Physiological Performance and Grain Yield Components of Common Buckwheat (Fagopyrum esculentum Moench) Cultivated Under Different N Rates
Abstract
1. Introduction
2. Results
2.1. Environmental Conditions
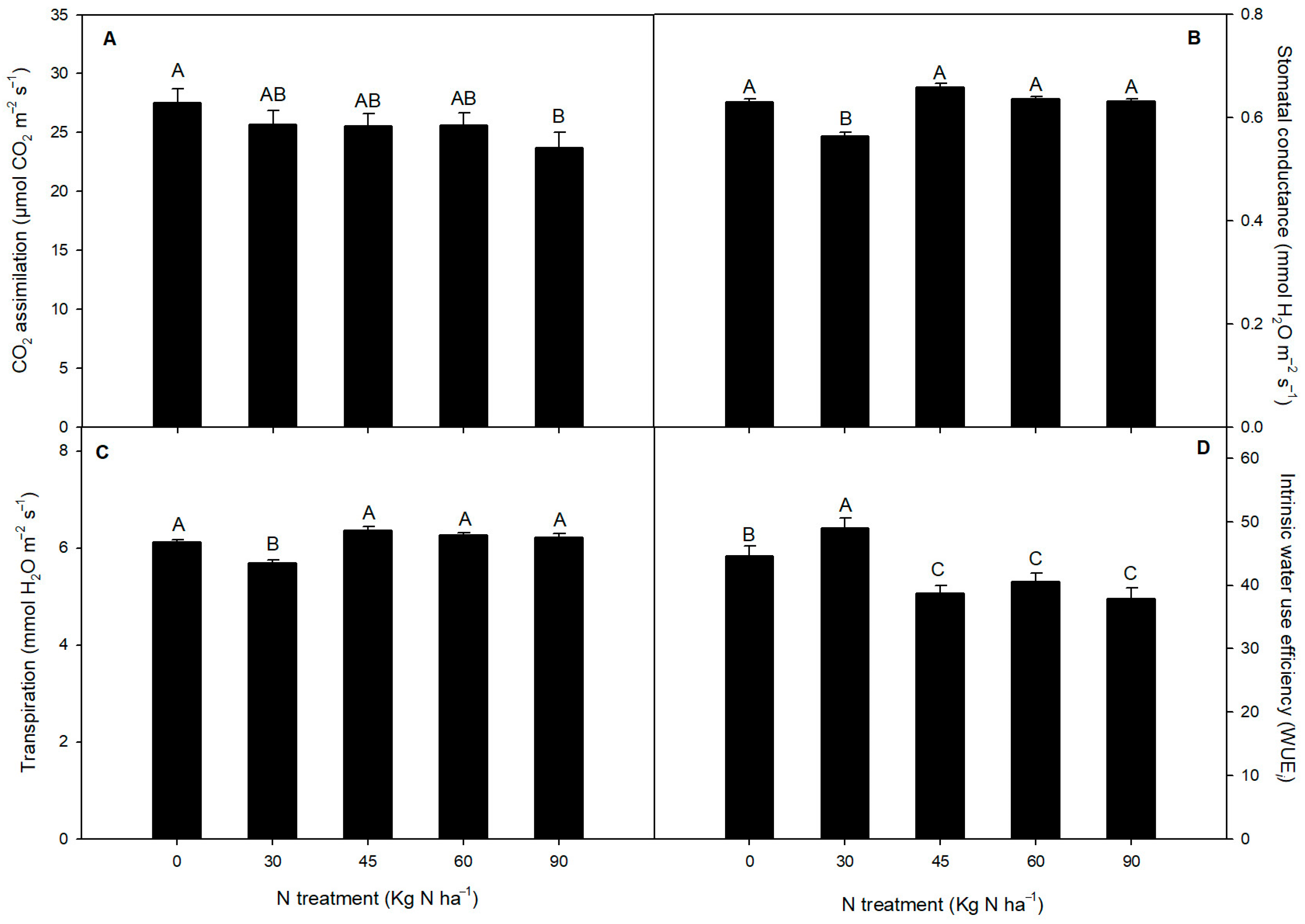
2.2. Chlorophyll Fluorescence and Gas Exchange Parameters
2.3. Plant Traits and Grain Yield Components
3. Discussion
4. Materials and Methods
4.1. Description of the Study Site and Weather Conditions
4.2. Plant Material and Experimental Conditions
4.3. Photosynthetic Performance
4.4. Morphological Traits and Grain Yield Components
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, F. Buckwheat starch: Structures, properties, and applications. Trends Food Sci. Technol. 2016, 49, 121–135. [Google Scholar] [CrossRef]
- Yilmaz, H.Ö.; Ayhan, N.Y.; Meriç, Ç.S. Buckwheat: A useful food and its effects on human health. Curr. Nutr. Food Sci. 2020, 16, 29–34. [Google Scholar] [CrossRef]
- Vieites-Álvarez, Y.; Hussain, M.I.; Reigosa, M.J.; Kolmanic, A.; Meglic, V.; Cepkova, P.H.; Zhou, M.; Janavska, D.; Sánchez-Moreiras, A. Potential of different common (Fagopyrum esculentum Moench) and Tartary (Fagopyrum tataricum (L.) Gaertn.) buckwheat accessions to sustainably manage surrounding weeds. Eur. J. Agron. 2024, 153, 127040. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Płażek, A.; Dziurka, M.; Słomka, A.; Kopeć, P. The Effect of Stimulants on Nectar Composition, Flowering, and Seed Yield of Common Buckwheat (Fagopyrum esculentum Moench). Int. J. Mol. Sci. 2023, 24, 12852. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Rahman, H.; Thushar, S.; Singh, R.K. Healthy and Resilient Cereals and Pseudo-Cereals for Marginal Agriculture: Molecular Advances for Improving Nutrient Bioavailability. Front. Genet. 2020, 11, 49. [Google Scholar] [CrossRef]
- Aubert, L.; Konrádova, D.; Barris, S.; Quinet, M. Different drought resistance mechanisms between two buckwheat species Fagopyrum esculentum and Fagopyrum tataricum. Physiol. Plant. 2021, 172, 577–586. [Google Scholar] [CrossRef]
- FAO STAT. 2024. Available online: https://www.fao.org/faostat/es/#home (accessed on 10 January 2025).
- Falquet, B.; Gfeller, A.; Pourcelot, M.; Tschuy, F.; Iirth, J.W. Weed suppression by common buckwheat: A review. Environ. Control Biol. 2015, 53, 1–6. [Google Scholar] [CrossRef]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of sowing time and soil water content on grain yield and phenolic profile of four buckwheat (Fagopyrum esculentum Moench) varieties in a Mediterranean environment. J. Food Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Jacquemart, A.-L.; Cawoy, V.; Kinet, J.-M.; Ledent, J.-F.; Quinet, M. Is buckwheat (Fagopyrum esculentum Moench) still a valuable crop today? Eur. J. Plant Sci. Biotechnol. 2012, 6, 1–10. [Google Scholar]
- Płażek, A.; Kopeć, P.; Dziurka, M. The yield of common buckwheat (Fagopyrum esculentum Moench) depends on the genotype but not on the Pin-to-Thrum ratio. Sci. Rep. 2023, 13, 16022. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M. A growth scale for the phasic development of common buckwheat. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2015, 66, 215–228. [Google Scholar] [CrossRef]
- Jung, G.H.; Kim, S.L.; Kim, M.J. Effect of sowing time on buckwheat (Fagopyrum esculentum Moench) growth and yield in central Korea. J. Crop Sci. Biotechnol. 2015, 18, 285–291. [Google Scholar] [CrossRef]
- Domingos, I.; Bilsborrow, P. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron. 2021, 126, 126264. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of selenium (Se) uptake on plant growth and yield in common buckwheat (Fagopyrum esculentum Moench). Field Crops Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Rotili, D.; Guglielmini, A.; Caccavo, M.; Antequera, S.; Rocca, C.; Miralles, D. Grain yield, yield components and nitrogen economy of irrigated second-crop common Buckwheat (Fagopyrum esculentum Moench) in a cold-temperate region. Eur. J. Agron. 2023, 144, 126750. [Google Scholar] [CrossRef]
- Wan, C.; Gao, L.; Wang, J.; Lei, X.; Tao, J.; Feng, B.; Gao, J. Effects of nitrogen fertilization on protein synthesis, accumulation, and physicochemical properties in common buckwheat. Crop J. 2023, 11, 941–950. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a major essential element of plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Seo, J.S.; Kim, S.H.; Shim, J.S.; Um, T.; Oh, N.; Park, T.; Kim, Y.S.; Oh, S.J.; Kim, J.K. The rice NUCLEAR FACTOR-YA5 and MICRORNA169a module promotes nitrogen utilization during nitrogen deficiency. Plant Physiol. 2024, 194, 491–510. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Adalibieke, W.; Cui, X.; Cai, H. Global crop-specific nitrogen fertilization dataset in 1961–2020. Sci. Data 2023, 10, 617. [Google Scholar] [CrossRef]
- Wang, C.; Wu Ruan, R.; Hui Yuan, X.; Hu, D.; Yang, H.; Li, Y.; Lin Yi, Z. Effects of Nitrogen Fertilizer and Planting Density on the Lignin Synthesis in the Culm in Relation to Lodging Resistance of Buckwheat. Plant Prod. Sci. 2015, 18, 218–227. [Google Scholar] [CrossRef]
- Walsh, O.; Raun, W.; Klatt, A.; Solie, J. Effect of delayed nitrogen fertilization on maize (Zea mays L.) grain yields and nitrogen use efficiency. J. Plant Nutr. 2012, 35, 538–555. [Google Scholar] [CrossRef]
- Stumpf, B.; Yan, F.; Honermeier, B. Influence of nitrogen fertilization on yield and phenolic compounds in wheat grains (Triticumm aestivum L. ssp. aestivum). J. Plant Nutr. Soil Sci. 2018, 182, 111–118. [Google Scholar] [CrossRef]
- Zhou, C.; Jia, B.; Wang, S. Effects of Nitrogen Fertilizer Applications on Photosynthetic Production and Yield of Japonica Rice. Int. J. Plant Prod. 2021, 15, 599–613. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Nie, J.; Wang, C.; Huang, K.; Zhang, Y.; Zhang, Y.; She, H.; Liu, X.; Ruan, R.; et al. Effects of nitrogen fertilizer and planting density on the leaf photosynthetic characteristics, agronomic traits and grain yield in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2018, 219, 160–168. [Google Scholar] [CrossRef]
- Liu, H.; Hu, C.; Sun, X. Interactive effects of molybdenum and phosphorus fertilizers on photosynthetic characteristics of seedlings and grain yield of Brassica napus. Plant Soil 2010, 326, 345–353. [Google Scholar] [CrossRef]
- Hornyák, M.; Dziurka, M.; Kula-Maximenko, M. Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci. Rep. 2022, 12, 257. [Google Scholar] [CrossRef]
- Solano, J.; González-Villagra, J.; Collinao, M.; Borie, F.; Castillo, C. Radical architecture and phenological stages of Andean crops quinoa, amaranth, lupine and buckwheat in an Andisol from Southern Chile. IDESIA 2021, 39, 23–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, J.; Wu, Q.; Wang, H.; Huang, X.; Liao, L.; Xie, H.; Peng, X. Systematic Identification of Phosphate Transporter Family 1 (PHT1) Genes and Their Expression Profiling in Response to Low Phosphorus and Related Hormones in Fagopyrum tataricum (L.) Gaertn. Agronomy 2025, 15, 576. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhao, Q.; Liao, H. Genetic improvement of legume roots for adaption to acid soils. Crop J. 2023, 11, 1022–1033. [Google Scholar] [CrossRef]
- Wan, C.; Gao, S.; Wang, J.; Lei, X.; Ge, J.; Tao, J. Optimal planting density combined with phosphorus input promotes common buckwheat resource use efficiency and productivity to increase grain yield. Agric. Water Manag. 2023, 287, 108468. [Google Scholar] [CrossRef]
- CIREN–Centro de Información de Recursos Naturales. Estudio Agrológico IX Región: Descripciones de Suelos, Materiales y Símbolos. (Pub. CIREN N°122). 343p. Available online: https://bibliotecadigital.ciren.cl/items/0c56d801-ba1e-476a-a634-b40b819b66e5 (accessed on 10 January 2025).
- Wang, C.; She, H.Z.; Liu, X.B.; Hu, D.; Ruan, R.W.; Shao, M.B.; Zhang, L.Y.; Zhou, L.B.; Zhang, C.B.; Wu, D.Q.; et al. Effects of fertilization on leaf photosynthetic characteristics and grain yield in trtary buckwheat Yunqiao1. Photosynthetica 2017, 55, 77–84. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef]
- Navarrete-Campos, D.; Bravo, L.A.; Rubilar, R.A.; Emhart, V.; Sanhueza, R. Drought effects on water use efficiency, freezing tolerance and survival of Eucalyptus globulus and Eucalyptus globulus x nitens cutting. New For. 2013, 44, 119–134. [Google Scholar] [CrossRef]


| N Rates (Kg N ha−1) | Plant Height (cm) | N° Branches | N° Internode | Shoot Diameter (cm) | Shoot Biomass (g) |
|---|---|---|---|---|---|
| 0 | 118.7 ± 1.9 b | 5.7 ± 0.5 b | 7.9 ± 0.7 b | 10.3 ± 1.0 a | 10.2 ± 1.1 b |
| 30 | 120.6 ± 1.7 b | 6.3 ± 0.3 b | 8.0 ± 0.7 b | 11.7 ± 0.6 a | 10.5 ± 1.0 b |
| 45 | 120.67 ± 1.1 b | 5.8 ± 0.5 b | 6.8 ± 0.6 c | 11.7 ± 0.3 a | 11.2 ± 1.7 b |
| 60 | 127.8 ± 1.5 a | 7.4 ± 0.7 a | 9.8 ± 0.8 a | 11.9 ± 0.2 a | 13.3 ± 0.1 a |
| 90 | 123.1 ± 1.6 a | 7.2 ± 0.5 a | 8.9 ± 0.4 a | 11.0 ± 0.5 a | 13.2 ± 0.3 a |
| N Rates (Kg N ha−1) | Grain per Plant (N°) | Yield per Plant (g) | 1000-Grain Weight (g) | Crop Yield (Kg ha−1) |
|---|---|---|---|---|
| 0 | 271.3 ± 30.6 c | 8.5 ± 0.9 c | 34.0 ± 0.2 a | 715.1 ± 50.4 b |
| 30 | 457.5 ± 30.8 a | 14.8 ± 0.6 a | 35.0 ± 0.3 a | 914.1 ± 45.5 a |
| 45 | 402.2 ± 14.5 a | 12.1 ± 1.9 b | 34.9 ± 0.5 a | 856.1 ± 19.4 a |
| 60 | 369.9 ± 40.1 b | 12.3 ± 0.4 b | 33.4 ± 0.4 a | 749.6 ± 28.7 b |
| 90 | 255.5 ± 37.5 c | 8.1 ± 1.8 c | 33.6 ± 0.2 a | 630.8 ± 30.9 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villagra, J.; Solano, J.; Ávila, K.; Tranamil-Manquein, J.; Tighe-Neira, R.; Ribera-Fonseca, A.; Inostroza-Blancheteau, C. Physiological Performance and Grain Yield Components of Common Buckwheat (Fagopyrum esculentum Moench) Cultivated Under Different N Rates. Plants 2025, 14, 2037. https://doi.org/10.3390/plants14132037
González-Villagra J, Solano J, Ávila K, Tranamil-Manquein J, Tighe-Neira R, Ribera-Fonseca A, Inostroza-Blancheteau C. Physiological Performance and Grain Yield Components of Common Buckwheat (Fagopyrum esculentum Moench) Cultivated Under Different N Rates. Plants. 2025; 14(13):2037. https://doi.org/10.3390/plants14132037
Chicago/Turabian StyleGonzález-Villagra, Jorge, Jaime Solano, Kevin Ávila, Jaime Tranamil-Manquein, Ricardo Tighe-Neira, Alejandra Ribera-Fonseca, and Claudio Inostroza-Blancheteau. 2025. "Physiological Performance and Grain Yield Components of Common Buckwheat (Fagopyrum esculentum Moench) Cultivated Under Different N Rates" Plants 14, no. 13: 2037. https://doi.org/10.3390/plants14132037
APA StyleGonzález-Villagra, J., Solano, J., Ávila, K., Tranamil-Manquein, J., Tighe-Neira, R., Ribera-Fonseca, A., & Inostroza-Blancheteau, C. (2025). Physiological Performance and Grain Yield Components of Common Buckwheat (Fagopyrum esculentum Moench) Cultivated Under Different N Rates. Plants, 14(13), 2037. https://doi.org/10.3390/plants14132037








