Evolutionary and Structural Analysis of the Aquaporin Gene Family in Rice
Abstract
1. Introduction
2. Evolutionary Dynamics of Aquaporin Genes in Rice
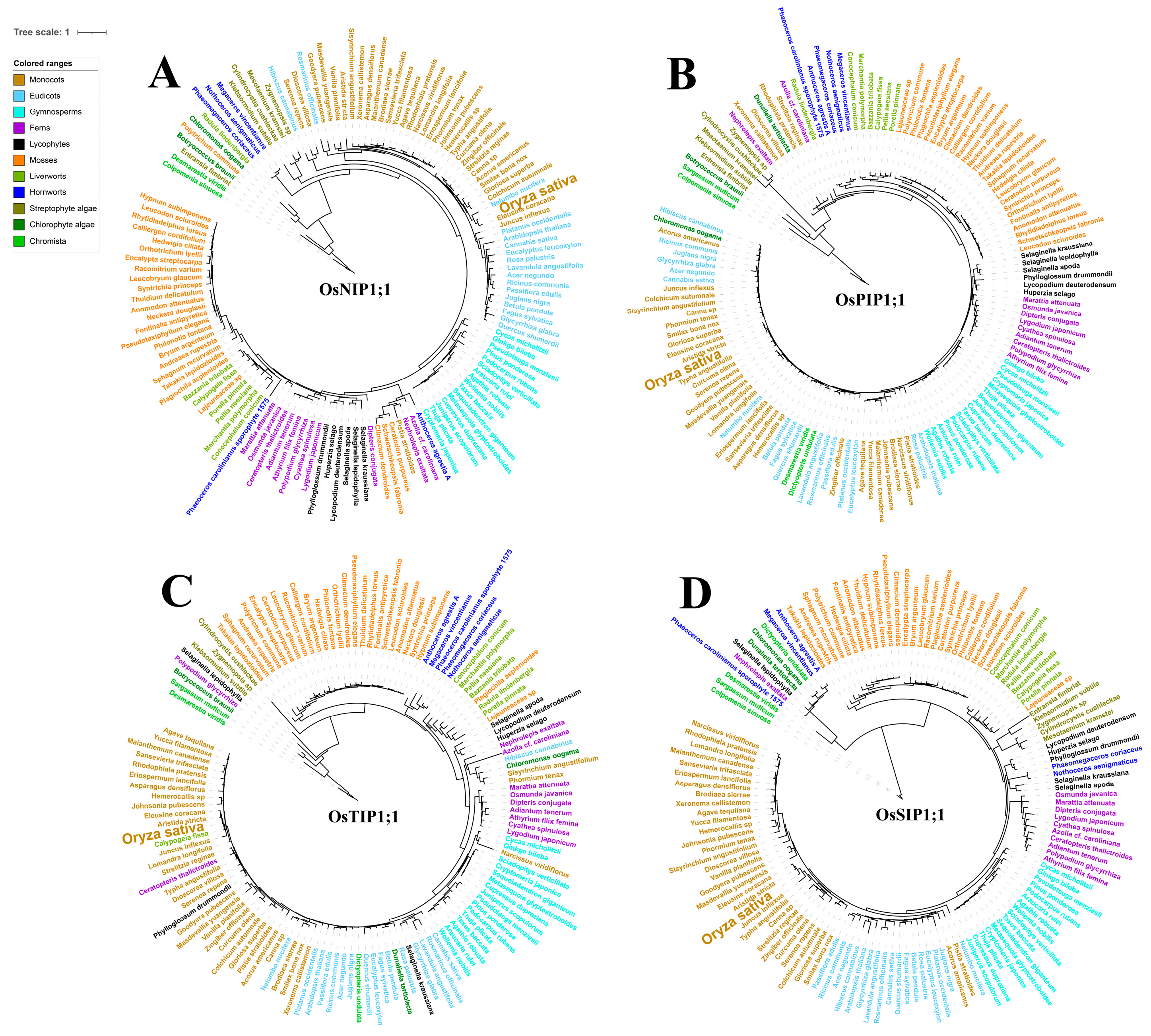
2.1. The Origin of Aquaporin in Green Plants
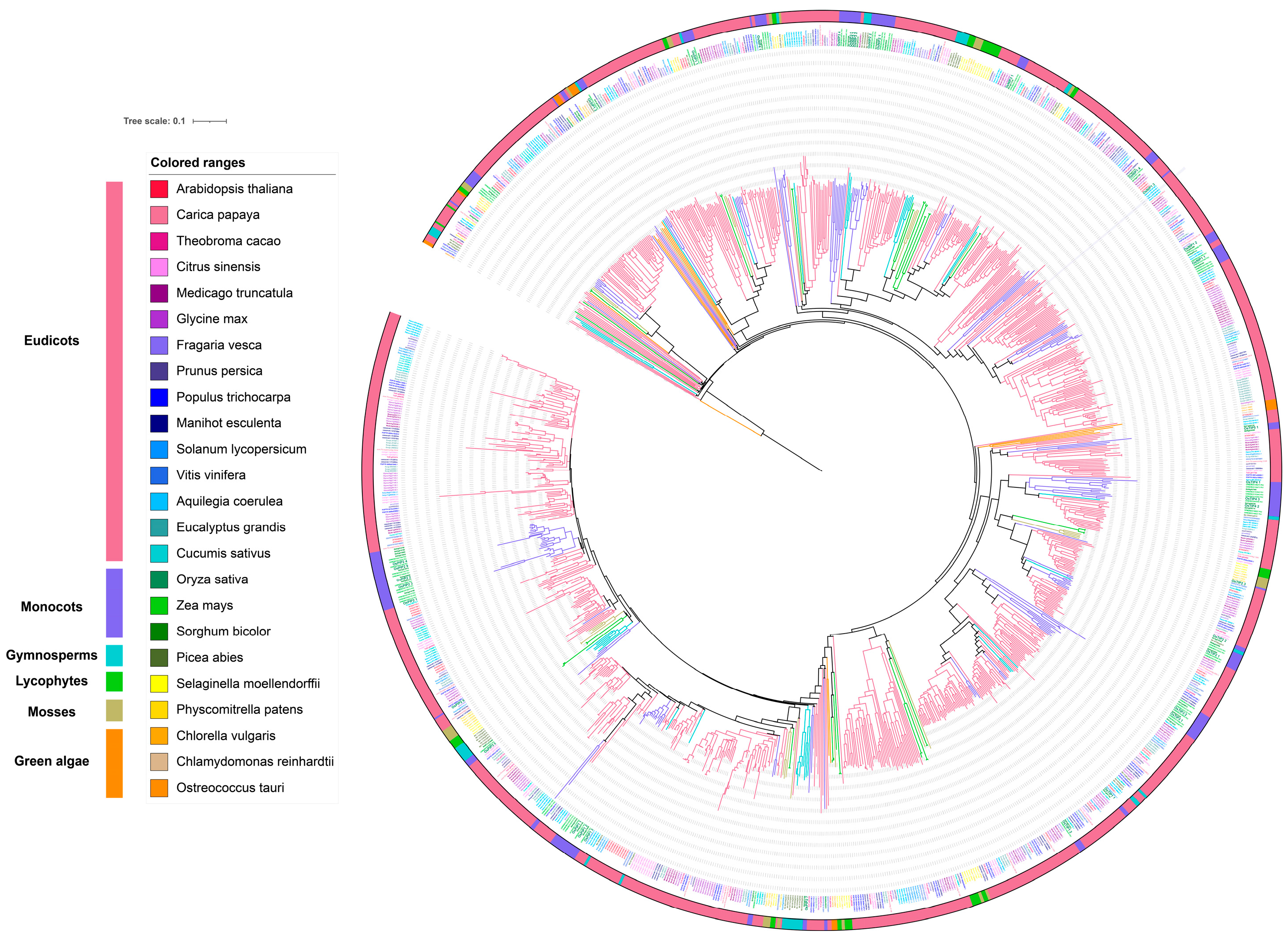
2.2. Gene Expansion and Diversification of Aquaporin in Rice and Other Plants
3. Structural Characteristics of Rice Aquaporins
3.1. Molecular Architecture and Evolutionary Adaptations Governing Water Transport and Substrate Selectivity
3.2. Three-Dimensional Structural Modeling Guides Functional Analysis of Rice Aquaporin
4. Functional Roles of Aquaporins in Rice Physiology
4.1. Aquaporin-Mediated Water Homeostasis and Nutrient Transport in Rice
4.2. Role of Aquaporins in Rice Abiotic Stress Responses
4.3. Regulation of Aquaporin Expression in Rice

5. Future Directions and Challenges in Rice Aquaporin Research
5.1. Gene Editing Technology and Functional Dissection Deepening
5.2. Multi-Omics Integration and Regulatory Network Revelation
5.3. Rice Aquaporin Evolution and Structure for Functional Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, X.; Kitagawa, Y.; Calamita, G.; Ding, X. Plant aquaporins: Their roles beyond water transport. Crop J. 2024, 12, 641–655. [Google Scholar] [CrossRef]
- Galaz, A.; Pérez-Donoso, A.G.; Gambardella, M. Leaf Aquaporin Expression in Grafted Plants and the Influence of Genotypes and Scion/Rootstock Combinations on Stomatal Behavior in Grapevines Under Water Deficit. Plants 2024, 13, 3427. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant Aquaporins: Membrane Channels with Multiple Integrated Functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Liu, F.; Sun, L.; Hao, F. Versatile Roles of Aquaporins in Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 9485. [Google Scholar] [CrossRef]
- Gautam, A.; Pandey, A. Aquaporins Responses under Challenging Environmental Conditions and Abiotic Stress Tolerance in Plants. Bot. Rev. 2021, 87, 467–495. [Google Scholar] [CrossRef]
- Yao, X.; Mu, Y.; Zhang, L.; Chen, L.; Zou, S.; Chen, X.; Lu, K.; Dong, H. AtPIP1;4 and AtPIP2;4 Cooperatively Mediate H2O2 Transport to Regulate Plant Growth and Disease Resistance. Plants 2024, 13, 1018. [Google Scholar] [CrossRef]
- Pawłowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef]
- Bellati, J.; Champeyroux, C.; Hem, S.; Rofidal, V.; Krouk, G.; Maurel, C.; Santoni, V. Novel Aquaporin Regulatory Mechanisms Revealed by Interactomics. Mol. Cell. Proteom. 2016, 15, 3473–3487. [Google Scholar] [CrossRef]
- Sun, F.; Deng, Y.; Ma, X.; Liu, Y.; Zhao, L.; Yu, S.; Zhang, L. Structure-based prediction of protein-protein interaction network in rice. Genet. Mol. Biol. 2024, 47, e20230068. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Zaplana, A. Deciphering Arabidopsis Aquaporin Networks: Comparative Analysis of the STRING and BioGRID Interactomes. Int. J. Plant Biol. 2025, 16, 28. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice—not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Nada, R.M.; Abogadallah, G.M. Aquaporins are major determinants of water use efficiency of rice plants in the field. Plant Sci. 2014, 227, 165–180. [Google Scholar] [CrossRef]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Ishikawa-Sakurai, J.; Murai-Hatano, M.; Ahamed, A.; Uemura, M. Aquaporins in developing rice grains. Biosci. Biotechnol. Biochem. 2015, 79, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Devi, K.; Baruah, A.; Sarma, R. Relationship of root aquaporin genes, OsPIP1;3, OsPIP2;4, OsPIP2;5, OsTIP2;1 and OsNIP2;1 expression with drought tolerance in rice. Indian J. Genet. Plant Breed. 2020, 80, 50–57. [Google Scholar] [CrossRef]
- Ahamed, A.; Murai-Hatano, M.; Ishikawa-Sakurai, J.; Hayashi, H.; Kawamura, Y.; Uemura, M. Cold stress-induced acclimation in rice is mediated by root-specific aquaporins. Plant Cell Physiol. 2012, 53, 1445–1456. [Google Scholar] [CrossRef]
- Raza, Q.; Rashid, M.A.R.; Waqas, M.; Ali, Z.; Rana, I.A.; Khan, S.H.; Khan, I.A.; Atif, R.M. Genomic diversity of aquaporins across genus Oryza provides a rich genetic resource for development of climate resilient rice cultivars. BMC Plant Biol. 2023, 23, 172. [Google Scholar] [CrossRef]
- Sabir, F.; Di Pizio, A.; Loureiro-Dias, M.; Casini, A.; Soveral, G.; Prista, C. Insights into the Selectivity Mechanisms of Grapevine NIP Aquaporins. Int. J. Mol. Sci. 2020, 21, 6697. [Google Scholar] [CrossRef]
- Anderberg, H.I.; Kjellbom, P.; Johanson, U. Annotation of Selaginella moellendorffii Major Intrinsic Proteins and the Evolution of the Protein Family in Terrestrial Plants. Front. Plant Sci. 2012, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Mitani-Ueno, N.; Saito, K.; Matsuki, K.; Huang, S.; Yang, L.; Yamaji, N.; Ishikita, H.; Shen, J.-R.; Ma, J.F.; et al. Structural basis for high selectivity of a rice silicon channel Lsi1. Nat. Commun. 2021, 12, 6236. [Google Scholar] [CrossRef]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Grosse, I.; Li, Z.; Melkonian, M.; Mirarab, S.; et al. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679–685. [Google Scholar]
- Deng, F.; Zeng, F.; Chen, G.; Feng, X.; Riaz, A.; Wu, X.; Gao, W.; Wu, F.; Holford, P.; Chen, Z.-H. Metalloid hazards: From plant molecular evolution to mitigation strategies. J. Hazard. Mater. 2021, 409, 124495. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Huang, Y.; Chen, F.; Zhang, X.; Sessa, E.; Zhao, C.; Marchant, D.B.; Xue, D.; Chen, G.; Dai, F.; et al. Evolution of rapid blue-light response linked to explosive diversification of ferns in angiosperm forests. New Phytol. 2021, 230, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R.; Ding, X.; Kitagawa, Y.; Chrispeels, M.J. Origin of plant glycerol transporters by horizontal gene transfer and functional recruitment. Proc. Natl. Acad. Sci. USA 2002, 99, 14893–14896. [Google Scholar] [CrossRef]
- Courty, P.E.; Penelope, S.; Sally, K.; Dirk, R.; Wipf, D. Inorganic Nitrogen Uptake and Transport in Beneficial Plant Root-Microbe Interactions. Crit. Rev. Plant Sci. 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Guenther, J.F.; Roberts, D.M. Water-selective and multifunctional aquaporins from Lotus japonicus nodules. Planta 2000, 210, 741–748. [Google Scholar] [CrossRef]
- Martins Cde, P.; Pedrosa, A.M.; Du, D.; Gonçalves, L.P.; Yu, Q.; Gmitter, F.G., Jr.; Costa, M.G. Genome-Wide Characterization and Expression Analysis of Major Intrinsic Proteins during Abiotic and Biotic Stresses in Sweet Orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0138786. [Google Scholar]
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007, 581, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tong, T.; Jiang, W.; Cheng, J.; Deng, F.; Wu, X.; Chen, Z.H.; Ouyang, Y.; Zeng, F. Highly Conserved Evolution of Aquaporin PIPs and TIPs Confers Their Crucial Contribution to Flowering Process in Plants. Front. Plant Sci. 2022, 12, 761713. [Google Scholar] [CrossRef]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Diehn, T.A.; Pommerrenig, B.; Bernhardt, N.; Hartmann, A.; Bienert, G.P. Genome-wide identification of aquaporin encoding genes in Brassica oleracea and their phylogenetic sequence comparison to Brassica crops and Arabidopsis. Front. Plant Sci. 2015, 6, 166. [Google Scholar] [CrossRef]
- Hove, R.M.; Ziemann, M.; Bhave, M. Identification and Expression Analysis of the Barley (Hordeum vulgare L.) Aquaporin Gene Family. PLoS ONE 2015, 10, e0128025. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.B.; Sankararamakrishnan, R. Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: Characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol. 2009, 9, 134. [Google Scholar] [CrossRef]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and evolution of membrane intrinsic proteins. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef]
- Hussain, A.; Tanveer, R.; Mustafa, G.; Farooq, M.; Amin, I.; Mansoor, S. Comparative phylogenetic analysis of aquaporins provides insight into the gene family expansion and evolution in plants and their role in drought tolerant and susceptible chickpea cultivars. Genomics 2020, 112, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.; Karplus, K.; Hughey, R.; Chothia, C. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 2001, 313, 903–919. [Google Scholar] [CrossRef]
- Gustavsson, S.; Lebrun, A.S.; Nordén, K.; Chaumont, F.; Johanson, U. A Novel Plant Major Intrinsic Protein in Physcomitrella patens Most Similar to Bacterial Glycerol Channels. Plant Physiol. 2005, 139, 287–295. [Google Scholar] [CrossRef]
- Anderberg, H.I.; Danielson, J.Å.; Johanson, U. Algal MIPs, High Diversity and Conserved Motifs. BMC Evol. Biol. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huangfu, L.; Lu, Y.; Fang, H.; Xu, Y.; Li, P.; Zhou, Y.; Xu, C.; Huang, J.; Yang, Z. Adaptive innovation of green plants by horizontal gene transfer. Biotechnol. Adv. 2020, 46, 107671. [Google Scholar] [CrossRef]
- Kirit, H.; Bollback, J.; Lagator, M. The Role of the Environment in Horizontal Gene Transfer. Mol. Biol. Evol. 2022, 39, msac220. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhang, Z.; Zhong, B. Horizontal gene transfer: Driving the evolution and adaptation of plants. J. Integr. Plant Biol. 2022, 64, 1576–1593. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Yang, J. Genome-wide comparison reveals divergence of cassava and rubber aquaporin family genes after the recent whole-genome duplication. BMC Genomics 2019, 20, 380. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; de Araújo, F.C.; Ferreira-Neto, J.R.C.; da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; de Oliveira Silva, R.L.; Kido, E.A.; Barbosa Amorim, L.L.; et al. Plant Aquaporins: Diversity, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bai, N.; Wang, P.; Su, J.; Chang, Q.; Zhang, Q. Co-Inoculation with Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes under Drought Stress: Synergistic or Competitive Effects on Maize Growth, Photosynthesis, Root Hydraulic Properties and Aquaporins? Plants 2023, 12, 2596. [Google Scholar] [CrossRef]
- Ndayambaza, B.; Si, J.; Zhou, D.; Bai, X.; Jia, B.; He, X.; Wang, C.; Qin, J.; Zhu, X.; Liu, Z.; et al. Genome-Wide Analysis of Aquaporins Gene Family in Populus euphratica and Its Expression Patterns in Response to Drought, Salt Stress, and Phytohormones. Int. J. Mol. Sci. 2024, 25, 10185. [Google Scholar] [CrossRef]
- Lin, R.; Zheng, J.; Pu, L.; Wang, Z.; Mei, Q.; Zhang, M.; Jian, S. Genome-wide identification and expression analysis of aquaporin family in Canavalia rosea and their roles in the adaptation to saline-alkaline soils and drought stress. BMC Plant Biol. 2021, 21, 333. [Google Scholar] [CrossRef]
- Maistriaux, L.; Laurent, M.; Jeanguenin, L.; Prado, A.; Nader, J.; Welcker, C.; Charcosset, A.; Tardieu, F.; Nicolas, S.; Chaumont, F. Genetic variability of aquaporin expression in maize: From eQTLs to a MITE insertion regulating PIP2;5 expression. Plant Physiol. 2024, 196, 368–384. [Google Scholar] [CrossRef]
- Gonen, T.; Walz, T. The structure of aquaporins. Q. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef]
- Kreida, S.; Törnroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef]
- Savage, D.; Egea, P.; Robles-Colmenares, Y.; O’Connell, J.D., III; Stroud, R. Architecture and Selectivity in Aquaporins: 2.5 Å X-Ray Structure of Aquaporin Z. PLoS Biol. 2003, 1, e72. [Google Scholar] [CrossRef]
- Wree, D.; Wu, B.; Zeuthen, T.; Beitz, E. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J. 2011, 278, 2991–2998. [Google Scholar] [CrossRef]
- Yong, J.; Tonghui, M. Importance of NPA motifs in the expression and function of water channel aquaporin-1. Chin. Sci. Bull. 2007, 52, 771–776. [Google Scholar]
- Miloshevsky, G.V.; Jordan, P.C. Water and Ion Permeation in bAQP1 and GlpF Channels: A Kinetic Monte Carlo Study. Biophys. J. 2004, 87, 3690–3702. [Google Scholar] [CrossRef]
- Jensen, M.; Tajkhorshid, E.; Schulten, K. Electrostatic tuning of permeation and selectivity in aquaporin water channels. Biophys. J. 2003, 85, 2884–2899. [Google Scholar] [CrossRef]
- Beitz, E.; Wu, B.; Holm, L.; Schultz, J.; Zeuthen, T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl. Acad. Sci. USA 2006, 103, 269–274. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Yamaji, N.; Zhao, F. The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J. Exp. Bot. 2011, 62, 4391–4398. [Google Scholar] [CrossRef]
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The Evolutionary Aspects of Aquaporin Family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef]
- Tania, S.S.; Utsugi, S.; Tsuchiya, Y.; Sasano, S.; Katsuhara, M.; Mori, I.C. Amino Acid Substitutions in Loop C of Arabidopsis PIP2 Aquaporins Alters the Permeability of CO2. Plant Cell Environ. 2025. [Google Scholar] [CrossRef]
- Azad, A.K.; Yoshikawa, N.; Ishikawa, T.; Sawa, Y.; Shibata, H. Substitution of a Single Amino Acid Residue in the Aromatic/Arginine Selectivity Filter Alters the Transport Profiles of Tonoplast Aquaporin Homologs. Biochim. Biophys. Acta 2012, 1818, 1–11. [Google Scholar] [CrossRef]
- Walz, T.; Fujiyoshi, Y.; Engel, A. The AQP structure and functional implications. Handb. Exp. Pharmacol. 2009, 190, 31–56. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Patoliya, J.; Thaker, K.; Rabadiya, K.; Patel, D.; Jain, N.K.; Joshi, R. Uncovering the Interaction Interface Between Harpin (Hpa1) and Rice Aquaporin (OsPIP1;3) Through Protein-Protein Docking: An In Silico Approach. Mol. Biotechnol. 2024, 66, 756768. [Google Scholar] [CrossRef]
- Xu, F.; Wang, K.; Yuan, W.; Xu, W.; Shuang, L.; Kronzucker, H.J.; Chen, G.; Miao, R.; Zhang, M.; Ding, M.; et al. Overexpression of rice aquaporin OsPIP1;2 improves yield by enhancing mesophyll CO2 conductance and phloem sucrose transport. J. Exp. Bot. 2019, 70, 671–681. [Google Scholar] [CrossRef]
- Chen, X.; Ma, J.; Wang, X.; Lu, K.; Liu, Y.; Zhang, L.; Peng, J.; Chen, L.; Yang, M.; Li, Y.; et al. Functional modulation of an aquaporin to intensify photosynthesis and abrogate bacterial virulence in rice. Plant J. 2021, 108, 330–346. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Dong, H. Plant Aquaporins in Infection by and Immunity Against Pathogens—A Critical Review. Front. Plant Sci. 2019, 10, 632. [Google Scholar] [CrossRef]
- Liu, S.; Fukumoto, T.; Gena, P.; Feng, P.; Sun, Q.; Li, Q.; Matsumoto, T.; Kaneko, T.; Zhang, H.; Zhang, Y.; et al. Ectopic expression of a rice plasma membrane intrinsic protein (OsPIP1;3) promotes plant growth and water uptake. Plant J. 2019, 102, 779–796. [Google Scholar] [CrossRef]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [CrossRef]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef]
- Maurel, C.; Reizer, J.; Schroeder, J.I.; Chrispeels, M.J. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993, 12, 2241–2247. [Google Scholar] [CrossRef]
- Shibasaka, M.; Horie, T.; Katsuhara, M. Mechanisms activating latent functions of PIP aquaporin water channels via the interaction between PIP1 and PIP2 proteins. Plant Cell Physiol. 2020, 62, 92–99. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Z.Y.; Lin, H.; Cui, W.E.; Chen, J.; Liu, M.; Chen, Z.L.; Qu, L.J.; Gu, H. Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 2006, 16, 277–286. [Google Scholar] [CrossRef]
- Li, G.W.; Zhang, M.H.; Cai, W.M.; Sun, W.N.; Su, W.A. Characterization of OsPIP2;7, a Water Channel Protein in Rice. Plant Cell Physiol. 2008, 49, 1851–1858. [Google Scholar] [CrossRef]
- Tran, S.T.H.; Katsuhara, M.; Mito, Y.; Onishi, A.; Higa, A.; Ono, S.; Paul, N.C.; Horie, R.; Harada, Y.; Horie, T. OsPIP2;4 aquaporin water channel primarily expressed in roots of rice mediates both water and nonselective Na+ and K+ conductance. Sci. Rep. 2025, 15, 12857. [Google Scholar] [CrossRef]
- Sakurai-Ishikawa, J.; Murai-Hatano, M.; Hayashi, H.; Ahamed, A.; Fukushi, K.; Matsumoto, T.; Kitagawa, Y. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant Cell Environ. 2011, 34, 1150–1163. [Google Scholar] [CrossRef]
- Li, G.; Peng, Y.-H.; Yu, X.-Y.; Zhang, M.; Cai, W.; Sun, W.; Su, W. Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J. Plant Physiol. 2008, 165, 1879–1888. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Vivancos, J.; Ramakrishnan, G.; Guérin, V.; Carpentier, G.; Sonah, H.; Labbé, C.; Isenring, P.; Belzile, F.J.; Bélanger, R.R. A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 2015, 83, 489–500. [Google Scholar] [CrossRef]
- Katsuhara, M.; Sasano, S.; Horie, T.; Matsumoto, T.; Rhee, J.; Shibasaka, M. Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol. 2014, 31, 213–219. [Google Scholar] [CrossRef]
- Muto, Y.; Segami, S.; Hayashi, H.; Sakurai, J.; Murai-Hatano, M.; Hattori, Y.; Ashikari, M.; Maeshima, M. Vacuolar proton pumps and aquaporins involved in rapid internode elongation of deepwater rice. Biosci. Biotechnol. Biochem. 2011, 75, 114–122. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.; Yao, X.; Chen, X.; Lu, K.; Hu, Y.; Wang, Z.; Mu, Y.; Zhang, L.; Dong, H. Rice aquaporin OsPIP2;2 is a water-transporting facilitator in relevance to drought-tolerant responses. Plant Direct 2021, 5, e338. [Google Scholar] [CrossRef]
- Sun, J.Y.; Liu, X.S.; Khan, I.U.; Wu, X.C.; Yang, Z.M. OsPIP2;3 as an aquaporin contributes to rice resistance to water deficit but not to salt stress. Environ. Exp. Bot. 2021, 183, 104342. [Google Scholar] [CrossRef]
- Nada, R.M.; Abogadallah, G.M. Contrasting root traits and native regulation of aquaporin differentially determine the outcome of overexpressing a single aquaporin (OsPIP2;4) in two rice cultivars. Protoplasma 2020, 257, 583–595. [Google Scholar] [CrossRef]
- Li, R.; Li, N.X.; Wen, M.Y.; Jie, X.F.; Sheng, L.Y. Functional Characterization of the Plasma Intrinsic Protein Gene OsPIP2;6 in Rice. Sci. Agric. Sin. 2013, 46, 3079–3086. [Google Scholar]
- Li, G.; Han, J.; Yi, C.; Luo, H.; Wang, Y.; Wang, F.; Wang, X.; Chen, L.; Zhang, Y. Global characterization of OsPIP aquaporins reveals that the H2O2 transporter OsPIP2;6 increases resistance to rice blast. Crop J. 2024, 12, 102–109. [Google Scholar] [CrossRef]
- Meng, D.; Fricke, W. Changes in root hydraulic conductivity facilitate the overall hydraulic response of rice (Oryza sativa L.) cultivars to salt and osmotic stress. Plant Physiol. Biochem. 2017, 113, 64–77. [Google Scholar] [CrossRef]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef]
- Tao, L.; Wang, B.; Xin, S.; Li, W.; Huang, S.; Liu, L.; Cui, J.; Zhang, Q.; Cheng, X.-G. A cluster of mutagenesis revealed an osmotic regulatory role of the OsPIP1 genes in enhancing rice salt tolerance. Crop J. 2023, 11, 1204–1217. [Google Scholar] [CrossRef]
- Matsumoto, T.; Lian, H.-L.; Su, W.; Tanaka, D.; Liu, C.; Iwasaki, I.; Kitagawa, Y. Role of the aquaporin PIP1 subfamily in the chilling tolerance of rice. Plant Cell Physiol. 2009, 50, 216–229. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Peng, Y.-H.; Zhang, M.; Shao, Y.; Su, W.; Tang, Z. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res. 2006, 16, 599–608. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, J.; Zhai, J. Plant Intron-Splicing Efficiency Database (PISE): Exploring splicing of ∼1,650,000 introns in Arabidopsis, maize, rice, and soybean from ∼57,000 public RNA-seq libraries. Sci. China Life Sci. 2023, 66, 602–611. [Google Scholar] [CrossRef]
- Karle, S.; Kumar, K. Rice tonoplast intrinsic protein member OsTIP1;2 confers tolerance to arsenite stress. J. Hazard. Mater. 2023, 465, 133078. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, P.; Li, L.; Yang, L.; Mu, D.; Yan, X.; Li, Z.; Lin, W. Serine hydroxymethyltransferase localised in the endoplasmic reticulum plays a role in scavenging H2O2 to enhance rice chilling tolerance. BMC Plant Biol. 2020, 20, 236. [Google Scholar] [CrossRef]
- Rabeh, K.; Sallami, A.; Gaboun, F.; Filali-Maltouf, A.; Sbabou, L.; Belkadi, B. Genome-wide analysis of aquaporin and their responses to abiotic stresses in plants: A systematic review and meta-analysis. Plant Stress 2024, 11, 100362. [Google Scholar] [CrossRef]
- Miao, M.; Shi, X.; Zheng, X.; Wu, B.; Miao, Y. Characterization of SIPs-Type Aquaporins and Their Roles in Response to Environmental Cues in Rice (Oryza sativa L.). BMC Plant Biol. 2024, 24, 305. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Pascovici, D.; Atwell, B.J.; Haynes, P.A. Differential regulation of aquaporins, small GTPases and V-ATPases proteins in rice leaves subjected to drought stress and recovery. Proteomics 2012, 12, 864–877. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Tang, X.; Ren, Q.; Yang, L.; Bao, Y.; Zhong, Z.; He, Y.; Liu, S.; Qi, C.; Liu, B.; Wang, Y.; et al. Single transcript unit CRISPR 2.0 systems for robust Cas9 and Cas12a mediated plant genome editing. Plant Biotechnol. J. 2019, 17, 1431–1445. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Z.; Huang, J.; Peng, S.; Xiong, D. Mesophyll conductance variability of rice aquaporin knockout lines at different growth stages and growing environments. Plant J. 2021, 107, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, A.; Singh, T.; Anto, S.; Indoliya, Y.; Tiwari, P.; Behera, S.K.; Chakrabarty, D. Targeting OsNIP3;1 via CRISPR/Cas9: A strategy for minimizing arsenic accumulation and boosting rice resilience. J. Hazard. Mater. 2024, 471, 134325. [Google Scholar] [CrossRef]
- Deshmukh, R.; Sonah, H.; Bélanger, R. Plant Aquaporins: Genome-Wide Identification, Transcriptomics, Proteomics, and Advanced Analytical Tools. Front. Plant Sci. 2016, 7, 1896. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zeng, Y.; Liang, Y.; Qian, Q.; Yang, C. Proteomic dissection of the rice-Fusarium fujikuroi interaction and the correlation between the proteome and transcriptome under disease stress. BMC Genomics 2019, 20, 91. [Google Scholar] [CrossRef]
- Cai, X.; He, W.; Qian, Q.; Shang, L. Genetic resource utilization in wild rice species: Genomes and gene bank. New Crops 2025, 2, 100065. [Google Scholar] [CrossRef]
- González, A.; Gómez-Silva, V.; Ramirez, M.; Fontúrbel, F. Meta-analysis of the differential effects of habitat fragmentation and degradation on plant genetic diversity. Conserv. Biol. 2019, 34, 711–720. [Google Scholar] [CrossRef]
- Seidel, D.; Claudino, P.; Sperotto, G.; Wendt, S.; Shomo, Z.; Mural, R.; Dias, H. Comprehensive analysis of the Aquaporin genes in Eucalyptus grandis suggests potential targets for drought stress tolerance. bioRxiv 2023, 17, 233–248. [Google Scholar] [CrossRef]
- Zou, Z.; Zheng, Y.; Xie, Z. Analysis of Carica papaya Informs Lineage-Specific Evolution of the Aquaporin (AQP) Family in Brassicales. Plants 2023, 12, 3847. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Zeng, F.; Ye, S.; Ji, Z.; Wang, Y.; Chen, Z.-H.; Ouyang, Y. Evolutionary and Structural Analysis of the Aquaporin Gene Family in Rice. Plants 2025, 14, 2035. https://doi.org/10.3390/plants14132035
Tong T, Zeng F, Ye S, Ji Z, Wang Y, Chen Z-H, Ouyang Y. Evolutionary and Structural Analysis of the Aquaporin Gene Family in Rice. Plants. 2025; 14(13):2035. https://doi.org/10.3390/plants14132035
Chicago/Turabian StyleTong, Tao, Fanrong Zeng, Shuzhen Ye, Zhijuan Ji, Yanli Wang, Zhong-Hua Chen, and Younan Ouyang. 2025. "Evolutionary and Structural Analysis of the Aquaporin Gene Family in Rice" Plants 14, no. 13: 2035. https://doi.org/10.3390/plants14132035
APA StyleTong, T., Zeng, F., Ye, S., Ji, Z., Wang, Y., Chen, Z.-H., & Ouyang, Y. (2025). Evolutionary and Structural Analysis of the Aquaporin Gene Family in Rice. Plants, 14(13), 2035. https://doi.org/10.3390/plants14132035







