Assessment of the Status of Cephalanthera longifolia Populations in Lithuania Derived from a Single-Census Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
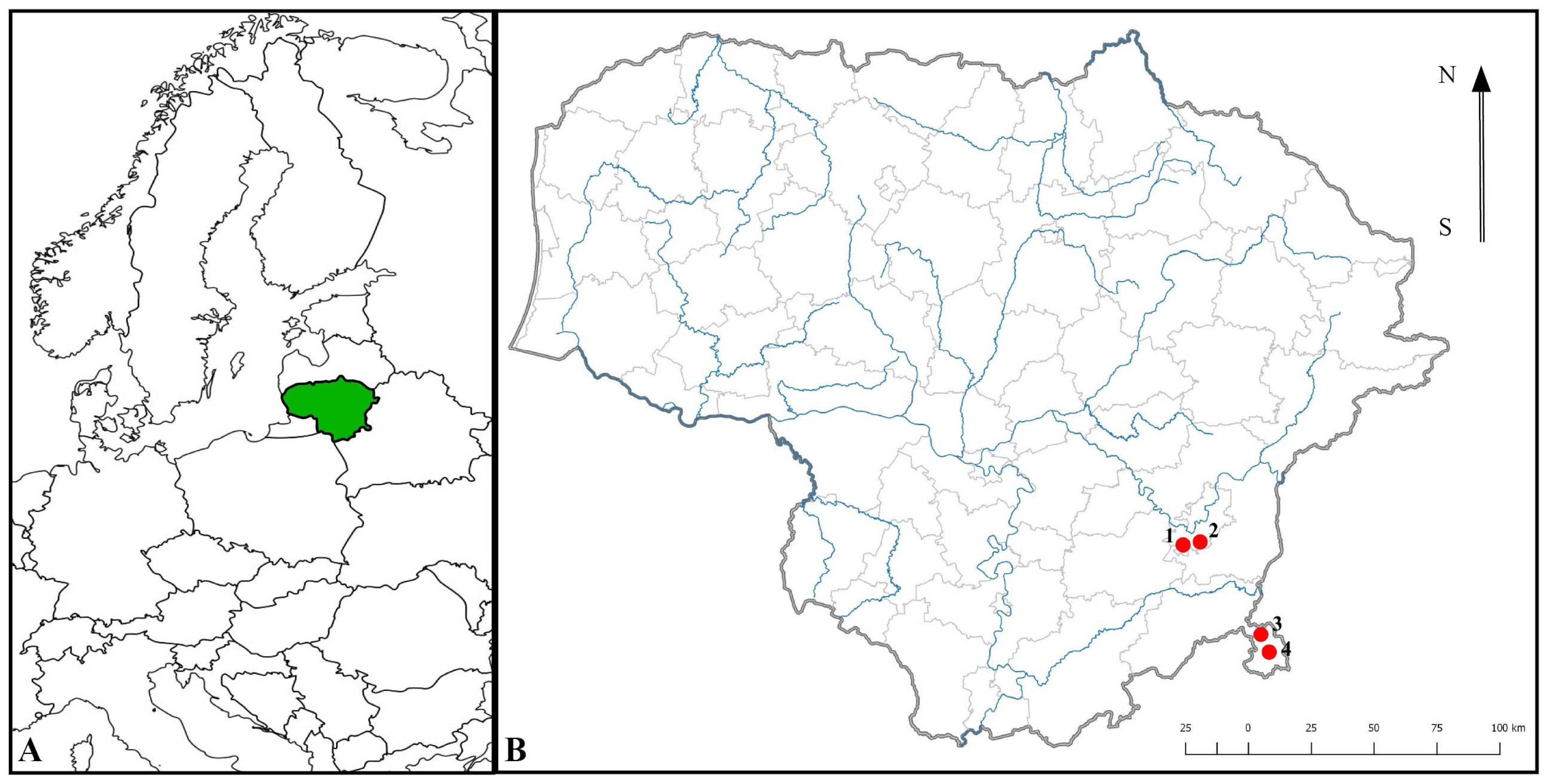
2.2. Study Sites
2.3. Field Surveys
2.4. Statistical Analysis
3. Results
3.1. Developmental Stages of Individuals
3.2. Density of Individuals
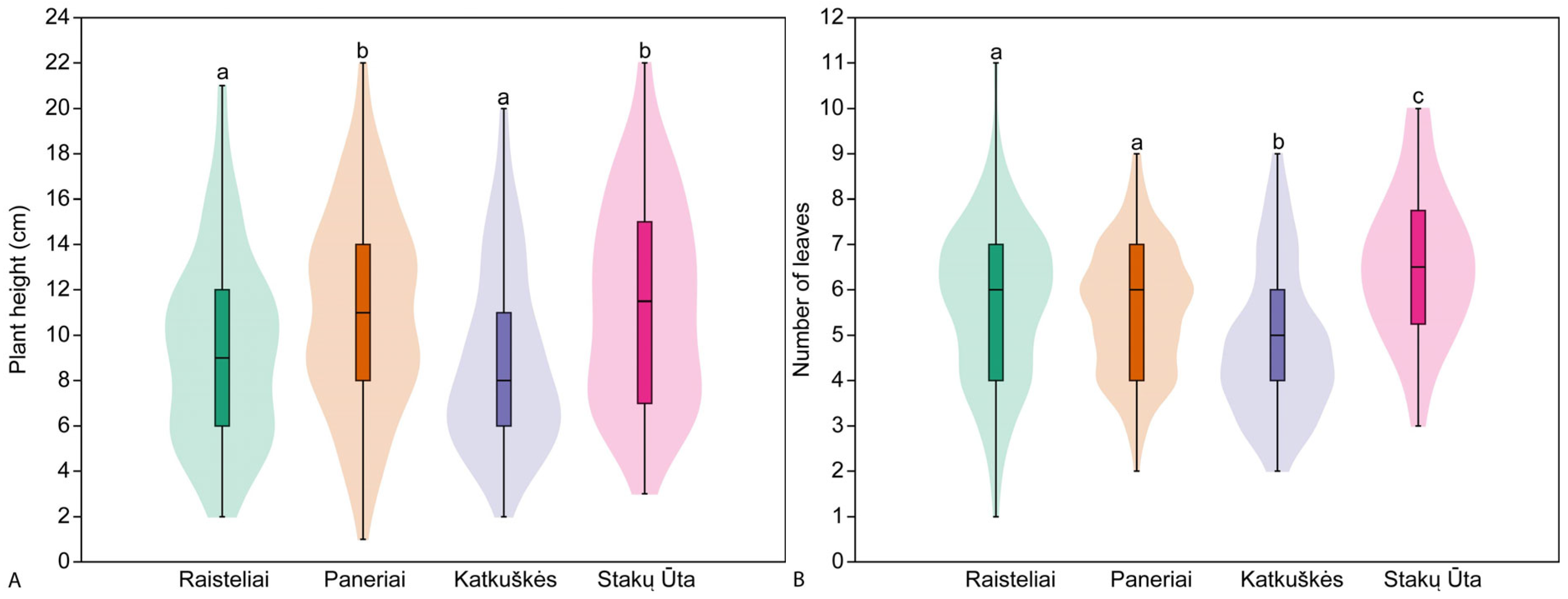
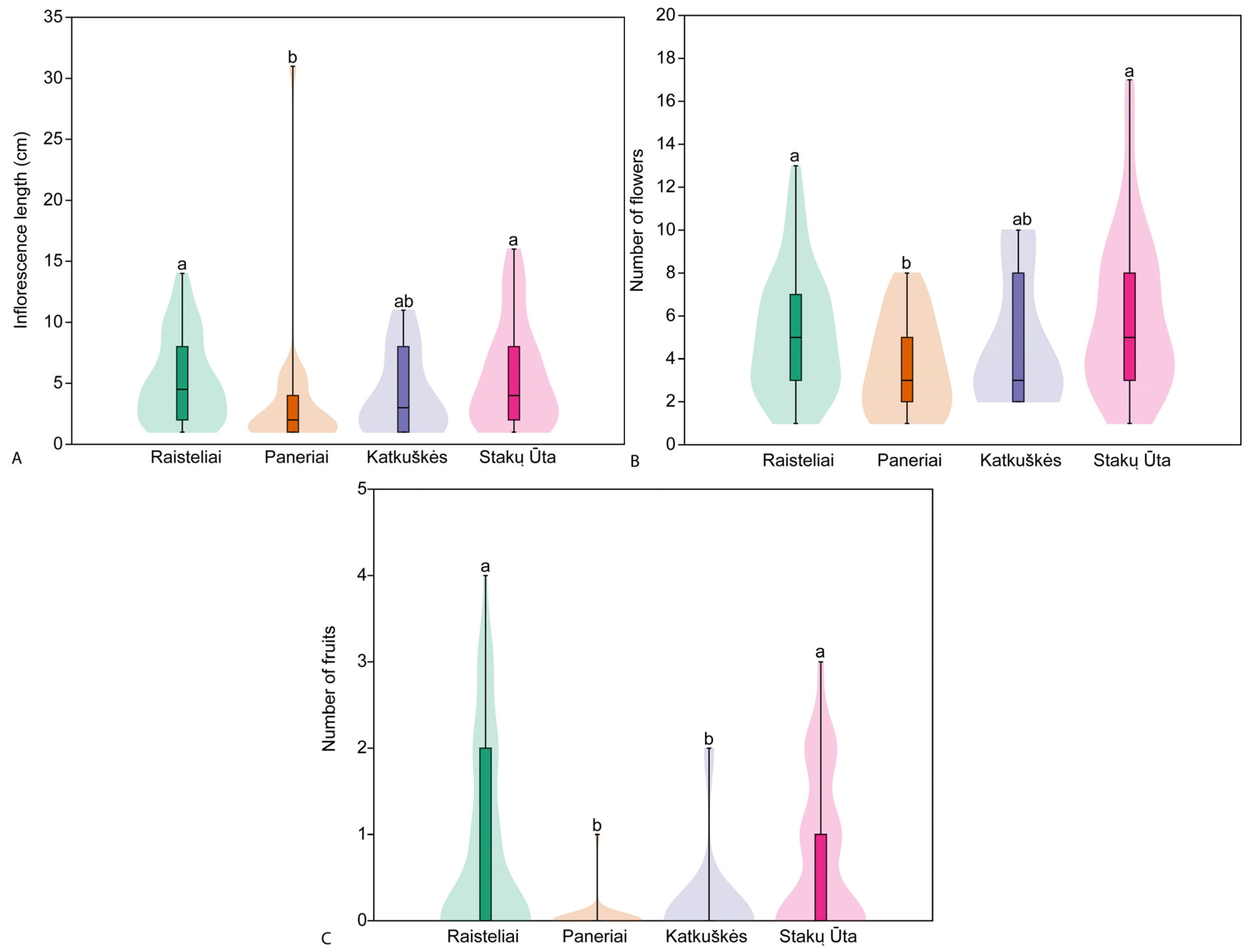
3.3. Plant Traits in Studied Populations
3.4. Species Richness in Habitats
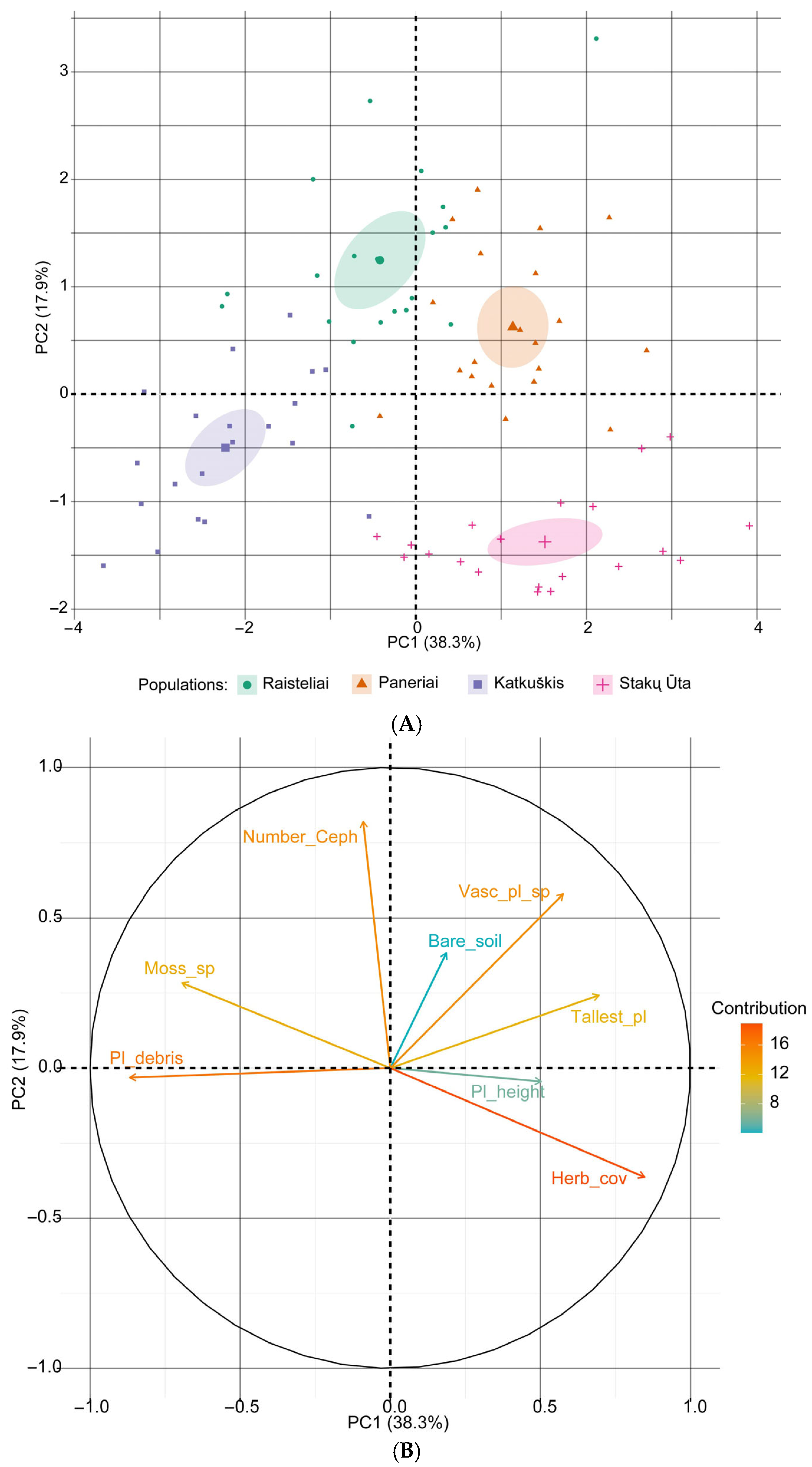
3.5. Effect of Community Structure
4. Discussion
4.1. Developmental Stages of Individuals
4.2. Density of Individuals
4.3. Species Richness in Habitats
4.4. Effect of Community Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Site | pH | P2O5 (mg/kg) | K2O (mg/kg) | N total (mg/kg) | Humus (%) |
|---|---|---|---|---|---|
| Raisteliai | 5.2 | 172 | 82 | 1.02 | 2.01 |
| Paneriai | 5.7 | 147 | 58 | 1.39 | 1.81 |
| Katkuškės | 4.2 | 13 | 37 | 0.80 | 2.00 |
| Stakų Ūta | 4.4 | 50 | 78 | 1.21 | 2.61 |
References
- Selwood, K.E.; McGeoch, M.A.; Mac Nally, R. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 2015, 90, 837–853. [Google Scholar] [CrossRef]
- Iler, A.M.; CaraDonna, P.J.; Forrest, J.R.; Post, E. Demographic consequences of phenological shifts in response to climate change. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 221–245. [Google Scholar] [CrossRef]
- Knapp, W.M.; Frances, A.; Noss, R.; Naczi, R.F.C.; Weakley, A.; Gann, G.D.; Baldwin, B.G.; Miller, J.; McIntyre, P.; Mishler, B.D.; et al. Vascular plant extinction in the continental United States and Canada. Conserv. Biol. 2021, 35, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Mehrabian, A.R.; Sayadi, S.; Kuhbenani, M.M.; Yeganeh, V.H.; Abdoljabari, M. Priorities for conservation of endemic trees and shrubs of Iran: Important Plant Areas (IPAs) and Alliance for Zero Extinction (AZE) in SW Asia. J. Asia-Pac. Biodivers. 2020, 13, 295–305. [Google Scholar] [CrossRef]
- Vegas-Vilarrúbia, T.; Rull, V.; Montoya, E.; Safont, E. Quaternary palaeoecology and nature conservation: A general review with examples from the Neotropics. Quat. Sci. Rev. 2011, 30, 2361–2388. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Rabitsch, W.; Hulme, P.E.; Pyšek, P.; Wilson, J.R.; Richardson, D.M. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Divers. Distrib. 2015, 21, 534–547. [Google Scholar] [CrossRef]
- Eichenberg, D.; Bowler, D.E.; Bonn, A.; Bruelheide, H.; Grescho, V.; Harter, D.; Jandt, U.; May, R.; Winter, M.; Jansen, F. Widespread decline in Central European plant diversity across six decades. Glob. Change Biol. 2021, 27, 1097–1110. [Google Scholar] [CrossRef]
- Franklin, J.; Serra-Diaz, J.M.; Syphard, A.D.; Regan, H.M. Global change and terrestrial plant community dynamics. Proc. Natl. Acad. Sci. USA 2016, 113, 3725–3734. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Búrquez, A.; Martínez-Yrízar, A.; Dovciak, M. A trait-based approach to the conservation of threatened plant species. Oryx 2019, 53, 429–435. [Google Scholar] [CrossRef]
- Kaya, O.F.; Ertekin, A.S. Evaluation of the taxonomy and conservation status of Podonosma sintenisii (Boraginaceae). Botanica 2024, 30, 31–41. [Google Scholar] [CrossRef]
- Turco, A.; Wagensommer, R.P.; Medagli, P.; D’Emerico, S.; Ippolito, F.; Scordella, G.; Albano, A. Centaurea pumilio (Asteraceae): Conservation status, threats and population size of a critically endangered species in Italy. Plants 2025, 14, 1074. [Google Scholar] [CrossRef] [PubMed]
- Oostermeijer, J.G.B.; Hartman, Y. Inferring population and metapopulation dynamics of Liparis loeselii from single-census and inventory data. Acta Oecol. 2014, 60, 30–39. [Google Scholar] [CrossRef]
- Hüls, J.; Otte, A.; Eckstein, R.L. Population life-cycle and stand structure in dense and open stands of the introduced tall herb Heracleum mantegazzianum. Biol. Invasions 2007, 9, 799–811. [Google Scholar] [CrossRef]
- Pergl, J.; Hüls, J.; Perglová, I.; Eckstein, R.L.; Pyšek, P.; Otte, A. Population dynamics of Heracleum mantegazzianum. In Ecology and Management of Giant Hogweed (Heracleum mantegazzianum); CAB International: Oxfordshire, UK, 2007; pp. 92–111. [Google Scholar]
- Crone, E.E.; Menges, E.S.; Ellis, M.M.; Bell, T.; Bierzychudek, P.; Ehrlén, J.; Williams, J.L. How do plant ecologists use matrix population models? Ecol. Lett. 2011, 14, 1–8. [Google Scholar] [CrossRef]
- Meekins, J.F.; McCarthy, B.C. Effect of population density on the demography of an invasive plant (Alliaria petiolata, Brassicaceae) population in a southeastern Ohio forest. Am. Midl. Nat. 2002, 147, 256–278. [Google Scholar] [CrossRef]
- Gudžinskas, Z.; Žalneravičius, E.; Norkevičienė, E.; Obelevičius, K. State and dynamics of the protected plant species in the south-western Lithuania under conditions of the climate changes. In Conservation of Botanical Diversity in South-Western Lithuania; Mildažienė, V., Stankevičienė, K., Balsevičius, A., Narijauskas, R., Gudžinskas, Z., Žalneravičius, E., Norkevičienė, E., Obelevičius, K., Eds.; Versus Aureus: Vilnius, Lithuania, 2016; pp. 140–159. [Google Scholar]
- Taura, L.; Gudžinskas, Z. Life stages and demography of invasive shrub Cytisus scoparius (Fabaceae) in Lithuania. Botanica 2020, 26, 1–14. [Google Scholar] [CrossRef]
- Gudžinskas, Z.; Kazlauskas, M. The first record of Heracleum mantegazzianum Sommier & Levier (Apiaceae) in Lithuania. BioInvasions Rec. 2022, 11, 320–329. [Google Scholar] [CrossRef]
- Hegland, S.J.; Van Leeuwen, M.; Oostermeijer, J.G.B. Population structure of Salvia pratensis in relation to vegetation and management of Dutch dry floodplain grasslands. J. Appl. Ecol. 2001, 38, 1277–1289. [Google Scholar] [CrossRef]
- Kalliovirta, M.; Ryttäri, T.; Heikkinen, R.K. Population structure of a threatened plant, Pulsatilla patens, in boreal forests: Modelling relationships to overgrowth and site closure. Biodivers. Conserv. 2006, 15, 3095–3108. [Google Scholar] [CrossRef]
- Rasimavičius, M.; Naujalis, J.R.; Gudžinskas, Z. Structure of Equisetum variegatum (Equisetaceae) populations in natural and anthropogenic habitats. Botanica 2023, 29, 96–109. [Google Scholar] [CrossRef]
- Brys, R.; Jacquemyn, H.; Endels, P.; Hermy, M.; De Blust, G. The relationship between reproductive success and demographic structure in remnant populations of Primula veris. Acta Oecol. 2003, 24, 247–253. [Google Scholar] [CrossRef]
- Colas, B.; Kirschner, F.; Riba, M.; Olivieri, I.; Mignot, A.; Imbert, E.; Fréville, H. Restoration demography: A 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa (Asteraceae). J. Appl. Ecol. 2008, 45, 1468–1476. [Google Scholar] [CrossRef]
- Jermakowicz, E.; Brzosko, E. Demographic responses of boreal-montane orchid Malaxis monophyllos (L.) Sw. populations to contrasting environmental conditions. Acta Soc. Bot. Pol. 2016, 85, 3488. [Google Scholar] [CrossRef]
- Pylypiv, Y. Structural features of the Orchidaceae populations. Sci. Horiz. 2020, 23, 33–46. [Google Scholar] [CrossRef]
- Cook, R.E. Clonal plant populations: A knowledge of clonal structure can affect the interpretation of data in a broad range of ecological and evolutionary studies. Am. Sci. 1983, 71, 244–253. [Google Scholar]
- Brys, R.; Jacquemyn, H.; Endels, P.; De Blust, G.; Hermy, M. The effects of grassland management on plant performance and demography in the perennial herb Primula veris. J. Appl. Ecol. 2004, 41, 1080–1091. [Google Scholar] [CrossRef]
- Kazlauskas, M.; Taura, L.; Gudžinskas, Z. Current state of critically endangered Neotinea ustulata (Orchidaceae) in Lithuania and report on a new record of the species. Botanica 2022, 28, 91–101. [Google Scholar] [CrossRef]
- El Karmoudi, Y.; Libiad, M.; Fahd, S. Diversity and conservation strategies of wild Orchidaceae species in the West Rif region (northern Morocco). Botanica 2025, 31, 13–24. [Google Scholar] [CrossRef]
- Štípková, Z.; Kindlmann, P. Distribution of population sizes in metapopulations of threatened organisms—Implications for conservation of orchids. Plants 2025, 14, 369. [Google Scholar] [CrossRef]
- Taura, L.; Gudžinskas, Z. Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) in Lithuania: Analysis of distribution, population dynamics and conservation issues. Botanica 2024, 30, 127–149. [Google Scholar] [CrossRef]
- Cabral, J.S.; Schurr, F.M. Estimating demographic models for the range dynamics of plant species. Glob. Ecol. Biogeogr. 2010, 19, 85–97. [Google Scholar] [CrossRef]
- Salguero-Gómez, R.; Siewert, W.; Casper, B.B.; Tielbörger, K. A demographic approach to study effects of climate change in desert plants. Philos. Trans. R. Soc. B 2012, 367, 3100–3114. [Google Scholar] [CrossRef] [PubMed]
- Ehrlén, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Kitajima, K.; Fenner, M. Ecology of seedling regeneration. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB Publishing: Oxfordshire, UK, 2000; pp. 331–359. [Google Scholar] [CrossRef]
- Whigham, D.F.; Willems, J.H. Demographic studies and life-history strategies of temperate terrestrial orchids as a basis for conservation. In Orchid Conservation; Dixon, K.W., Barrett, S.P., Cribb, P., Eds.; Natural History Publications: Research Triangle Park, NC, USA, 2003; pp. 137–158. [Google Scholar]
- Gregg, K.B. Recovery from bud disappearance explains prolonged dormancy in Cleistes bifaria (Orchidaceae). Am. J. Bot. 2011, 98, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Juárez, L.; Montaña, C.; Franco, M. The viability of two populations of the terrestrial orchid Cyclopogon luteoalbus in a fragmented tropical mountain cloud forest: Dormancy delays extinction. Biol. Conserv. 2014, 170, 162–168. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Mizuta, R.; Hutchings, M.J. Predicting evolution in response to climate change: The example of sprouting probability in three dormancy-prone orchid species. R. Soc. Open Sci. 2017, 4, 160647. [Google Scholar] [CrossRef]
- Chauhan, P.; Attri, L. Mycorrhizal associations in orchids: A review. Asian J. Biol. Life Sci. 2024, 13, 278–286. [Google Scholar] [CrossRef]
- Mawinei, N.; Paramitha, Q. Comparative anatomy of roots and leaves in epiphytic and terrestrial orchids: Insights into adaptations and ecological strategies. Law Econ. 2024, 18, 73–85. [Google Scholar]
- Ray, H.; Gillett-Kaufman, J. By land and by tree: Pollinator taxa diversity of terrestrial and epiphytic orchids. J. Pollinat. Ecol. 2022, 32, 174–185. [Google Scholar]
- Shefferson, R.P. Survival costs of adult dormancy and the confounding influence of size in lady’s slipper orchids, genus Cypripedium. Oikos 2006, 115, 253–262. [Google Scholar] [CrossRef]
- Rock-Blake, R.; McCormick, M.K.; Brooks, H.E.A.; Jones, C.S.; Whigha, D.F. Symbiont abundance can affect host plant population dynamics. Am. J. Bot. 2017, 104, 72–82. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Sandercock, B.K.; Proper, I.; Beissinger, S.R. Estimating dormancy and survival of a rare herbaceous perennial using mark-recapture models. Ecology 2001, 82, 145–156. [Google Scholar] [CrossRef]
- Kindlmann, P.; Willems, J.H.; Whigham, D.F. (Eds.) Trends and Fluctuations and Underlying Mechanisms in Terrestrial Orchid Populations; Backhuys Publishers: Kerkwerve, The Netherlands, 2002. [Google Scholar]
- Shefferson, R.P.; Jacquemyn, H.; Kull, T.; Hutchings, M.J. The demography of terrestrial orchids: Life history, population dynamics and conservation. Bot. J. Linn. Soc. 2020, 192, 315–332. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 234, 7–17. [Google Scholar] [CrossRef]
- Willems, J.H. Establishment and development of a population of Orchis simia (Lamk.) in The Netherlands, 1972 to 1981. New Phytol. 1982, 91, 757–765. [Google Scholar] [CrossRef]
- Kolon, K.; Dudzic, J.; Krawczyk, J.; Sadowska, A. Ekologiczna charakterystyka populacji Cephalanthera longifolia (L.) Fritsch na Łysej Górze k. Rząśnika. Acta Univ. Wratislav. Pr. Bot. 1993, 62, 99–104. [Google Scholar]
- Kolon, K.; Krawczyk, J.; Krawczyk, A. Charakterystyka ekologiczna populacji Epipactis palustris (L.) Crantz znad jeziora Pomorze w Puszczy Augustowskiej. Acta Univ. Wratislav. Pr. Bot. 1995, 172, 91–99. [Google Scholar]
- Ryla, M.; Čiuplys, R. Populations of Cephalanthera longifolia (L.) Fritsch in Lithuania. In Biodiversity in Relation to Vegetation Zones in Europe; Czyżewska, K., Hereźniak, J., Eds.; Łódź University Press: Łódź, Poland, 2005; pp. 41–55. [Google Scholar]
- Brzosko, E.; Wróblewska, A. Genetic variation and clonal diversity in island Cephalanthera rubra populations from the Biebrza National Park, Poland. Bot. J. Linn. Soc. 2003, 143, 99–108. [Google Scholar] [CrossRef]
- Sundberg, S. Present status for Cephalanthera rubra and Chimaphila umbellata in Sweden. Sven. Bot. Tidskr. 2017, 111, 90–104. [Google Scholar]
- Žalneravičius, E. Cephalanthera rubra, (L.) Rich. In Red Data Book of Lithuania: Animals, Plants, Fungi; Rašomavičius, V., Ed.; Aplinkos Ministerija: Vilnius, Lithuania, 2021; 395p. [Google Scholar]
- Taura, L.; Gudžinskas, Z. What factors determine the natural fruit set of Cephalanthera longifolia and Cephalanthera rubra? Diversity 2024, 16, 333. [Google Scholar] [CrossRef]
- Pykälä, J. Habitat loss and deterioration explain the disappearance of populations of threatened vascular plants, bryophytes and lichens in a hemiboreal landscape. Glob. Ecol. Conserv. 2019, 18, e00610. [Google Scholar] [CrossRef]
- Püttsepp, Ü.; Kull, T. Cephalanthera longifolia and Cephalanthera rubra in Estonia. Bot. Lith. 1997, 1, 133–135. [Google Scholar]
- Abadie, J.C.; Püttsepp, Ü.; Gebauer, G.; Faccio, A.; Bonfante, P.; Selosse, M.-A. Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: A comparative study between green and nonphotosynthetic individuals. Can. J. Bot. 2006, 84, 1462–1477. [Google Scholar] [CrossRef]
- Taura, L.; Gudžinskas, Z. Effect of simulated autogamy and allogamy on the success of Cephalanthera longifolia and Cephalanthera rubra (Orchidaceae) fruit set. Diversity 2025, 17, 73. [Google Scholar] [CrossRef]
- Dafni, A. Orchids of Israel: Notes on distribution, ecology and local variation. Mitt. Arb. Heim. Orchid. Baden-Württ. 1979, 11, 206–222. [Google Scholar]
- Delforge, P. Guide des orchidées d’Europe, d’Afrique du Nord et du Proche-Orient, 3rd ed.; Delachaux et Niestlé: Paris, France, 2005. [Google Scholar]
- Galvonaitė, A.; Valiukas, D.; Kilpys, J.; Kitrienė, Z.; Misiūnienė, M. Climate Atlas of Lithuania; Lithuanian Hydrometeorological Service Under the Ministry of Environment: Vilnius, Lithuania, 2013; 176p. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde. Dritte Auflage; Springer: New York, NY, USA, 1964; p. 865. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 16 February 2025).
- Thein, S.; Roscher, C.; Schulze, E.-D. Effects of trait plasticity on aboveground biomass production depend on species identity in experimental grasslands. Basic. Appl. Ecol. 2008, 9, 475–484. [Google Scholar] [CrossRef]
- Barry, K.E.; de Kroon, H.; Dietrich, P.; Harpole, S.W.; Roeder, A.; Schmid, B.; Clark, A.T.; Mayfield, M.M.; Wagg, C.; Roscher, C. Linking species coexistence to ecosystem functioning—A conceptual framework from ecological first principles in grassland ecosystems. Adv. Ecol. Res. 2019, 61, 265–296. [Google Scholar] [CrossRef]
- Roeder, A.; Schweingruber, F.H.; Ebeling, A.; Eisenhauer, N.; Fischer, M.; Roscher, C. Plant diversity effects on plant longevity and their relationships to population stability in experimental grasslands. J. Ecol. 2021, 109, 2566–2579. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Feng, J.Q.; Zhang, F.P.; Huang, J.L.; Hu, H.; Zhang, S.B. Allometry between vegetative and reproductive traits in orchids. Front. Plant Sci. 2021, 12, 728843. [Google Scholar] [CrossRef] [PubMed]

| Site | Tree Layers | Shrubs | Herbs | Bryophytes | Plant Debris | Bare Soil | ||
|---|---|---|---|---|---|---|---|---|
| First | Second | Total | ||||||
| Raisteliai | 30 | 10 | 30 | 50 | 50 | 10 | 40 | 0 |
| Paneriai | 40 | 20 | 50 | 40 | 70 | 60 | 40 | 1 |
| Katkuškės | 40 | 60 | 70 | 30 | 30 | 40 | 60 | 5 |
| Stakų Ūta | 30 | 30 | 40 | 20 | 80 | 30 | 30 | 3 |
| Population | Total Number | Mean ± SD | Vegetative | Mean ± SD | Generative | Mean ± SD |
|---|---|---|---|---|---|---|
| Raisteliai | 221 | 11.1 ± 4.3 a | 135 | 6.8 ± 3.9 a | 86 | 4.3 ± 2.4 a |
| Paneriai | 160 | 8.0 ± 3.5 ac | 132 | 6.6 ± 3.6 a | 28 | 1.4 ± 1.2b cd |
| Katkuškės | 121 | 6.1 ± 2.6 bc | 103 | 5.2 ± 2.6 a | 18 | 0.9 ± 1.9 c |
| Stakų Ūta | 75 | 3.8 ± 2.3 d | 44 | 2.2 ± 1.8 b | 31 | 1.5 ± 1.3 d |
| Pooled | 577 | 7.2 ± 4.2 | 414 | 5.2 ± 3.5 | 163 | 2.0 ± 2.2 |
| Percentage | 100% | 71.8% | 28.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taura, L.; Gudžinskas, Z. Assessment of the Status of Cephalanthera longifolia Populations in Lithuania Derived from a Single-Census Study. Plants 2025, 14, 2039. https://doi.org/10.3390/plants14132039
Taura L, Gudžinskas Z. Assessment of the Status of Cephalanthera longifolia Populations in Lithuania Derived from a Single-Census Study. Plants. 2025; 14(13):2039. https://doi.org/10.3390/plants14132039
Chicago/Turabian StyleTaura, Laurynas, and Zigmantas Gudžinskas. 2025. "Assessment of the Status of Cephalanthera longifolia Populations in Lithuania Derived from a Single-Census Study" Plants 14, no. 13: 2039. https://doi.org/10.3390/plants14132039
APA StyleTaura, L., & Gudžinskas, Z. (2025). Assessment of the Status of Cephalanthera longifolia Populations in Lithuania Derived from a Single-Census Study. Plants, 14(13), 2039. https://doi.org/10.3390/plants14132039







