchB, PchC, PchD, PchEPchF, ICM, BDH, and PchK, among others [47]. Additionally, genome mining reveals that Priestia megaterium 53B2 possesses a sophisticated, multi-tiered iron acquisition system featuring (i) heme biosynthesis/transport (hemABCDL, feuA), (ii) ferric citrate uptake (fecABCD), and (iii) ferrous iron transport (efeUOB) (Table S1) [48,49,50,51,52]. While this genetic repertoire suggests metabolic flexibility, the absence of CAS reactivity indicates conditional silencing of siderophore production, a phenomenon observed in bacteria that prioritize alternative iron sources when available [48,49]. This finding might position Priestia megaterium 53B2 to study metal homeostasis, where genomic potential and phenotypic expression diverge based on environmental cues.
On the other hand, several genes related to phosphate solubilization were identified. These include pqq and gdh, which participate in the oxidation of glucose to gluconic acid (the main organic acid responsible for dissolving insoluble phosphate compounds [53,54]. Also, the presence of genes such as phoA, ppx, phoD, phn, ppk, and other related genes suggests the potential for organic phosphorus mineralization via the production of phosphatases; many of the carrier genes encode different enzymes, such as alkaline phosphatases, extracellular polyphosphatase, and polyphosphate kinase [55,56]. The functionality of these genes was supported by in vitro assays, where Priestia megaterium 53B2 demonstrated a phosphate solubilization capacity of 36.83 ± 11.06%. This trait is highly desirable in plant growth-promoting bacteria, as phosphorus is an essential macronutrient involved in numerous metabolic and physiological processes. In arid and semi-arid soils, where phosphorus availability is frequently a limiting factor for crop productivity, such microbial traits offer promising solutions for sustainable agriculture [57].
Moreover, genome annotation of Priestia megaterium 53B2 using the RAST server revealed five CDSs associated with auxin biosynthesis: APRT, PRAI, IGS, TSa, and TSb. Several genes from the tryptophan biosynthesis pathway were also identified, including trpA, trpB, trpC, trpD, trpE, trpF, trpR, and trpS, which contribute not only to the synthesis of indole-3-acetic acid (IAA) but are also involved in ethylene and ammonia enzymes in the indole-3-pyruvic acid (IPA) pathway, one of the most widespread IAA biosynthetic routes among microorganisms [47]. In this pathway, tryptophan is first converted into IPA, which is then transformed into indole-3-acetaldehyde (IAAld), and subsequently oxidized into IAA via the action of aldehyde dehydrogenases [58]. The presence and functionality of these genes were supported by metabolic assays, where Priestia megaterium produced 2.29 µg/mL of IAA, as determined by the Salkowski colorimetric method. This trait is particularly valuable in plant-beneficial microorganisms, as IAA plays a pivotal role in regulating key developmental processes such as cell elongation, division, root initiation, fruit development, and senescence [58,59].
Given the arid and semi-arid conditions of the Yaqui Valley, Mexico [60], the ability of Priestia megaterium 53B2 to tolerate abiotic stressors such as salinity, drought, and temperature fluctuations is of particular interest. The strain exhibited tolerance levels of 34.24% to salinity, 99.23% to water deficit, and 99.22% to thermal stress, respectively [61]. These phenotypic traits are likely supported by the presence of 17 CDSs related to osmotic stress identified in its genome. The genomic landscape reveals a coordinated network of stress-responsive pathways centered on redox balance, ion homeostasis, and energy metabolism. Notable genes include betA, betB, betT, betC, betI, opuAA, opuAB, and opuAC, which are involved in the synthesis and transport of osmoprotectants. Specifically, these genes facilitate the uptake and conversion of choline into glycine betaine, a well-known osmoprotectant that enables bacterial survival under high salinity and drought conditions [62,63]. The presence of the nadABCDE operon ensures robust NAD+ biosynthesis, critical for maintaining redox poise under oxidative stress [64], while its linkage to the mnhABCDEFG operon (encoding a multisubunit NA+/H+ antiporter) suggests coupled NADH recycling and pH homeostasis during osmotic stress [65,66]. Sulfur assimilation via cys genes (cysACEGHIJKSTW) further supports resilience, as cysteine is a precursor for glutathione (GSH) and iron–sulfur (Fe-S) clusters required for oxidative defense [67]. Notably, the clpBCEPXR protease system, regulated by NAD+-dependent sirtuins, links protein quality control to metabolic status, degrading misfolded proteins during heat shock [68]. Further work should probe the transcriptional coordination of these systems under combinational stresses (e.g., oxidative + osmotic) to elucidate regulatory checkpoints.
Genome mining of P. megaterium 53B2 on AntiSMASH revealed low similarity scores (<70%) to known biosynthetic gene clusters, including schizokinen (62%), carotenoid (50%), and surfactin (13%). These values suggest that although homologous sequences exist, the cluster may be incomplete, divergent, or non-functional, and such variation is not uncommon at the intraspecies level [69,70]. Priestia species are widely recognized for their biocontrol potential through a variety of mechanisms associated with plant protection, including the production of (i) organic volatile compounds (VOCs), such as polyketones [71]; (ii) lipopeptides like surfactin and iturin [25]; (iii) siderophores such as schizokinen [72]; and (iv) organic compounds like phenazine [73]. Nevertheless, the results of genomic and in vitro assays indicate that strain 53B2, despite belonging to a genus known for its plant-beneficial properties, lacks biocontrol capacity.
Thus, based on genome mining and metabolic tests, Priestia megaterium 53B2 demonstrated significant improvements (p ≤ 0.05) in stem height by 22.8%, root length by 35.7%, stem and root fresh weights by 39.6% and 66.1%, and stem and root dry weights by 33.7% and 44.7%, respectively. These effects suggest the presence of multiple plant growth-promotion traits in this strain. Genomic analysis supported this, revealing genes associated with auxin biosynthesis, phosphate solubilization, and abiotic stress tolerance. These genomic features, along with confirmed IAA production and phosphate solubilization in vitro, likely underpin the observed improvements in plant biomass and morphology.
Although functional validation at the transcriptomic or metabolomic level is still required, the simultaneous enhancement of both shoot and root biomass suggests a balanced and potentially adaptive plant growth-promotion mechanism. This differentiates P. megaterium 53B2 from strains that tend to favor root development over aerial growth.
To contextualize these findings, other Priestia strains have shown similar traits. For example, P. megaterium HY-01 promoted Centella asiatica leaf elongation via auxin production, and strain ZS-3 enhanced cotton biomass [74,75]. However, P. megaterium 53B2 showed dry weight increment (33.66% stem, 44.73% root), suggesting enhanced nutrient transporters, which parallels P. megaterium BP-R2’s IAA-mediated root architecture modifications but with a more balanced shoot–root enhancement, diverging from root-prioritizing strains like P. aryabhattai C1-9 [76,77,78]. The observed growth promotion likely stems from Priestia megaterium 53B2’s multifaceted PGP traits. Auxin biosynthesis, a hallmark of P. megaterium, such as HY-01’s tryptophan-dependent IAA pathway, may explain root elongation and stem cell expansion. Concurrent phosphate solubilization, documented in ZS-3 through gene expression, could drive biomass accumulation. Notably, the performance of P. megaterium 53B2 surpasses typical P. megaterium strains (e.g., 20–50% dry weight gains), potentially due to its origin in an intensive maize agroecosystem, which may have been selected for genomic adaptations related to nutrient acquisition and stress tolerance (for example, pqq-like operons).
Isolated from maize fields in the Yaqui Valley, P. megaterium 53B2 may offer valuable support in addressing two regional agricultural challenges: fertilizer dependence and climate volatility. Its performance under stress conditions, along with its genomic potential, supports its use as a versatile bacterial inoculant candidate for nutrient-poor or saline soils. Nonetheless, further studies are needed to clarify the precise mechanisms driving its plant-growth-promoting effects and to evaluate its efficacy under field conditions.
4. Materials and Methods
4.1. Bacterial Isolation and Cultivation
Strain 53B2 was isolated from the soil of a maize (Zea mays L.) commercial field located in the Yaqui Valley, Mexico (coordinates 27° 25′ 38.3′′ N, 110° 06′ 27.8′′ W). To isolate this strain, a composite soil sampling weighing 10 kg was collected from a one-hectare area, comprising ten subsamples. Then, 10 g were mixed with 90 mL of sterile distilled water and subjected to serial dilutions up to 10−4. Subsequently, 100 µL of each dilution was inoculated, in triplicate, onto Petri dishes containing nutrient agar (NA) and incubated at 28 °C for 48 h. Morphological traits, such as colony form, pigmentation, elevation, opacity, and cellular shape, were used to identify and describe one of the most abundant bacterial strains, designated as 53B2. To obtain microscopic images, strain 53B2 was cultured in NB at 30 °C for 24 h and subsequently fixed with 1% formaldehyde. The samples were then dehydrated through a graded ethanol series. Microscopic observations were performed using a JSM-6400 scanning electron microscope (JEOL, Peabody, MA, USA). The presence of endospores was assessed using the Schaeffer–Fulton staining method [79,80]. This strain was preserved in nutrient broth (NB) with 30% glycerol at −80 °C and is currently stored in the Culture Collection of Native Soil and Endophytic Microorganisms (itson.mx/colmena, accessed on 1 April 2025) [13].
4.2. Genomic Analysis
Examining the genomic background of strain 53B2, particularly regarding traits advantageous for agriculture, started with obtaining high-quality DNA from a freshly cultured sample grown in NB for 24 h at 30 °C with constant agitation at 120 rpm, reaching a concentration of 1 × 108 CFU/mL. Then, 40 µL of the cellular suspension was treated with 120 µL of TE buffer containing lysozyme (0.1 mg/mL final concentration) and RNAse A (0.1 mg/mL, ITW Reagents, Barcelona, Spain), followed by incubation at 37 °C for 25 min. Next, proteinase K (0.1 mg/mL, VWR Chemicals, Solon, OH, USA) and 0.5% SDS (v/v, Sigma-Aldrich, St. Louis, MO, USA) were added, and the mixture was incubated at 65 °C for 5 min. DNA purification was performed using SPRI beads, and the resulting DNA was eluted in EB buffer (10 mM Tris-HCl, pH 8.0). DNA concentration (≥20 ng/µL) and yield (≥1 µg) were determined using the Quant-iT dsDNA HS kit (ThermoFisher Scientific, Waltham, MA, USA) and read on an Eppendorf AF2200 plate reader (Eppendorf UK Ltd., Stevenage, Hertfordshire, UK), followed by appropriate dilution [81].
For sequencing, libraries were prepared from the high-quality DNA using the Nextera XT Library Prep Kit (Illumina, San Diego, CA, USA) with modifications to the manufacturer’s protocol, including doubling the input DNA and extending the PCR elongation step to 45 s. Quantification and library preparation were automated using a Hamilton Microlab STAR system (Hamilton Bonaduz AG, Bonaduz, Switzerland) [82]. Sequencing was carried out using a 2 × 250 bp paired-end protocol on the Illumina NovaSeq 6000 platform. Raw reads were quality-filtered and trimmed using Trimmomatic version 0.30, applying a sliding window approach with a quality threshold of 15. A de novo assembly of the genome was generated with SPAdes version 3.15.4, using the “careful” parameter for error correction in reads [83].
The resulting contigs were organized using Mauve Contig Mover version 2.4.0 [84], referencing the Priestia megaterium ATCC 14581T genome (GenBank: GCA_006094495.1), showing 99.92% similarity and 100% completeness based on the 16S rRNA gene sequence, which was submitted to the EzBioCloud database [85] to identify the closest phylogenetic relatives, using the established species delimitation threshold of >98.7% similarity. The 16S rRNA gene sequence of strain 53B2 was submitted to the NCBI GenBank under accession number PV037199. A phylogenetic tree based on the 16S rRNA gene was generated using the neighbor-joining method in CLC Sequence Viewer version 8.0 (Qiagen, Aarhus, Denmark), employing Bacillus vallismortis DV1-F-3T (GenBank: JH600237) as the outgroup. To determine the species-level classification of strain 53B2, its genome was analyzed in comparison with those of the closest related strains (with >98.7% 16S rRNA gene sequence similarity), using overall genome relatedness indices (OGRI), such as average nucleotide identity (ANI), calculated using the OrthoANI algorithm, ANIb using the BLAST+ version 2.2.29 algorithm, ANIm by the MUMmer version 3.0 algorithm, and digital DNA-DNA hybridization, estimated through the Genome-to-Genome Distance Calculator (GGDC) version 3.0 via BLAST+ [86,87,88,89,90,91]. Finally, plasmid sequences were identified using PlasmidFinder 2.0 [92].
4.3. Genome Annotation
Genome annotation for strain 53B2 was carried out using the Rapid Annotation Using Subsystem Technology (RAST) server version 2.0 (https://rast.nmpdr.org/), employing the RASTk pipeline integrated within the PathoSystem Resources Integration Center (PATRIC) platform (accessed on 13 February 2025) [93]. In addition, the Proksee platform (https://proksee.ca/, accessed on 13 February 2025) [94], which utilizes Rapid Prokaryotic Genome Annotation (Prokka) [95], was used to construct a circular chromosome map of strain 53B2, illustrating coding sequences (CDSs), tRNAs, rRNAs, and GC skew.
4.4. Genome Mining
Plant growth-promoting traits were inferred using the PGPT-Pred tool available in PLaBAse (version 1.02) (https://plabase.cs.uni-tuebingen.de/pb/plabase.php, accessed on 24 March 2025) [96]. For this analysis, the protein sequence file obtained from genome annotation using RAST was uploaded to the PGPT-Pred interface to detect genetic elements in strain 53B2. Additionally, to explore biosynthetic gene clusters (BGCs) potentially involved in biocontrol, the genome of strain 53B2 was analyzed through AntiSMASH version 7.0 (https://antismash.secondarymetabolites.org, accessed on 19 February 2025), using the “relaxed” detection settings. This approach allowed the identification of diverse biosynthetic pathways related to secondary metabolite production, including those coding for non-ribosomal peptide synthetases (NRPSs), polyketide synthases (PKSs), types I and II, and ribosomally synthesized and post-translationally modified peptides (RiPP) such as lanthipeptides, lasso peptides, sactipeptides, and thiopeptides [22].
4.5. Metabolic Characterization
The metabolic characterization of strain 53B2 related to plant growth promotion was carried out to validate putative functions identified by genome mining. Thus, indole-3-acetic acid (IAA) production was assessed by inoculating 1 × 104 CFU into 10 mL of nutrient broth (NB) enriched with 100 ppm of L-tryptophan, in triplicate [97]. Cultures were incubated at 30 ± 2 °C for five days with constant shaking at 120 rpm. After incubation, 1 mL of each culture was centrifuged at 13,000 rpm for 10 min. Then, 100 µL of the resulting supernatant was mixed with 200 µL of Salkowski reagent, incubated in the dark at room temperature for 30 min, and the absorbance was measured at 530 nm using a BioTek ELx800 Absorbance™ Microplate Reader (BioTek Instruments, Winooski, VT, USA). IAA concentration was calculated based on a standard calibration curve. Phosphate solubilization ability was evaluated using Pikovskaya (PVK) agar [98], where Petri dishes were inoculated with 1 × 104 CFU of the studied strain, in triplicate, and incubated at 28 °C for seven days. The formation of a clear halo around colonies indicated positive solubilization. Phosphate solubilization efficiency (PSE) was calculated using the formula %PSE = , where HD is the halo diameter including the bacteria and CD is the bacterial colony diameter. Siderophore production was determined using Chrome Azurol S (CAS) agar [99]. Inoculations of 1 × 104 CFU in triplicate, followed by incubation at 28 °C for seven days. A yellow-to-orange halo around the colony indicated positive siderophore activity. Siderophore production efficiency (SPE) was calculated as %SPE = , as mentioned before. Abiotic stress tolerance was also assessed by inoculating 1 × 104 CFU of strain 53B2 on Falcon tubes with nutrient broth (NB) supplemented with (i) 5% sodium chloride (68 dS/m) for salt stress and (ii) 10% polyethylene glycol 6000 (PEG 6000; −0.84 MPa) to simulate water deficit, both incubated at 28 °C and 120 rpm for three days [100]; the control treatment did not include sodium chloride or polyethylene glycol. For heat stress, the studied strain was inoculated on Falcon tubes with NB and incubated at 43.5 °C and 120 rpm for three days. Control tubes were incubated at 28 °C for three days [101]. Each stress test was conducted in triplicate. Stress tolerance (ST) was expressed as a percentage using the formula %ST = , where BGs means the colony-forming units obtained under stress conditions and BG represents the colony-forming units obtained in the control treatment [102].
4.6. Biocontrol In Vitro Assay
Confrontations of Priestia megaterium 53B2 against Fusarium verticillioides, Curvularia sp. H3-5, and Fusarium sp. H2-3 were carried out. For each phytopathogen, the assay was performed using Petri dishes containing PDA. In the center, a circle with a diameter of 0.5 cm was sown from a dish with fresh mycelium; two slices of bacterial biomass were taken and placed at two equidistant points, with 1 cm of separation from the edge of the plate. Incubation was for five days at 30 °C [12,103]. The assays were conducted with three independent replicates, and control treatments were only inoculated with the phytopathogens.
4.7. Maize Growth Promotion Assay
To evaluate the interaction between maize plants and strain 53B2, a greenhouse experiment was conducted. Twenty-four maize seeds (var. Hipopótamo) were sown in a forest tray with cavities, 4.7 cm in diameter, filled with commercial GTX PRO-MIX substrate (PRO-MIX, Québec, QC, Canada). Strain 53B2 was grown in a 250 mL Erlenmeyer flask containing 100 mL of NB and incubated at 30 °C for 48 h with constant agitation at 120 rpm. Following incubation, the bacterial culture was centrifuged at 3600 rpm for 10 min. The pellet was washed three times with sterile water and then resuspended in 100 mL of sterile water. The optical density of the final suspension was adjusted to 0.5 at 630 nm, corresponding to 8.5 × 106 CFU/mL. Each maize seed was inoculated with 3 mL of the bacterial suspension, while the control group was treated with 3 mL of sterile distilled water. After three weeks, plant growth parameters were measured, including stem height, root length, and both root and stem dry and fresh biomass [104].
4.8. Statistical Analysis
All statistics were performed using STATGRAPHICS Plus version 5.1. Data were analyzed through one-way analysis of variance (ANOVA), considering differences significant at p ≤ 0.05.
5. Conclusions
The bioprospection of plant growth-promoting bacteria (PGPB) through integrative genomic and metabolic approaches represents a valuable strategy for identifying effective microbial inoculants aimed at fostering sustainable agriculture. Priestia megaterium strain 53B2 exhibited a diverse repertoire of genes associated with plant growth promotion. These genetic features were confirmed through targeted metabolic assays and validated in planta under greenhouse trials, showing a significant positive impact on maize development. The integration of genome mining with phenotypic and plant interaction studies provides a robust framework for selecting microbial candidates with high agronomic potential. This study constitutes the first report on the plant-beneficial potential of P. megaterium 53B2 in maize. However, further research on modes of action under field conditions is essential to ensure the successful implementation of this strain in sustainable crop production systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14132081/s1, Table S1: Features of strain 53B2 genome by PLaBAse.
Author Contributions
Conceptualization, S.d.l.S.-V. and F.I.P.-C.; methodology, all authors; software, A.E.-B., P.H.M.-S., C.B.G.-A. and A.C.M.-M.; formal analysis, all authors; data curation, A.E.-B., P.H.M.-S., C.B.G.-A. and A.C.M.-M.; writing—original draft preparation, all authors; writing—review and editing, all authors; visualization, all authors; supervision, F.I.P.-C. and S.d.l.S.-V.; project administration, F.I.P.-C. and S.d.l.S.-V.; funding acquisition, F.I.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Project Number 11175835959: Los microorganismos nativos del suelo como una estrategia para incrementar la tolerancia a estrés biótico y abiótico en cereales [Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP)]. In addition, we acknowledge funding from GetGenome (a newly formed charitable organization that provides equitable access to genomics technology for early-career researchers all over the world), as well as PROFAPI-ITSON (2025-001).
Data Availability Statement
The draft genome sequence has been deposited in DDBJ/ENA/GenBank under accession number JBLZXA000000000. The version described in this paper is the first version, JBLZXA010000000, under BioProject number PRJNA1080047 and BioSample number SAMN45897227.
Acknowledgments
The authors thank all members of the research node LBRM-COLMENA (www.itson.mx/LBRM, accessed on 13 February 2025), as well as the funding by the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). A.C.M.-M. acknowledges Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) for a postdoctoral fellowship (application number: 2306476).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization (FAO). How to Feed the World in 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050, Rome, Italy, 24–26 June 2009. [Google Scholar]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Byerlee, D.; Fanzo, J. The SDG of Zero Hunger 75 years on: Turning Full Circle on Agriculture and Nutrition. Glob. Food Secur. 2019, 21, 52–59. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Viewpoint: Agri-Nutrition Research: Revisiting the Contribution of Maize and Wheat to Human Nutrition and Health. Food Policy 2021, 100, 101976. [Google Scholar] [CrossRef]
- Yamini, V.; Singh, K.; Antar, M.; El Sabagh, A. Sustainable Cereal Production through Integrated Crop Management: A Global Review of Current Practices and Future Prospects. Front. Sustain. Food Syst. 2025, 9, 1428687. [Google Scholar] [CrossRef]
- SIAP (Servicio de Información Agroalimentaria y Pesquera). Anuario Estadístico de La Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 30 January 2025).
- de los Santos-Villalobos, S.; Parra-Cota, F.I.; Ayala-Zepeda, M.; Díaz-Rodríguez, A.M. Tecnologías Para Una Producción Sostenible de Trigo: El Caso Del Valle Del Yaqui, México, 1st ed.; Fontamara: Mexico City, Mexico, 2023. [Google Scholar]
- Parra-Cota, F.I.; Coronel-Acosta, C.-B.; Amézquita-Avilés, C.F.; de los Santos-Villalobos, S.; Escalante-Martínez, D.I. Diversidad Metabólica de Microorganismos Edáficos Asociados al Cultivo de Maíz En El Valle Del Yaqui, Sonora. Rev. Mex. Cienc. Agric. 2018, 9, 431–442. [Google Scholar]
- Ayala-Zepeda, M.; Parra-Cota, F.I.; Chinchilla-Soto, C.; De La Cruz-Torres, E.; Ibba, M.I.; Estrada-Alvarado, M.I.; de los Santos-Villalobos, S. 15N-Nitrogen Use Efficiency, Productivity, and Quality of Durum Wheat Integrating Nitrogen Management and an Indigenous Bacterial Inoculant in a Single Growing Season. Appl. Sci. 2025, 15, 1429. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Gándara-Ledezma, A.; Godoy-Flores, A.D.; Herrera-Sepúlveda, A.; Díaz-Rodríguez, A.M.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Salt-Tolerant Bacillus Species as a Promising Strategy to Mitigate the Salinity Stress in Wheat (Triticum turgidum Subsp. Durum). J. Arid. Environ. 2021, 186, 104399. [Google Scholar] [CrossRef]
- Figueroa-Brambila, K.M.; Escalante-Beltrán, A.; Montoya-Martínez, A.C.; Díaz-Rodríguez, A.M.; López-Montoya, N.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Bacillus cabrialesii: Five Years of Research on a Novel Species of Biological Control and Plant Growth-Promoting Bacteria. Plants 2023, 12, 2419. [Google Scholar] [CrossRef]
- Ortega-Urquieta, M.E.; Valenzuela-Ruiz, V.; Mitra, D.; Hyder, S.; Elsheery, N.I.; Kumar Das Mohapatra, P.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Draft Genome Sequence of Priestia Sp. Strain TSO9, a Plant Growth-Promoting Bacterium Associated with Wheat (Triticum turgidum Subsp. Durum) in the Yaqui Valley, Mexico. Plants 2022, 11, 2231. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Díaz-Rodríguez, A.M.; Ávila-Mascareño, M.F.; Martínez-Vidales, A.D.; Parra-Cota, F.I. COLMENA: A Culture Collection of Native Microorganisms for Harnessing the Agro-Biotechnological Potential in Soils and Contributing to Food Security. Diversity (Basel) 2021, 13, 337. [Google Scholar] [CrossRef]
- García-Montelongo, A.M.; Montoya-Martínez, A.C.; Morales-Sandoval, P.H.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Beneficial Microorganisms as a Sustainable Alternative for Mitigating Biotic Stresses in Crops. Stresses 2023, 3, 210–228. [Google Scholar] [CrossRef]
- Montoya-Martínez, A.C.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Beneficial Microorganisms in Sustainable Agriculture: Harnessing Microbes’ Potential to Help Feed the World. Plants 2022, 11, 372. [Google Scholar] [CrossRef]
- Valenzuela-Ruiz, V.; Gálvez-Gamboa, G.T.; Villa-Rodríguez, E.D.; Parra-Cota, F.I.; Santoyo, G.; de los Santos-Villalobos, S. Lipopeptides Produced by Biological Control Agents of the Genus Bacillus: A Review of Analytical Tools Used for Their Study. Rev. Mex. Cienc. Agric. 2020, 11, 419–432. [Google Scholar]
- Hyder, S.; Rizvi, Z.F.; de los Santos-Villalobos, S.; Santoyo, G.; Gondal, A.S.; Khalid, N.; Fatima, S.N.; Nadeem, M.; Rafique, K.; Rani, A. Applications of Plant Growth-Promoting Rhizobacteria for Increasing Crop Production and Resilience. J. Plant Nutr. 2023, 46, 2551–2580. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Pesticide Residues in Food; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Biedendieck, R.; Knuuti, T.; Moore, S.J.; Jahn, D. The “Beauty in the Beast”-the Multiple Uses of Priestia Megaterium in Biotechnology. Appl. Microbiol. Biotechnol. 2021, 105, 5719–5737. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust Demarcation of 17 Distinct Bacillus Species Clades, Proposed as Novel Bacillaceae Genera, by Phylogenomics and Comparative Genomic Analyses: Description of Robertmurraya kyonggiensis Sp. Nov. and Proposal for an Emended Genus Bacillus Limiting It Only to the Members of the Subtilis and Cereus Clades of Species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef] [PubMed]
- Almirón, C.; Petitti, T.D.; Ponso, M.A.; Romero, A.M.; Areco, V.A.; Bianco, M.I.; Espariz, M.; Yaryura, P.M. Functional and Genomic Analyses of Plant Growth Promoting Traits in Priestia aryabhattai and Paenibacillus Sp. Isolates from Tomato Rhizosphere. Sci. Rep. 2025, 15, 3498. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.K.; Kaur, J.; Singh, G.B.; Chauhan, A.; Tamang, J.; Lakhara, N.; Asyakina, L.; Atuchin, V.; Mudgal, G.; Abdi, G. Novel Bacillus and Prestia Isolates from Dwarf Century Plant Enhance Crop Yield and Salinity Tolerance. Sci. Rep. 2024, 14, 14645. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Steven, R.; Natanael, Y.; Nugrahapraja, H.; Radjasa, S.K.; Kristianti, T.; Moeis, M.R.; Trinugroho, J.P.; Suharya, H.B.; Rachmatsyah, A.O.; et al. From the Depths of the Java Trench: Genomic Analysis of Priestia Flexa JT4 Reveals Bioprospecting and Lycopene Production Potential. BMC Genom. 2024, 25, 1259. [Google Scholar] [CrossRef]
- Cui, Z.; Hu, L.; Zeng, L.; Meng, W.; Guo, D.; Sun, L. Isolation and Characterization of Priestia Megaterium KD7 for the Biological Control of Pear Fire Blight. Front. Microbiol. 2023, 14, 1099664. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.S.; Ram, H.; Dastager, S.G. Priestia veravalensis Sp. Nov., Isolated from Coastal Sample. Arch. Microbiol. 2021, 203, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Robles-Montoya, R.I.; Valenzuela-Ruiz, V.; Parra-Cota, F.I.; Santoyo, G.; de los Santos-Villalobos, S. Description of a Polyphasic Taxonomic Approach for Plant Growth-Promoting Rhizobacteria (PGPR). In Microbial Services in Restoration Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–269. ISBN 9780128199787. [Google Scholar]
- Morales-Sandoval, P.H.; Valenzuela-Ruiz, V.; Ortega-Urquieta, M.E.; Martínez-Vidales, A.D.; Félix-Pablos, C.M.; Chávez-Luzanía, R.A.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Taxonomía Bacteriana Basada En Índices Relacionados al Genoma Completo. La Sociedad Académica 2021, 58, 39–50. [Google Scholar]
- Rode, L.J. Correlation Between Spore Structure and Spore Properties in Bacillus Megaterium. J. Bacteriol. 1968, 95, 1979–1986. [Google Scholar] [CrossRef]
- Freer, J.H.; Levlnson, H.S. Fine Structure of Bacillus Megaterium during Microcycle Sporogenesis. J. Bacteriol. 1967, 94, 441–457. [Google Scholar] [CrossRef]
- Zarazúa-Osorio, B.; Srivastava, P.; Marathe, A.; Zahid, S.H.; Fujita, M. Autoregulation of the Master Regulator Spo0A Controls Cell-Fate Decisions in Bacillus Subtilis. Mol. Microbiol. 2025, 123, 305–329. [Google Scholar] [CrossRef]
- Fujita, M.; Losick, R. Evidence That Entry into Sporulation in Bacillus Subtilis Is Governed by a Gradual Increase in the Level and Activity of the Master Regulator Spo0A. Genes Dev. 2005, 19, 2236–2244. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Piggot, P.J. Compartmentalization of Gene Expression during Bacillus Subtilis Spore Formation. Microbiol. Mol. Biol. Rev. 2004, 68, 234–262. [Google Scholar] [CrossRef]
- Marathe, A.; Zarazúa-Osorio, B.; Srivastava, P.; Fujita, M. The Master Regulator for Entry into Sporulation in Bacillus Subtilis Becomes a Mother Cell-Specific Transcription Factor for Forespore Engulfment. Mol. Microbiol. 2023, 120, 439–461. [Google Scholar] [CrossRef]
- Chastanet, A.; Vitkup, D.; Yuan, G.C.; Norman, T.M.; Liu, J.S.; Losick, R.M. Broadly Heterogeneous Activation of the Master Regulator for Sporulation in Bacillus Subtilis. Proc. Natl. Acad. Sci. USA 2010, 107, 8486–8491. [Google Scholar] [CrossRef]
- Chastanet, A.; Losick, R. Just-in-Time Control of Spo0A Synthesis in Bacillus Subtilis by Multiple Regulatory Mechanisms. J. Bacteriol. 2011, 193, 6366–6374. [Google Scholar] [CrossRef]
- Iber, D. A Quantitative Study of the Benefits of Co-Regulation Using the SpoIIA Operon as an Example. Mol. Syst. Biol. 2006, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Panda, D.; Samanta, R.; Boro, R.C.; Modi, M.K.; Bujarbaruah, K.M.; Barooah, M. Bacillus Megaterium Adapts to Acid Stress Condition through a Network of Genes: Insight from a Genome-Wide Transcriptome Analysis. Sci. Rep. 2018, 8, 16105. [Google Scholar] [CrossRef]
- Fawcett, P.; Eichenberger, P.; Losick, R.; Youngman, P. The Transcriptional Profile of Early to Middle Sporulation in Bacillus Subtilis. Proc. Natl. Acad. Sci. USA 2000, 97, 8063–8068. [Google Scholar] [CrossRef]
- Molle, V.; Fujita, M.; Jensen, S.T.; Eichenberger, P.; González-Pastor, J.E.; Liu, J.S.; Losick, R. The Spo0A Regulon of Bacillus Subtilis. Mol. Microbiol. 2003, 50, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Reder, A.; Gerth, U.; Hecker, M. Integration of ΣB Activity into the Decision-Making Process of Sporulation Initiation in Bacillus Subtilis. J. Bacteriol. 2012, 194, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.B.; Orsaria, L.M.; Philippe, V.; Pedrido, M.E.; Grau, R.R. Novel Roles of the Master Transcription Factors Spo0A and σ B for Survival and Sporulation of Bacillus Subtilis at Low Growth Temperature. J. Bacteriol. 2004, 186, 989–1000. [Google Scholar] [CrossRef]
- Abhyankar, W.R.; Kamphorst, K.; Swarge, B.N.; van Veen, H.; van der Wel, N.N.; Brul, S.; de Koster, C.G.; de Koning, L.J. The Influence of Sporulation Conditions on the Spore Coat Protein Composition of Bacillus Subtilis Spores. Front. Microbiol. 2016, 7, 1636. [Google Scholar] [CrossRef]
- Saggese, A.; Scamardella, V.; Sirec, T.; Cangiano, G.; Isticato, R.; Pane, F.; Amoresano, A.; Ricca, E.; Baccigalupi, L. Antagonistic Role of CotG and CotH on Spore Germination and Coat Formation in Bacillus Subtilis. PLoS ONE 2014, 9, e104900. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Du, G.; Zhou, J.; Chen, J. Sporulation and Spore Stability of Bacillus Megaterium Enhance Ketogulonigenium Vulgare Propagation and 2-Keto-l-Gulonic Acid Biosynthesis. Bioresour. Technol. 2012, 107, 399–404. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Dahar, G.Y.; Wang, H.W.; Rajer, F.U.; Jin, P.; Xu, P.; Abro, M.A.; Qureshi, A.S.; Karim, A.; Miao, W. Comparative Genomic Analysis of Bacillus Atrophaeus HAB-5 Reveals Genes Associated with Antimicrobial and Plant Growth-Promoting Activities. Front. Microbiol. 2024, 15, 1384691. [Google Scholar] [CrossRef]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial Iron Homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Miethke, M.; Monteferrante, C.G.; Marahiel, M.A.; van Dijl, J.M. The Bacillus Subtilis EfeUOB Transporter Is Essential for High-Affinity Acquisition of Ferrous and Ferric Iron. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2267–2278. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Benaki, D.; Koukounia, M.; Zervakou, A.; Tzima, A.; Diallinas, G.; Hatzinikolaou, D.G.; Skandalis, N. Direct Antibiotic Activity of Bacillibactin Broadens the Biocontrol Range of Bacillus Amyloliquefaciens MBI600. mSphere 2021, 6, e0037621. [Google Scholar] [CrossRef]
- Nakatsuji, S.; Okumura, K.; Takase, R.; Watanabe, D.; Mikami, B.; Hashimoto, W. Crystal Structures of EfeB and EfeO in a Bacterial Siderophore-Independent Iron Transport System. Biochem. Biophys. Res. Commun. 2022, 594, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Feng, Y.; He, J.; Zhang, H.; Jia, X.; Hu, Y.; Ye, J.; Gu, X.; Zhang, X.; Chen, H. Phosphate Solubilizing Microorganisms: A Sustainability Strategy to Improve Urban Ecosystems. Front. Microbiol. 2023, 14, 1320853. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Shen, R.F. Stronger Effects of Maize Rhizosphere than Phosphorus Fertilization on Phosphatase Activity and Phosphorus-Mineralizing-Related Bacteria in Acidic Soils. Rhizosphere 2022, 23, 1320853. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-Term Nutrient Inputs Shift Soil Microbial Functional Profiles of Phosphorus Cycling in Diverse Agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic Pathways and Functions of Indole-3-Acetic Acid in Microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K. Auxin Herbicides: Current Status of Mechanism and Mode of Action. Pest. Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Ibarra-Villarreal, A.L.; Isela Parra-Cota, F.; Yépez, E.A.; Gutiérrez-Coronado, M.A.; Valdez-Torres, L.C.; de los Santos-Villalobos, S. Impacto Del Cambio En El Manejo Del Cultivo de Trigo de convencional a Orgánico Sobre Las Comunidades Fúngicas Del Suelo En El Valle Del Yaqui, México. Agrociencia 2020, 54, 643–659. [Google Scholar] [CrossRef]
- Córdova-Albores, L.C.; Zelaya-Molina, L.X.; Ávila-Alistac, N.; Valenzuela-Ruiz, V.; Cortés-Martínez, N.E.; Parra-Cota, F.I.; Burgos-Canul, Y.Y.; Chávez-Díaz, I.F.; Fajardo-Franco, M.L.; de los Santos-Villalobos, S. Omics Sciences Potential on Bioprospecting of Biological Control Microbial Agents: The Case of the Mexican Agro-Biotechnology. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2020, 39, 147–184. [Google Scholar] [CrossRef]
- Hoffmann, T.; Wensing, A.; Brosius, M.; Steil, L.; Völker, U.; Bremer, E. Osmotic Control of OpuA Expression in Bacillus Subtilis and Its Modulation in Response to Intracellular Glycine Betaine and Proline Pools. J. Bacteriol. 2013, 195, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Gregory, G.J.; Boyd, E.F. Stressed out: Bacterial Response to High Salinity Using Compatible Solute Biosynthesis and Uptake Systems, Lessons from Vibrionaceae. Comput. Struct. Biotechnol. J. 2021, 19, 1014–1027. [Google Scholar] [CrossRef]
- Gazzaniga, F.; Stebbins, R.; Chang, S.Z.; McPeek, M.A.; Brenner, C. Microbial NAD Metabolism: Lessons from Comparative Genomics. Microbiol. Mol. Biol. Rev. 2009, 73, 529–541. [Google Scholar] [CrossRef]
- Ito, M.; Morino, M.; Krulwich, T.A. Mrp Antiporters Have Important Roles in Diverse Bacteria and Archaea. Front. Microbiol. 2017, 8, 2325. [Google Scholar] [CrossRef]
- Kosono, S.; Haga, K.; Tomizawa, R.; Kajiyama, Y.; Hatano, K.; Takeda, S.; Wakai, Y.; Hino, M.; Kudo, T. Characterization of a Multigene-Encoded Sodium/Hydrogen Antiporter (Sha) from Pseudomonas Aeruginosa: Its Involvement in Pathogenesis. J. Bacteriol. 2005, 187, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Kredich, N.M. Biosynthesis of Cysteine. EcoSal Plus 2008, 3, 10–1128. [Google Scholar] [CrossRef]
- Kirstein, J.; Molière, N.; Dougan, D.A.; Turgay, K. Adapting the Machine: Adaptor Proteins for Hsp100/Clp and AAA+ Proteases. Nat. Rev. Microbiol. 2009, 7, 589–599. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into Secondary Metabolism from a Global Analysis of Prokaryotic Biosynthetic Gene Clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene Cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, G.; Bernacchi, A.; Amata, S.; Bechini, A.; Berti, F.; Calonico, C.; Catania, V.; Esposito, A.; Puglia, A.M.; Piccionello, A.P.; et al. Antibacterial Properties of Bacterial Endophytes Isolated from the Medicinal Plant Origanum heracleoticum L. Front. Biosci.- Landmark 2024, 29, 111. [Google Scholar] [CrossRef]
- Lam, V.B.; Ibrahim, H.M.M.; Oni, F.E.; Argüelles-Arias, A.; Marahatta, B.; Zhou, L.; Ferrarini, E.; De Coninck, B.; Cottyn, B.; Ongena, M.; et al. Diversity of Bacillaceae on Rice Grown in Acid Sulfate Soils in Vietnam: Taxonomy, Specialized Metabolites, and Inhibitory Effects on Fungal Pathogens. Phytobiomes J. 2024, 8, 469–483. [Google Scholar] [CrossRef]
- Khalifa, A.; Alsowayeh, N. Whole-Genome Sequence Insight into the Plant-Growth-Promoting Bacterium Priestia Filamentosa Strain AZC66 Obtained from Zygophyllum Coccineum Rhizosphere. Plants 2023, 12, 1944. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, X.; Qian, T.; Du, J.; Du, Y.; Ye, J. Mechanism of Salt Tolerance and Plant Growth Promotion in Priestia Megaterium ZS-3 Revealed by Cellular Metabolism and Whole-Genome Studies. Int. J. Mol. Sci. 2023, 24, 15751. [Google Scholar] [CrossRef]
- Jo, H.W.; Lim, K.; Ibal, J.C.; Kim, M.C.; Kim, H.B.; Baek, C.; Heo, Y.M.; Lee, H.; Kang, S.; Lee, D.G.; et al. Growth Increase in the Herbaceous Plant Centella Asiatica by the Plant Growth-Promoting Rhizobacteria Priestia Megaterium HyangYak-01. Plants 2023, 12, 2398. [Google Scholar] [CrossRef]
- Bhat, M.A.; Mishra, A.K.; Jan, S.; Bhat, M.A.; Kamal, M.A.; Rahman, S.; Shah, A.A.; Jan, A.T. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants 2023, 12, 629. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, V.F.; Fadiji, A.E.; Agbodjato, N.A.; Babalola, O.O. Isolation and Characterization of Plant-Growth-Promoting, Drought-Tolerant Rhizobacteria for Improved Maize Productivity. Plants 2024, 13, 1298. [Google Scholar] [CrossRef]
- Hwang, H.H.; Chien, P.R.; Huang, F.C.; Yeh, P.H.; Hung, S.H.W.; Deng, W.L.; Huang, C.C. A Plant Endophytic Bacterium Priestia Megaterium StrainBP-R2 Isolated from the Halophyte Bolboschoenus Planiculmis Enhances Plant Growth under Salt and Drought Stresses. Microorganisms 2022, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.B.; Fulton, M.D. A Simplified Method of Staining Endospores. Science (1979) 1933, 77, 194. [Google Scholar] [CrossRef]
- Moirangthem Bidyaswori, D.; Dhar, R.; Bhattacharjee, A.; Dibyendu, P. Screening of Different Media and Heat Shock Treatment Regimens for Enhancing Sporulation in Bacillus Licheniformis. Int. J. Curr. Sci. Res. Rev. 2023, 6, 2666–2674. [Google Scholar] [CrossRef]
- Valenzuela-Aragón, B.; Montoya-Martínez, A.C.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Genomic Insight into a Potential Biological Control Agent for Fusarium-Related Diseases in Potatoes: Bacillus Cabrialesii Subsp. Cabrialesii Strain PE1. Horticulturae 2024, 10, 357. [Google Scholar] [CrossRef]
- Campos-Avelar, I.; Montoya-Martínez, A.C.; Escalante-Beltrán, A.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Do Organic Amendments Foster Only Beneficial Bacteria in Agroecosystems?: The Case of Bacillus Paranthracis TSO55. Plants 2025, 14, 1019. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.-M.; Lim, J.; Kwon, S.; Chun, J. A Large-Scale Evaluation of Algorithms to Calculate Average Nucleotide Identity. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M.G. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-Based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and Open Software for Comparing Large Genomes. Genome Biol. 2004, 5, 12. [Google Scholar] [CrossRef]
- Carattoli, A.; Hasman, H. Identification and Typing of Plasmid Replicons in Whole-Genome Sequencing (WGS). In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2075, pp. 285–294. [Google Scholar]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding Data and Analysis Capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Patz, S.; Gautam, A.; Becker, M.; Ruppel, S.; Rodríguez-Palenzuela, P.; Huson, D.H. PLaBAse: A Comprehensive Web Resource for Analyzing the Plant Growth-Promoting Potential of Plant-Associated Bacteria. bioRxiv 2021. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; de Folter, S.; Délano-Frier, J.P.; Gómez-Lim, M.A.; Guzmán-Ortiz, D.A.; Peña-Cabriales, J.J. Growth Promotion and Flowering Induction in Mango (Mangifera Indica L. Cv “Ataulfo”) Trees by Burkholderia and Rhizobium Inoculation: Morphometric, Biochemical, and Molecular Events. J. Plant Growth Regul. 2013, 32, 615–627. [Google Scholar] [CrossRef]
- Onyia, C.E.; Anyanwu, C.U. Comparative Study on Solubilization of Tri-Calcium Phosphate (TCP) by Phosphate Solubilizing Fungi (PSF) Isolated from Nsukka Pepper Plant Rhizosphere and Root Free Soil. J. Yeast Fungal Res. 2013, 4, 52–57. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of Chrome Azurol S Reagents to Evaluate Siderophore Production by Rhizosphere Bacteria. Biol. Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Meléndez-García, M.; Zárate-Camargo, G.; Meza-Contreras, J.J.; Herrera-Sepúlveda, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I. Abiotic Stress Tolerance of Microorganisms Associated with Oregano (Origanum vulgare L.) in the Yaqui Valley, Sonora. Open Agric. 2017, 2, 260–265. [Google Scholar] [CrossRef]
- Valenzuela-Aragón, B.; Parra-Cota, F.I.; Santoyo, G.; Arellano-Wattenbarger, G.L.; de los Santos-Villalobos, S. Plant-Assisted Selection: A Promising Alternative for in Vivo Identification of Wheat (Triticum turgidum L. Subsp. Durum) Growth Promoting Bacteria. Plant Soil. 2019, 435, 367–384. [Google Scholar] [CrossRef]
- Morales-Sandoval, P.H.; Valenzuela-Ruiz, V.; Santoyo, G.; Hyder, S.; Mitra, D.; Zelaya-Molina, L.X.; Ávila-Alistac, N.; Parra-Cota, F.I.; de los Santos-Villalobos, S. Draft Genome of a Biological Control Agent against Bipolaris Sorokiniana, the Causal Phytopathogen of Spot Blotch in Wheat (Triticum turgidum L. Subsp. Durum): Bacillus Inaquosorum TSO22. Open Agric. 2024, 9, 20220309. [Google Scholar] [CrossRef]
- Villa-Rodríguez, E.; Parra-Cota, F.; Castro-Longoria, E.; López-Cervantes, J.; de los Santos-Villalobos, S. Bacillus Subtilis TE3: A Promising Biological Control Agent against Bipolaris Sorokiniana, the Causal Agent of Spot Blotch in Wheat (Triticum turgidum L. Subsp. Durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Rojas-Padilla, J.; Chaparro-Encinas, L.A.; Robles-Montoya, R.I.; de los Santos-Villalobos, S. Growth Promotion on Wheat (Triticum turgidum L. Subsp. Durum) by Co-Inoculation of Native Bacillus Strains Isolated from the Yaqui Valley, Mexico. Nova Scientia 2020, 12. Available online: https://www.scielo.org.mx/scielo.php?pid=S2007-07052020000100006&script=sci_abstract&tlng=en (accessed on 13 February 2025). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).