Antimicrobial Effects of Abies alba Essential Oil and Its Application in Food Preservation
Abstract
1. Introduction
2. Results
2.1. Volatile Constituents’ Evaluation
2.2. Antioxidant Activity Examination
2.3. In Vitro Antimicrobial Activity Evaluations
2.4. In Situ Antimicrobial Activity Evaluations
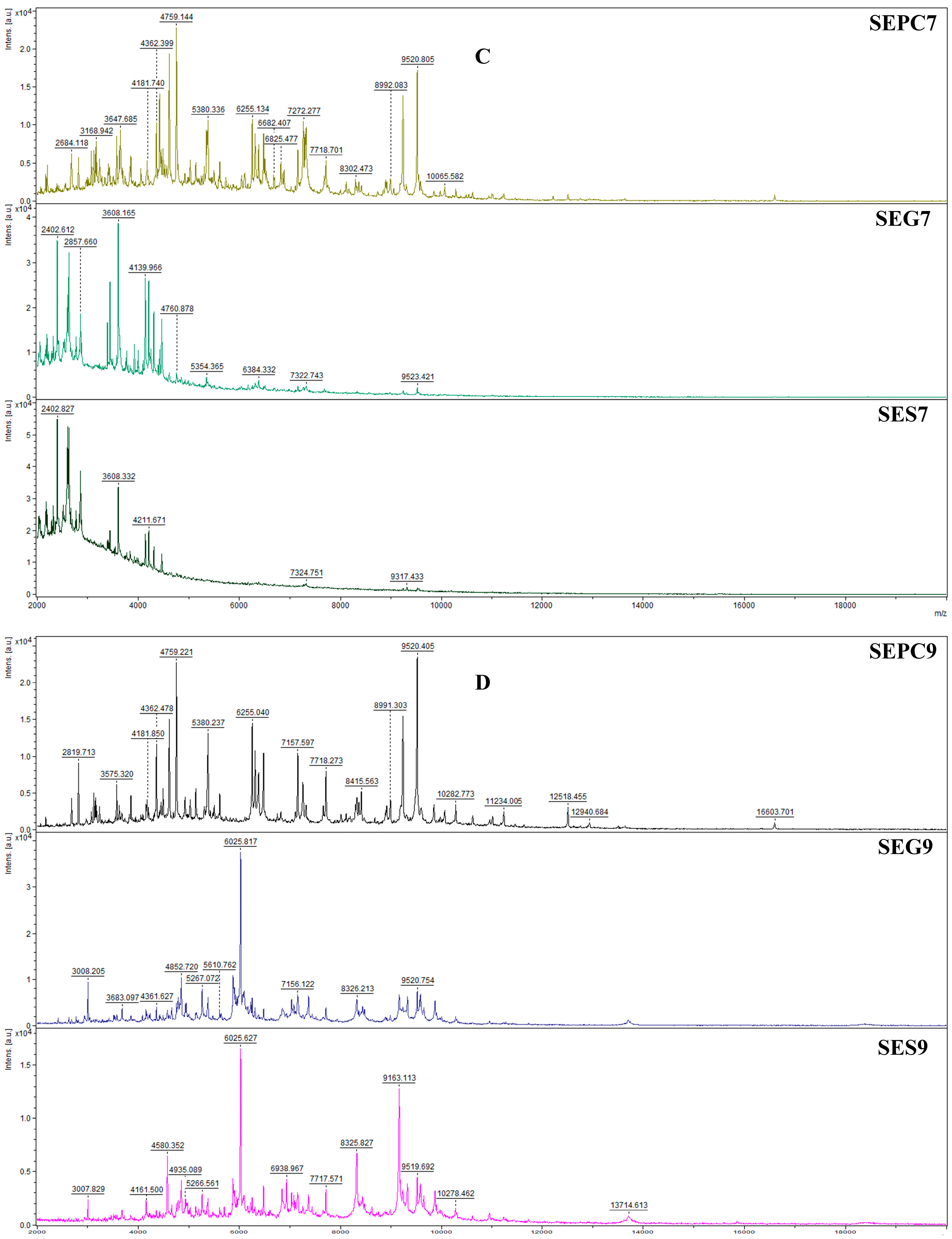
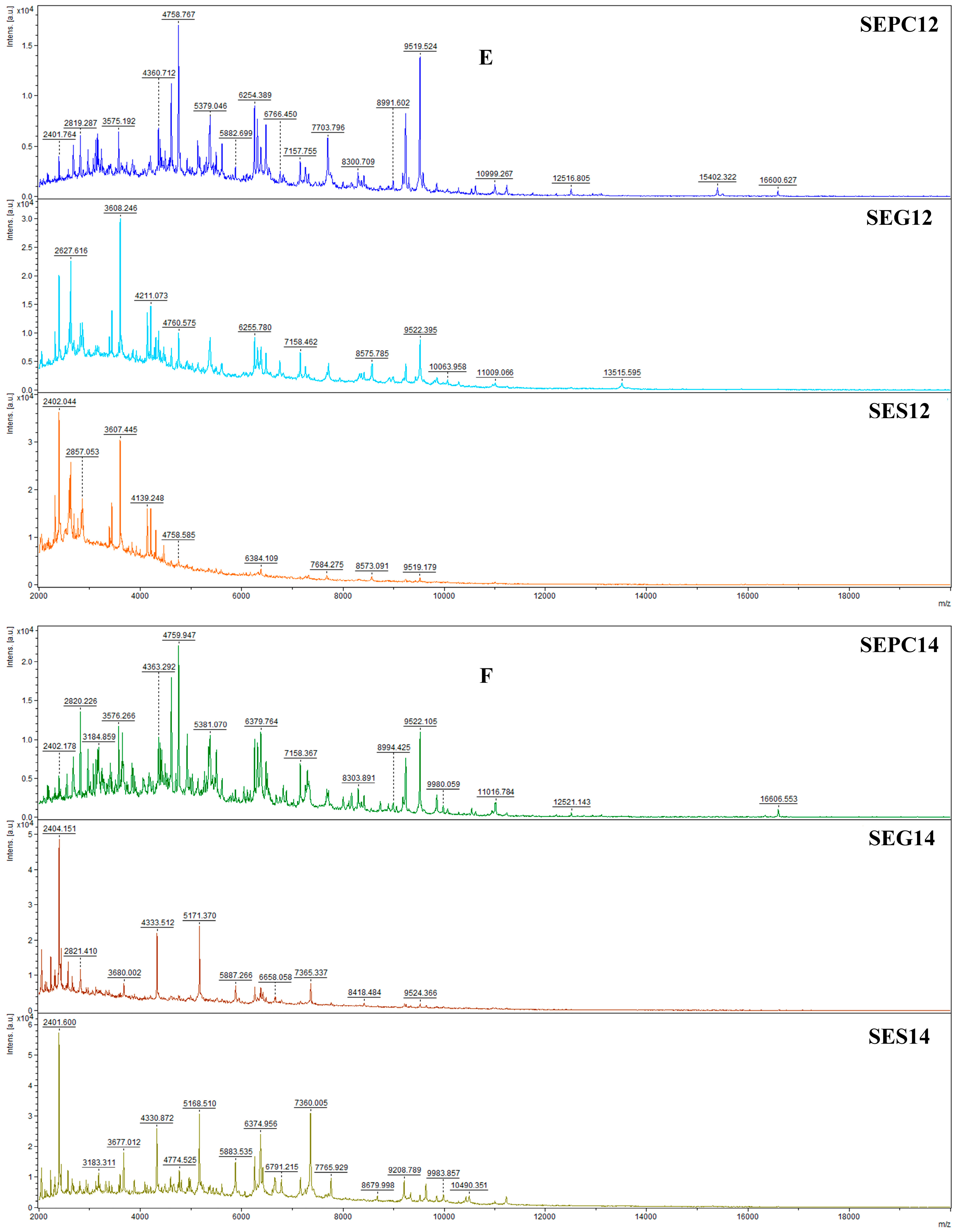
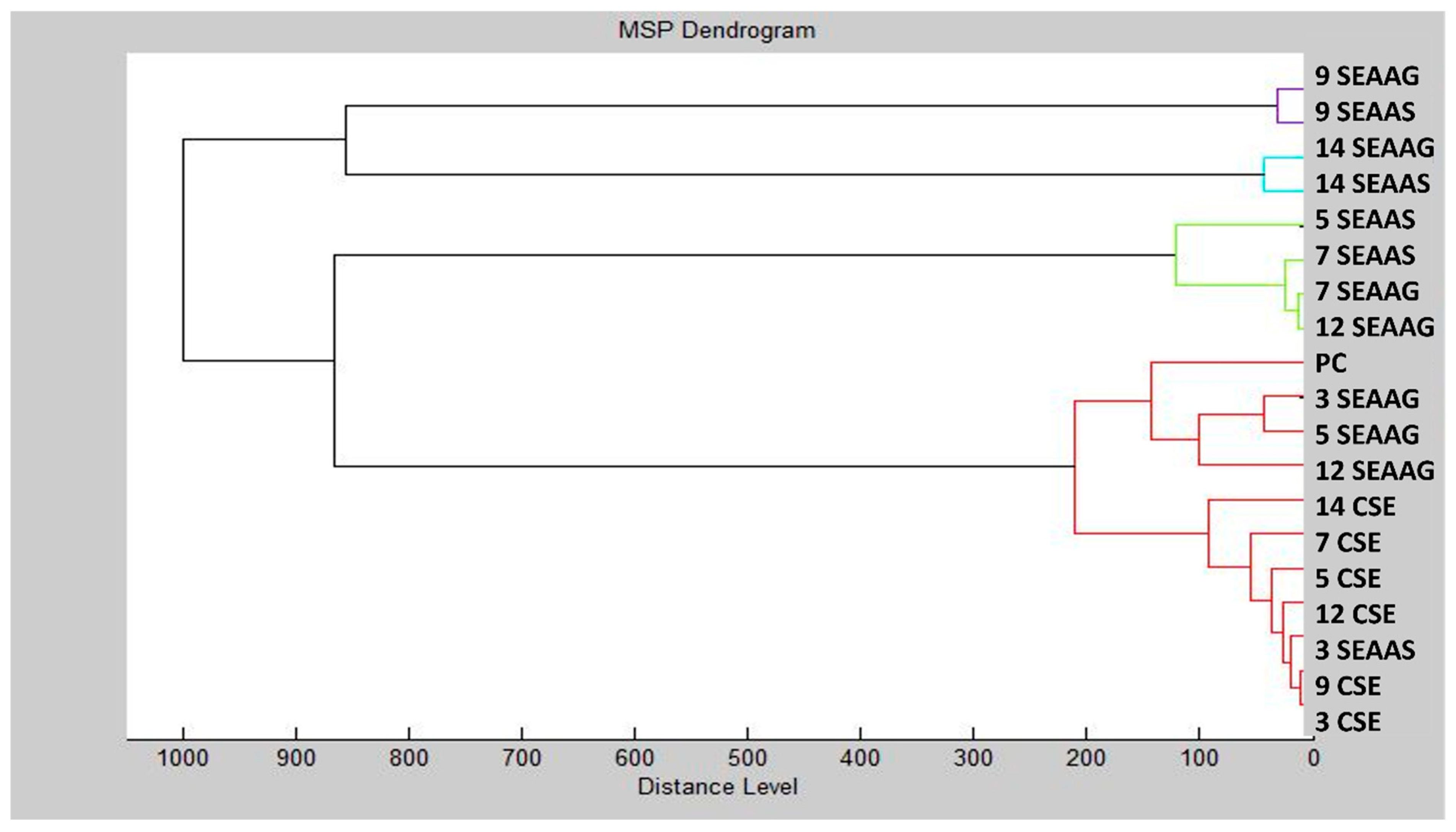
2.5. Antibiofilm Activity Evaluations
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Essential Oil
4.3. Determination of the Chemical Profile by Employing GC/MS Analysis
- MS ion source temperature at 230 °C;
- MS quadrupole temperature at 150 °C;
- Split mode set with a split ratio of 40.8:1;
- Flow rate of the carrier gas (Helium 5.0) at 1 mL/min;
- Electron-impact mass spectrometric data (EI-MS; 70 eV) were acquired in scan mode over the m/z range 35–550.
4.4. Antioxidant Activity
4.5. In Vitro Antimicrobial Evaluations
4.5.1. Disc Diffusion Method
4.5.2. Broth Dilution Method
4.6. In Situ Antimicrobial Evaluations
4.7. Antibiofilm Evaluations
4.7.1. Crystal Violet Assay
4.7.2. MALDI-TOF MS Study
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A.; et al. Antimicrobial Potentials of Natural Products against Multidrug Resistance Pathogens: A Comprehensive Review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Wu-Wu, J.W.F.; Guadamuz-Mayorga, C.; Oviedo-Cerdas, D.; Zamora, W.J. Antibiotic Resistance and Food Safety: Perspectives on New Technologies and Molecules for Microbial Control in the Food Industry. Antibiotics 2023, 12, 550. [Google Scholar] [CrossRef]
- Li, S.; Jiang, S.; Jia, W.; Guo, T.; Wang, F.; Li, J.; Yao, Z. Natural Antimicrobials from Plants: Recent Advances and Future Prospects. Food Chem. 2024, 432, 137231. [Google Scholar] [CrossRef]
- Chaves, R.D.; Kumazawa, S.H.; Khaneghah, A.M.; Alvarenga, V.O.; Hungaro, H.M.; Sant’Ana, A.S. Comparing the Susceptibility to Sanitizers, Biofilm-Forming Ability, and Biofilm Resistance to Quaternary Ammonium and Chlorine Dioxide of 43 Salmonella Enterica and Listeria Monocytogenes Strains. Food Microbiol. 2024, 117, 104380. [Google Scholar] [CrossRef] [PubMed]
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm Formation, and Related Impacts on Healthcare, Food Processing and Packaging, Industrial Manufacturing, Marine Industries, and Sanitation–A Review. Appl. Microbiol. 2023, 3, 629–665. [Google Scholar] [CrossRef]
- Jadhav, R.; Pawar, P.; Choudhari, V.; Topare, N.; Raut-Jadhav, S.; Bokil, S.; Khan, A. An Overview of Antimicrobial Nanoparticles for Food Preservation. Mater. Today Proc. 2023, 72, 204–216. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in Efficient Extraction of Essential Oils from Spices and Its Application in Food Industry: A Critical Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 11482–11503. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.P.; Gupta, V.; Raghuvanshi, T.S. Essential Oils as Green Promising Alternatives to Chemical Preservatives for Agri-Food Products: New Insight into Molecular Mechanism, Toxicity Assessment, and Safety Profile. Food Chem. Toxicol. 2024, 183, 114241. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Hovaneț, M.V.; Miron, A.; Anghel, A.I.; Dinu, M. Phytochemistry, Biological, and Pharmacological Properties of Abies Alba Mill. Plants 2023, 12, 2860. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.L.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and Vapor Phase of Four Conifer-Derived Essential Oils: Comparison of Chemical Compositions and Antimicrobial and Antioxidant Properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Broznić, D.; Ratkaj, I.; Malenica Staver, M.; Kraljević Pavelić, S.; Žurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies Alba Mill.) Honeydew Honey Collected from Gorski Kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Ďúranová, H.; Vukovic, N.L.; Vukic, M.; Kluz, M.; Kačániová, M. Assessment of Chemical Composition and Anti-Penicillium Activity of Vapours of Essential Oils from Abies Alba and Two Melaleuca Species in Food Model Systems. Molecules 2022, 27, 3101. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Ferraro, M.J. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Stojanović-Radić, Z.Z.; Jovanović, S.Č.; Cvetković, V.J.; Nikolić, J.S.; Ickovski, J.D.; Mitrović, T.L.; Nikolić, B.M.; Zlatković, B.K.; Stojanović, G.S. Essential Oils of Three Balkan Abies Species: Chemical Profiles, Antimicrobial Activity and Toxicity toward Artemia Salina and Drosophila Melanogaster. Chem. Biodivers. 2022, 19, e202200235. [Google Scholar] [CrossRef]
- Roussis, V.; Couladis, M.; Tzakou, O.; Loukis, A.; Petrakis, P.V.; Dukic, N.M.; Jancic, R. A Comparative Study on the Needle Volatile Constituents of Three Abies Species Grown in South Balkans. J. Essent. Oil Res. 2000, 12, 41–46. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Castola, V.; Casanova, J. Composition and Chemical Variability of the Twig Oil of Abies Alba Miller from Corsica. Flavour. Fragr. J. 2007, 22, 293–299. [Google Scholar] [CrossRef]
- Wajs, A.; Urbańska, J.; Zaleśkiewicz, E.; Bonikowski, R. Composition of Essential Oil from Seeds and Cones of Abies Alba. Nat. Prod. Commun. 2010, 5, 1934578X1000500830. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Maciąg, A.; Szoka, Ł.; Karna, E. Chemical Composition and Biological Activity of Abies Alba and A. Koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodivers. 2015, 12, 407–418. [Google Scholar] [CrossRef]
- Marjanovic-Balaban, Z.; Cvjetkovic, V.G.; Stanojevic, L.; Stanojevic, J.; Nikolic, L.; Danilovic, B. Quality Testing of Industrially Produced Essential Oil of Fir (Abies Alba L.) from the Republic of Srpska. J. Essent. Oil Bear. Plants 2020, 23, 503–513. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukic, M.; Čmiková, N.; Bianchi, A.; Garzoli, S.; Saad, R.B.; Hsouna, A.B.; Kluz, M.I.; Waszkiewicz-Robak, B.; Branković, J.; et al. Exploring the Bioactive Potential of Pinus Mugo Turra Essential Oil: Volatile Composition, Antioxidant, Antimicrobial, Antibiofilm and Insecticidal Activities. Flavour. Fragr. J. 2025, 40, 349–364. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Melkina, O.E.; Plyuta, V.A.; Khmel, I.A.; Zavilgelsky, G.B. The Mode of Action of Cyclic Monoterpenes (−)-Limonene and (+)-α-Pinene on Bacterial Cells. Biomolecules 2021, 11, 806. [Google Scholar] [CrossRef]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Im, N.K.; Jhee, K.H.; Lee, S.P. Radical Scavenging Activity of the Essential Oil of Silver Fir (Abies alba). J. Clin. Biochem. Nutr. 2009, 44, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of Stress and Defense in Plant Secondary Metabolites Production. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 151–195. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Ezzariga, N.; Moukal, A.; Asdadi, A.; Lemkhente, Z.; Moustaoui, F.; Kaaya, A.; Aghrouch, M. Evaluation of the Antimicrobial Activity of 20 Essential Oils and Their Combinations on Bacterial and Fungal Strains. Cureus 2025, 17, e79499. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Viljoen, A.M. Antimicrobial Activity of Limonene Enantiomers and 1,8-cineole Alone and in Combination. Flavour. Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef] [PubMed]
- Leite-Sampaio, N.F.; Gondim, C.N.F.L.; Martins, R.A.A.; Siyadatpanah, A.; Norouzi, R.; Kim, B.; Sobral-Souza, C.E.; Gondim, G.E.C.; Ribeiro-Filho, J.; Coutinho, H.D.M. Potentiation of the Activity of Antibiotics against ATCC and MDR Bacterial Strains with (+)- α -Pinene and (-)-Borneol. Biomed. Res. Int. 2022, 2022, 8217380. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, D.; Bautista-Baños, S. A Review on the Use of Essential Oils for Postharvest Decay Control and Maintenance of Fruit Quality during Storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Cavaco, A.M. The Use of Essential Oils for Postharvest Decay Control. A Review. Flavour. Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Garner, C.M. A Review of Essential Oils as Antimicrobials in Foods with Special Emphasis on Fresh Produce. J. Food Prot. 2022, 85, 1300–1319. [Google Scholar] [CrossRef]
- Popa, G.L.; Popa, M.I. Salmonella Spp. Infection—A Continuous Threat Worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.; Zoumpoulakis, P.; Sinanoglou, V. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological Evaluation of Several Coumarin Derivatives Designed as Possible Anti-Inflammatory/Antioxidant Agents. J. Enzym. Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef]
- Kundu, T.; Pramanik, A. Expeditious and Eco-Friendly Synthesis of New Multifunctionalized Pyrrole Derivatives and Evaluation of Their Antioxidant Property. Bioorg Chem. 2020, 98, 103734. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella Enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S.; et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum sativum L.) Essential Oil for Its Application in Foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]

| No | RI (Lit.) a | RI (Calc.) b | Compound c | % d |
|---|---|---|---|---|
| 1 | 939 | 935 | α-pinene | 36.2 |
| 2 | 954 | 951 | camphene | tr |
| 3 | 967 | 954 | verbenene | 1.4 |
| 4 | 979 | 977 | β-pinene | 5.3 |
| 5 | 990 | 988 | β-myrcene | 1.5 |
| 6 | 1026 | 1027 | o-cymene | tr |
| 7 | 1029 | 1033 | limonene | 52.2 |
| 8 | 1205 | 1205 | verbenone | 0.8 |
| 9 | 1523 | 1517 | δ-cadinene | 1.5 |
| total | 98.9 |
| IC50 (mg/mL) | TEAC | |
|---|---|---|
| ABTS+• | 1.18 ± 0.05 | 0.0014 |
| DPPH• | 2.33 ± 0.01 | 0.0020 |
| Microorganism | Inhibition Zone (mm) | |
|---|---|---|
| AAEO | ATB/AMC | |
| Gram-positive bacteria | ||
| Listeria monocytogenes CCM 4699 | 14.67 ± 0.58 d | 27.67 ± 0.58 a,b,c |
| Micrococcus luteus CCM 732 | 14.33 ± 0.58 d | 29.67 ± 0.58 d,e |
| Staphylococcus aureus CCM 3953 | 13.67 ± 0.58 d | 28.67 ± 0.58 b,c,d |
| Gram-negative bacteria | ||
| Enterobacter aerogenes CCM 2531 | 9.33 ± 0.58 b,c | 27.33 ± 0.58 a,b |
| Escherichia coli CCM 3953 | 10.67 ± 0.58 c | 30.67 ± 0.58 e |
| Yersinia enterocolitica CCM 5671 | 8.67 ± 0.58 b | 29.33 ± 0.58 c,d,e |
| Yeast | ||
| Candida albicans CCM 8186 | 5.67 ± 0.58 a | 27.67 ± 0.58 a,b,c |
| Candida glabrata CCM 8270 | 7.67 ± 0.58 b | 26.67 ± 0.59 a |
| Candida krusei CCM 8271 | 8.67 ± 0.58 b | 27.33 ± 0.58 a,b |
| Candida tropicalis CCM 8223 | 7.67 ± 0.58 b | 26.67 ± 0.58 a |
| Biofilm-forming bacteria (BFB) | ||
| Salmonella enterica | 10.67 ± 0.58 c | 27.67 ± 0.58 a,b,c |
| Microorganism | MIC50 | MIC90 |
|---|---|---|
| Gram-positive bacteria | ||
| Listeria monocytogenes CCM 4699 | 0.67 ± 0.09 a | 0.77 ± 0.10 a |
| Micrococcus luteus CCM 732 | 0.54 ± 0.06 a | 0.64 ± 0.06 a |
| Staphylococcus aureus CCM 3953 | 0.59 ± 0.06 a | 0.63 ± 0.03 a |
| Gram-negative bacteria | ||
| Enterobacter aerogenes CCM 2531 | 3.07 ± 0.53 b,c | 4.19 ± 0.46 d,e |
| Escherichia coli CCM 3953 | 2.63 ± 0.26 b | 3.19 ± 0.59 b,c |
| Yersinia enterocolitica CCM 5671 | 4.49 ± 0.14 d | 5.09 ± 0.30 f |
| Yeast | ||
| Candida albicans CCM 8186 | 7.62 ± 0.22 f | 8.05 ± 0.24 h |
| Candida glabrata CCM 8270 | 6.54 ± 0.2 e | 7.07 ± 0.23 g |
| Candida krusei CCM 8271 | 4.34 ± 0.22 d | 4.81 ± 0.05 e,f |
| Candida tropicalis CCM 8223 | 3.49 ± 0.29 c | 3.95 ± 0.05 c,d |
| Biofilm-forming bacteria (BFB) | ||
| Salmonella enterica | 2.45 ± 0.10 b | 2.74 ± 0.27 b |
| Food Model | Microorganisms | Percent of Inhibition of EO (μg/L) | |||
|---|---|---|---|---|---|
| Strawberries | 62.5 | 125 | 250 | 500 | |
| G+ | Listeria monocytogenes | 33.26 ± 1.14 b | 45.40 ± 0.55 b | 55.51 ± 1.49 a | 66.88 ± 2.01 a |
| Micrococcus luteus | 45.11 ± 2.99 c | 53.89 ± 2.78 c | 65.63 ± 1.16 b | 75.66 ± 1.72 a,b | |
| Staphylococcus aureus | 6.26 ± 1.14 d | 34.76 ± 2.03 d | 55.68 ± 2.02 c | 85.63 ± 0.95 c,d | |
| G− | Enterobacter aerogenes | 35.07 ± 3.14 b | 54.13 ± 3.56 c | 74.63 ± 3.99 c | 94.26 ± 3.50 d |
| Escherichia coli | 13.92 ± 0.55 a | 34.60 ± 1.89 a | 55.70 ± 2.66 a | 77.62 ± 1.79 b,c | |
| Yersinia enterocolitica | 34.00 ± 0.59 b | 45.17 ± 1.04 b | 55.75 ± 1.82 a | 73.82 ± 2.56 a,b | |
| Yeast | Candida albicans | 29.33 ± 5.72 b | 45.51 ± 1.01 b | 66.04 ± 2.15 b | 86.36 ± 2.43 c,d |
| Candida glabrata | 44.37 ± 2.52 c | 55.95 ± 0.64 c | 65.33 ± 2.20 b | 74.97 ± 3.78 a,b | |
| Candida krusei | 33.92 ± 1.66 b | 44.37 ± 1.13 b | 57.20 ± 2.17 a | 73.18 ± 6.40 a,b | |
| Candida tropicalis | 32.63 ± 1.27 b | 43.95 ± 2.02 b | 54.93 ± 2.17 a | 66.73 ± 2.90 a | |
| BFB | Salmonella enterica | 43.81 ± 1.64 c | 54.10 ± 2.77 c | 63.78 ± 2.22 b | 75.60 ± 2.97 a,b |
| Kiwi | |||||
| G+ | Listeria monocytogenes | 36.08 ± 1.31 c | 46.11 ± 0.76 b | 56.77 ± 1.00 b | 66.40 ± 1.56 b |
| Micrococcus luteus | 24.85 ± 2.22 b | 35.37 ± 1.39 a | 44.33 ± 1.25 a | 57.14 ± 1.67 a | |
| Staphylococcus aureus | 43.10 ± 0.40 d | 55.34 ± 2.23 c | 63.56 ± 2.28 c | 73.89 ± 2.51 c | |
| G− | Enterobacter aerogenes | 17.44 ± 0.58 a | 33.56 ± 1.06 a | 46.07 ± 2.62 a | 75.96 ± 1.22 c |
| Escherichia coli | 24.60 ± 1.89 b | 37.63 ± 3.06 a | 55.71 ± 1.86 b | 76.58 ± 2.05 c | |
| Yersinia enterocolitica | 24.44 ± 1.32 b | 43.60 ± 2.06 b | 64.92 ± 3.03 c | 84.55 ± 1.84 d | |
| Yeast | Candida albicans | 33.66 ± 1.01 c | 44.27 ± 1.23 b | 55.11 ± 2.08 b | 64.56 ± 2.94 b |
| Candida glabrata | 17.14 ± 1.68 a | 35.63 ± 3.04 a | 54.07 ± 1.12 b | 73.39 ± 1.20 c | |
| Candida krusei | 43.74 ± 1.75 d | 58.43 ± 1.52 c | 64.60 ± 1.17 c | 76.03 ± 2.09 c | |
| Candida tropicalis | 33.63 ± 0.96 c | 47.44 ± 1.14 b | 55.07 ± 1.58 b | 66.05 ± 1.63 b | |
| BFB | Salmonella enterica | 45.40 ± 3.50 d | 53.41 ± 2.92 c | 64.42 ± 1.09 c | 76.13 ± 1.98 c |
| White radish | |||||
| G+ | Listeria monocytogenes | 44.29 ± 1.45 b | 56.29 ± 2.46 b | 64.02 ± 1.25 b | 75.71 ± 1.11 b |
| Micrococcus luteus | 36.14 ± 1.66 a | 45.30 ± 1.84 a | 56.04 ± 2.32 a | 67.39 ± 1.80 a | |
| Staphylococcus aureus | 45.26 ± 1.87 b | 55.33 ± 2.30 b | 64.81 ± 0.91 b | 74.52 ± 2.94 b | |
| G− | Enterobacter aerogenes | 56.59 ± 2.16 c | 64.56 ± 3.21 c | 75.22 ± 2.18 c | 84.45 ± 3.17 c |
| Escherichia coli | 55.18 ± 4.80 c | 65.43 ± 2.24 c | 77.61 ± 1.23 c | 86.62 ± 1.12 c | |
| Yersinia enterocolitica | 44.19 ± 1.30 b | 56.60 ± 2.17 b | 64.74 ± 1.06 b | 85.32 ± 3.14 c | |
| Yeast | Candida albicans | 55.36 ± 1.27 c | 65.91 ± 1.67 c | 76.19 ± 2.22 c | 85.90 ± 0.59 c |
| Candida glabrata | 33.43 ± 1.20 a | 45.61 ± 2.31 a | 56.70 ± 2.04 a | 66.70 ± 2.04 a | |
| Candida krusei | 35.23 ± 2.19 a | 45.74 ± 1.89 a | 55.37 ± 2.27 a | 66.85 ± 1.78 a | |
| Candida tropicalis | 35.30 ± 1.70 a | 44.71 ± 0.95 a | 53.93 ± 2.09 a | 66.56 ± 1.46 a | |
| BFB | Salmonella enterica | 54.56 ± 2.01 c | 64.71 ± 0.98 c | 76.43 ± 1.70 c | 85.96 ± 1.78 c |
| Beetroot | |||||
| G+ | Listeria monocytogenes | 67.37 ± 1.70 a | 55.33 ± 1.29 a | 45.14 ± 1.53 a | 36.44 ± 2.28 a,b |
| Micrococcus luteus | 66.77 ± 1.24 a | 56.34 ± 1.82 a | 44.71 ± 1.06 a | 35.40 ± 1.37 a | |
| Staphylococcus aureus | 75.98 ± 2.56 b | 66.48 ± 2.23 b | 55.11 ± 2.08 b | 42.55 ± 1.20 b,c | |
| G− | Enterobacter aerogenes | 77.63 ± 1.89 b | 56.34 ± 1.82 a | 46.30 ± 2.13 a | 35.04 ± 2.64 a |
| Escherichia coli | 75.41 ± 1.16 b | 66.55 ± 2.90 b | 55.63 ± 1.24 b | 45.29 ± 1.91 c | |
| Yersinia enterocolitica | 77.26 ± 2.34 b | 65.95 ± 2.80 b | 55.49 ± 2.71 b | 46.10 ± 2.55 c | |
| Yeast | Candida albicans | 75.78 ± 1.64 b | 66.21 ± 2.58 b | 56.40 ± 2.78 b | 44.77 ± 1.75 c |
| Candida glabrata | 74.52 ± 1.23 b | 64.12 ± 1.13 b | 54.44 ± 1.56 b | 36.36 ± 2.03 a,b | |
| Candida krusei | 66.96 ± 2.18 a | 57.17 ± 0.62 a | 44.70 ± 1.00 a | 34.92 ± 2.26 a | |
| Candida tropicalis | 67.33 ± 2.53 a | 53.80 ± 0.76 a | 44.04 ± 1.48 a | 35.00 ± 2.69 a | |
| BFB | Salmonella enterica | 76.73 ± 2.21 b | 67.29 ± 1.67 b | 56.62 ± 2.22 b | 45.70 ± 1.96 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukić, M.D.; Vuković, N.L.; Jakovljević, M.R.; Ristić, M.S.; Kačániová, M. Antimicrobial Effects of Abies alba Essential Oil and Its Application in Food Preservation. Plants 2025, 14, 2071. https://doi.org/10.3390/plants14132071
Vukić MD, Vuković NL, Jakovljević MR, Ristić MS, Kačániová M. Antimicrobial Effects of Abies alba Essential Oil and Its Application in Food Preservation. Plants. 2025; 14(13):2071. https://doi.org/10.3390/plants14132071
Chicago/Turabian StyleVukić, Milena D., Nenad L. Vuković, Marina Radović Jakovljević, Marija S. Ristić, and Miroslava Kačániová. 2025. "Antimicrobial Effects of Abies alba Essential Oil and Its Application in Food Preservation" Plants 14, no. 13: 2071. https://doi.org/10.3390/plants14132071
APA StyleVukić, M. D., Vuković, N. L., Jakovljević, M. R., Ristić, M. S., & Kačániová, M. (2025). Antimicrobial Effects of Abies alba Essential Oil and Its Application in Food Preservation. Plants, 14(13), 2071. https://doi.org/10.3390/plants14132071









