Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants
Abstract
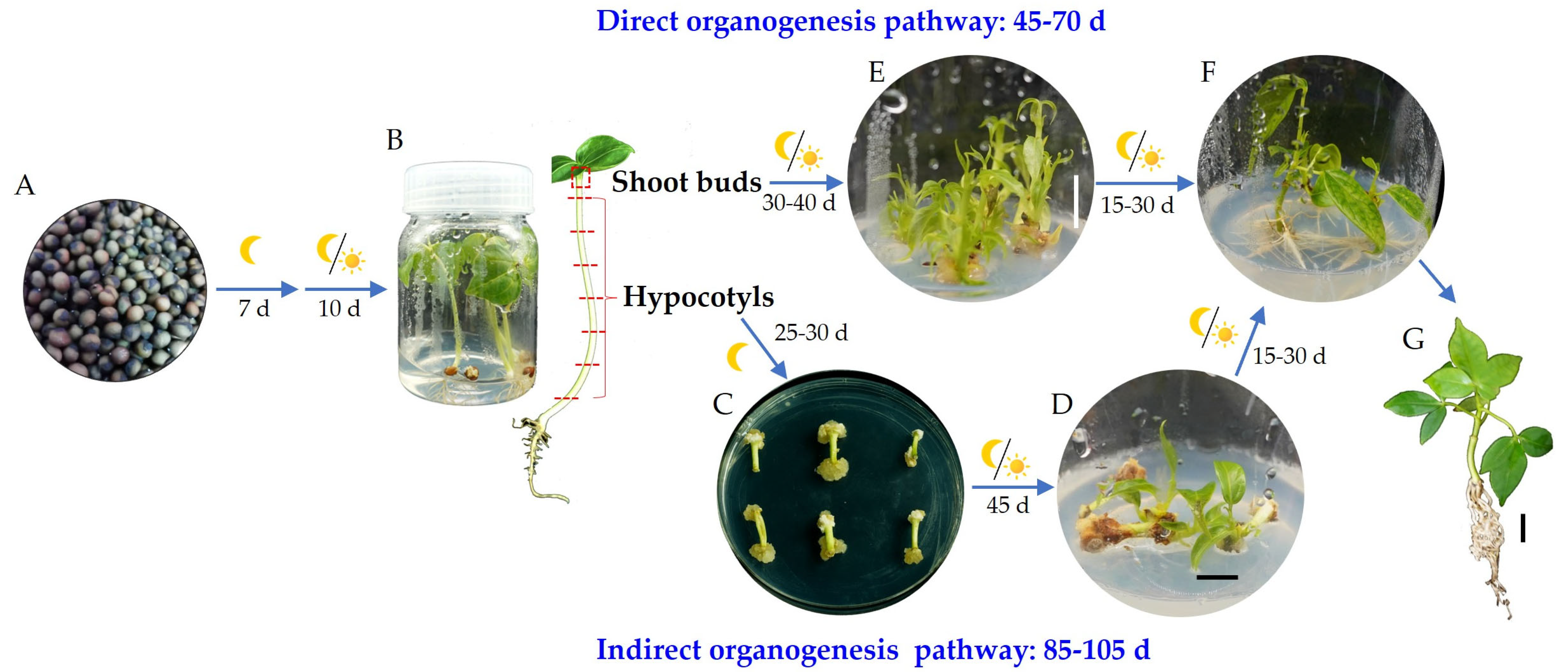
1. Introduction
2. Results
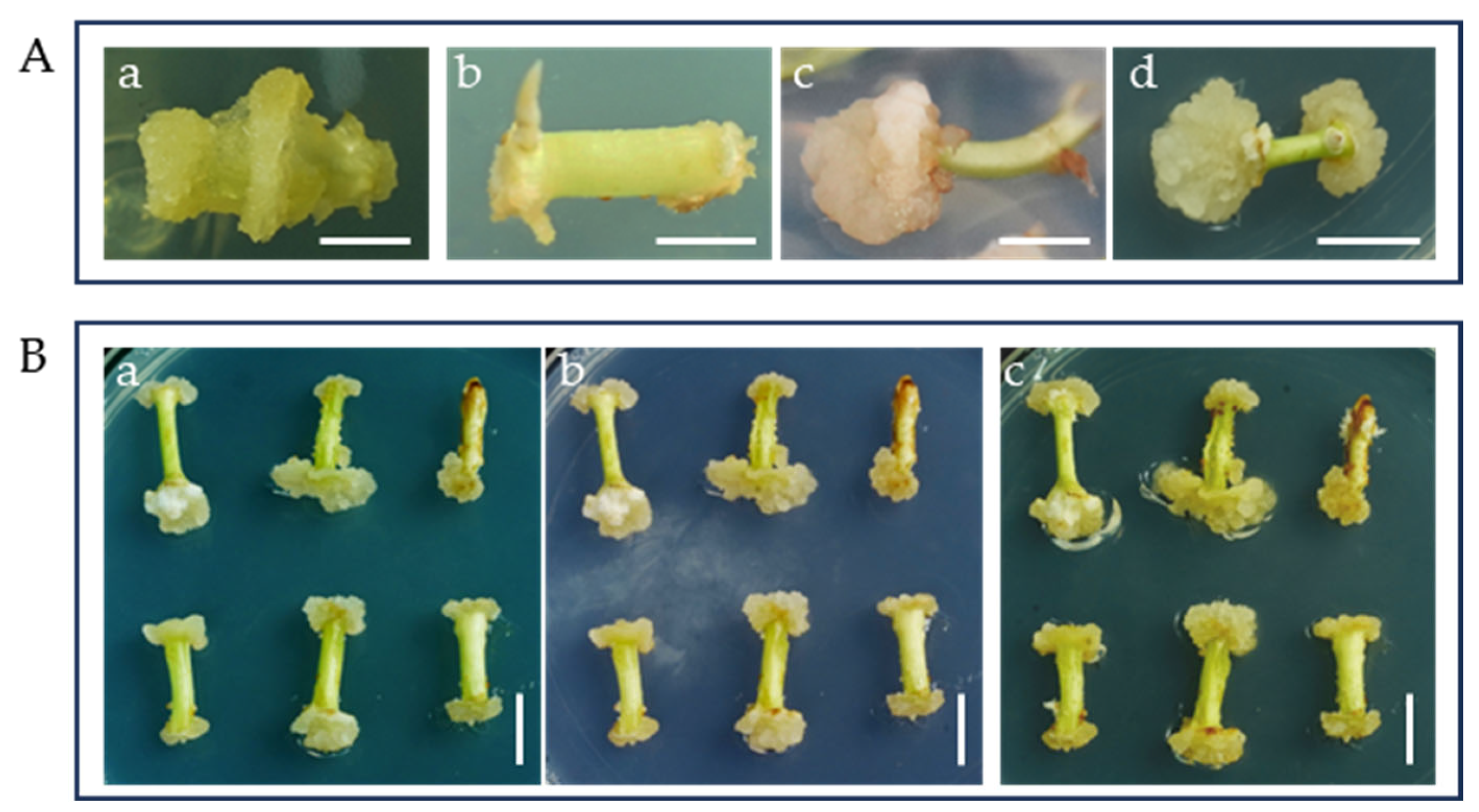
2.1. Optimal PGRs, Basal Medium, and Time for Callus Induction
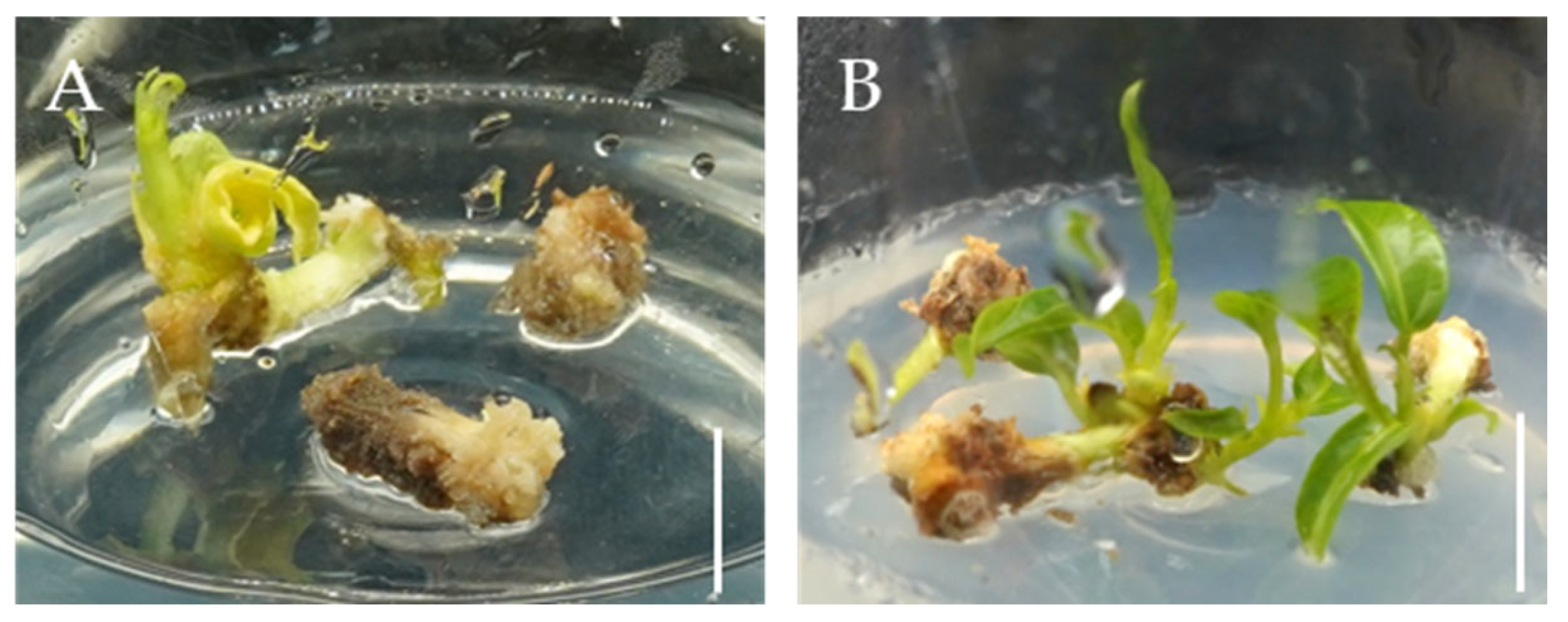
2.2. The Effect of PGRs on Callus Differentiation
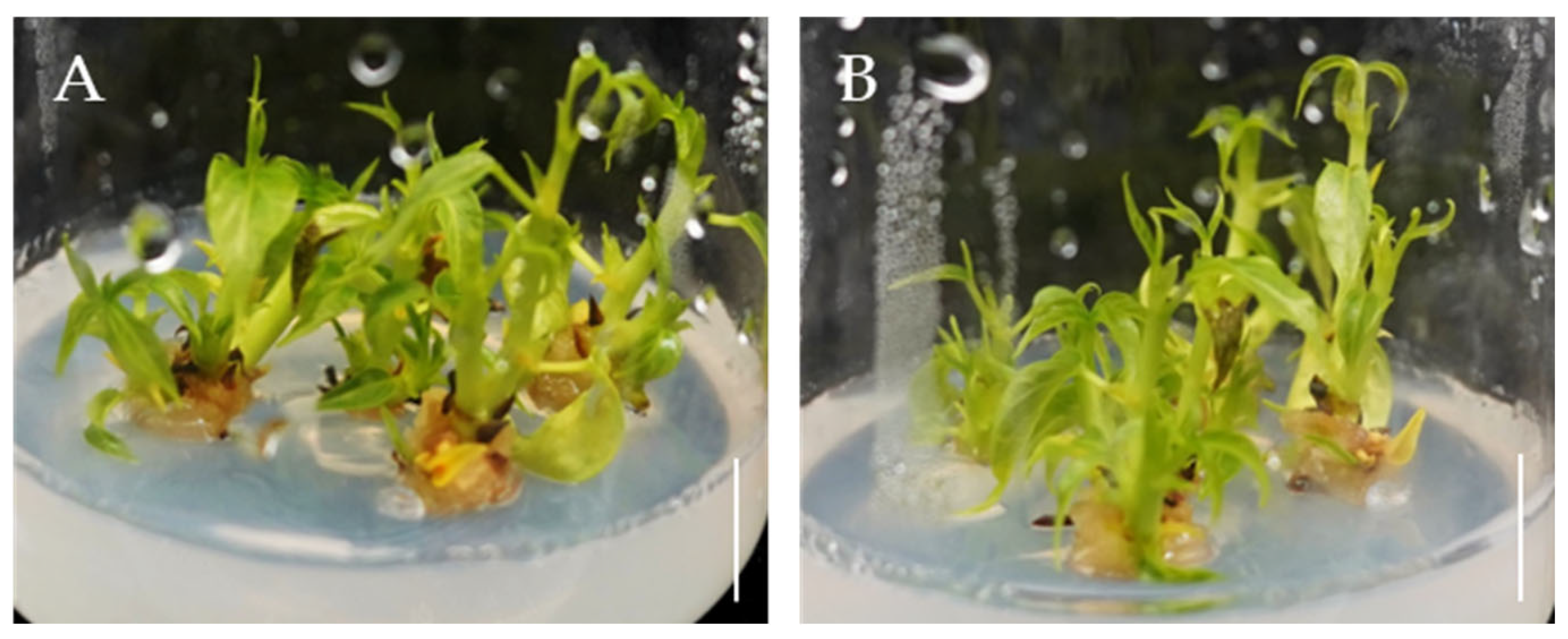
2.3. The Effects of 6-BA and IBA on Direct Organogenesis from Bombax ceiba Buds
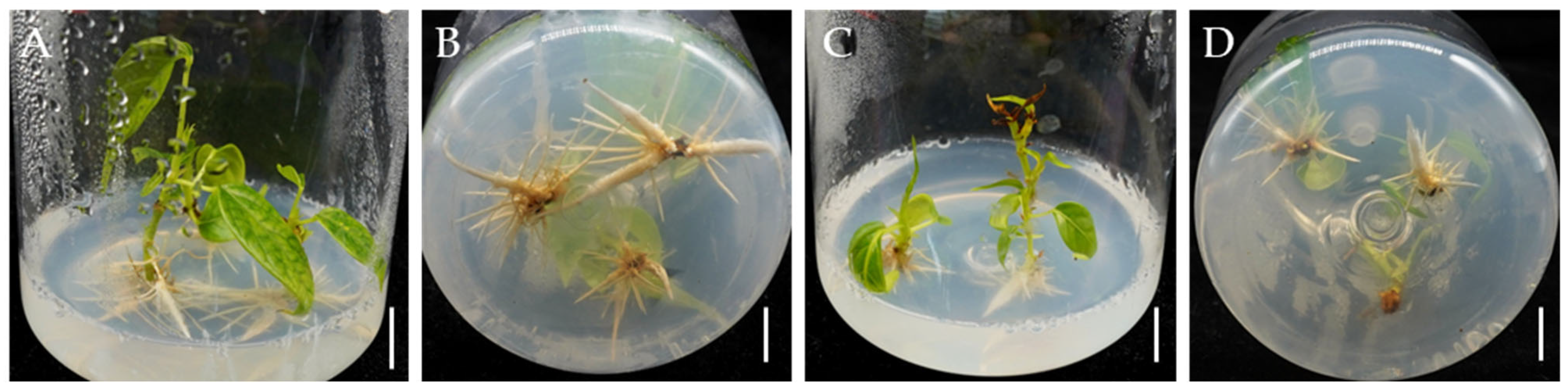
2.4. NAA Promotes Rooting of Bombax ceiba Adventitious Shoots
2.5. Plantlets Growth in Soil
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Explant Preparation
4.2. Medium Preparation and Tissue Culture
4.3. Callus Induction and Differentiation
4.4. Buds Proliferation into Adventitious Shoots
4.5. Rooting of Adventitious Shoots
4.6. Hardening and Transfer of Regenerated Plantlets in Soil
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Hong, W.; Yang, J.; Jiang, K.; Tan, Z.; He, Q.; Zhang, H.; Cui, J. Bombax ceiba is a good native tree species for performing reforestation to restore highly degraded tropical forests in Hainan island, China. Front. Environ. Sci. 2021, 9, 569428. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, Z.; Xu, F.; Wang, H.; Li, W.; Yang, Z.; Chen, X.; Zhu, B. Functional annotation and analysis of transcriptome of Bombax ceiba with different flower colors. J. Beijing For. Univ. 2022, 44, 1–11. [Google Scholar] [CrossRef]
- Bisht, A.S.; Gupta, A.K. Chapter 4—Bombax ceiba (Red silk cotton). In Edible Flowers; Academic Press: Cambridge, MA, USA, 2024; pp. 45–63. [Google Scholar] [CrossRef]
- Kriintong, N.; Katisart, T. In vitro antioxidant and antidiabetic activities of leaf and flower extracts from Bombax ceiba. Pharmacogn. Res. 2020, 12, 194–198. [Google Scholar] [CrossRef]
- Shah, S.S.; Shah, S.S.; Iqbal, A.; Ahmed, S.; Khan, W.M.; Hussain, S.; Li, Z. Report: Phytochemical screening and antimicrobial activities of red silk cotton tree (Bombax ceiba L.). Pak. J. Pharm. Sci. 2018, 31, 947–952. [Google Scholar] [PubMed]
- Yasien, S.; Iqbal, M.M.; Javed, M.; Alnuwaiser, M.A.; Iqbal, S.; Mahmood, Q.; Elkaeed, E.B.; Dera, A.A.; Alrbyawi, H.; Pashameah, R.A.; et al. Comparative evaluation of various extraction techniques for secondary metabolites from Bombax ceiba L. flowering plants along with in vitro anti-diabetic performance. Bioengineering 2022, 9, 486. [Google Scholar] [CrossRef]
- Das, P.; Puzari, A.; Devi, N. Cotton (Bombax ceiba) Seed Oil: Applications in the Synthesis of Polymer Resins and Blends. In Encyclopedia of Green Materials; Springer Nature: Singapore, 2025; pp. 471–479. [Google Scholar] [CrossRef]
- Ding, Y.; Gao, W.; Qin, Y.; Li, X.; Zhang, Z.; Lai, W.; Yang, Y.; Guo, K.; Li, P.; Zhou, S.; et al. Single-cell RNA landscape of the special fiber initiation process in Bombax ceiba. Plant Commun. 2023, 4, 100554. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Philips, A.; Godjuwa, C. Harvest of Bombax ceiba for the Aboriginal arts industry, central Arnhem Land, Australia. Biol. Conserv. 2003, 113, 295–305. [Google Scholar] [CrossRef]
- Dai, S.; Wang, S.; Ruan, L.; Zhou, Y.; Wu, W.; Liu, Y.; Shi, S.; Zhou, R. Extremely low genetic diversity and extensive genetic admixture at the northern range margins of Bombax ceiba. Biochem. Syst. Ecol. 2015, 60, 177–185. [Google Scholar] [CrossRef]
- Koenig, J.; Griffiths, A. The population ecology of two tropical trees, Brachychiton diversifolius (Malvaceae) and Bombax ceiba (Bombaceae), harvested by Indigenous woodcarvers in Arnhem Land, Australia. Environ. Manag. 2012, 50, 555–565. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Mao, K.; Li, W.; Cheng, X. Morphological responses of Bombax ceiba to habitat heterogeneity in Southwest China. Front. Ecol. Evol. 2023, 10, 1118045. [Google Scholar] [CrossRef]
- Omoyemi, O.M.; Olatunji, A.I. Effects of provenances, storage temperature and duration on seed germination of Bombax costatum Pellegr & Vuillet. J. For. Environ. Sci. 2021, 37, 235–242. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Plant stem cells and de novo organogenesis. New Phytol. 2018, 218, 1334–1339. [Google Scholar] [CrossRef]
- Hongxing, P.; Weiwei, W. Establishment of tissue culture rapid propagation system of Bombax malabaricum Dc. Trop. For. 2022, 50, 29–32. [Google Scholar] [CrossRef]
- Chand, S.; Singh, A.K. In vitro propagation of Bombax ceiba L. (Silkcotton). Silvae Genet. 1999, 48, 313–317. [Google Scholar]
- Fei, L.; Yong, K.; Dawood, M.; Chenran, Z.; Yamei, L.; Yuhua, G.; Junmei, Y. Efficient tissue culture and propagation technology of Bombax ceiba. Chin. J. Trop. Crops 2024, 46, 1167–1174. [Google Scholar] [CrossRef]
- Vyas, M.; Bansal, Y.K. Somatic embryogenesis and plantlet regeneration in semul (Bombax ceiba). Plant Cell Tissue Organ Cult. 2004, 79, 115–118. [Google Scholar] [CrossRef]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H. Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 2014, 108, 1–33. [Google Scholar]
- Mei, G.; Chen, A.; Wang, Y.; Li, S.; Wu, M.; Hu, Y.; Liu, X.; Hou, X. A simple and efficient in planta transformation method based on the active regeneration capacity of plants. Plant Commun. 2024, 5, 100822. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Liu, C.; Chu, H.; Dai, D.; Song, S.; Yu, L.; Han, L.; Fu, Y.; Tian, B.; et al. De novo genome assembly of the red silk cotton tree (Bombax ceiba). GigaScience 2018, 7, giy051. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Jin, S.; Chen, J.; Yang, G.; Fan, R.; Zhang, Z.; Deng, Q.; Han, J.; Ma, X.; Dong, Z.; et al. High-quality genomes of Bombax ceiba and Ceiba pentandra provide insights into the evolution of Malvaceae species and differences in their natural fiber development. Plant Commun. 2024, 5, 100832. [Google Scholar] [CrossRef] [PubMed]
- Lizhou, T.; Honglong, C.; Chao, L.; Haibo, W.; Yong, G. Faming Zhuanli Shenqing Gongkai. Shuomingshu. Patent No. CN107864862A, 3 April 2018. (In Chinese). [Google Scholar]
- Yamei, L.; Zhiqing, L.; Fei, L.; Yuhua, G.; Yong, K.; Guangsui, Y.; Junmei, Y. Effect of different disinfection methods and growth medium on obtaining sterile seedlings of Bombax ceiba. Mol. Plant Breed. 2024, 1–8. Available online: http://kns.cnki.net/kcms/detail/46.1068.s.20231114.1447.010.html (accessed on 15 November 2023).
- Pathi, K.M.; Sprink, T. From Petri Dish to Field: Plant Tissue Culture and Genetic Engineering of Oats for Improved Agricultural Outcomes. Plants 2023, 12, 3782. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L.; Canhoto, J. Plant tissue culture: Industrial relevance and future directions. Adv. Biochem. Eng. Biotechnol. 2024, 188, 1–15. [Google Scholar] [CrossRef]
- Wu, H.; Qu, X.; Dong, Z.; Luo, L.; Shao, C.; Forner, J.; Lohmann, J.U.; Su, M.; Xu, M.; Liu, X.; et al. WUSCHEL triggers innate antiviral immunity in plant stem cells. Science 2020, 370, 227–231. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Qin, M.; He, Y.; Zhang, J.; Gong, W.; Xiao, X.; Li, P.; Zhou, W. Establishment of in vitro efficient regeneration system for leaves and hypocotyls of Toona sinensis, a multifunctional woody plants. Ind. Crop. Prod. 2025, 224, 120328. [Google Scholar] [CrossRef]
- Hnatuszko-Konka, K.; Kowalczyk, T.; Gerszberg, A.; Glińska, S.; Grzegorczyk-Karolak, I. Regeneration of Phaseolus vulgaris from epicotyls and hypocotyls via direct organogenesis. Sci. Rep. 2019, 9, 6248. [Google Scholar] [CrossRef]
- Divya, K.; Swathi Anuradha, T.; Jami, S.K.; Kirti, P.B. Efficient regeneration from hypocotyl explants in three cotton cultivars. Biol. Plant. 2008, 52, 201–208. [Google Scholar] [CrossRef]
- Yuceer, S.U.; Koc, N.K. Agrobacterium-mediated transformation and regeneration of cotton plants. Russ. J. Plant Physiol. 2006, 53, 413–417. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Z.; Wang, G.; Cong, Y.; Yu, L.; Jia, R.; Qin, Y.; Zhang, G.; Li, B.; Yuan, D.; et al. Single-cell resolution analysis reveals the preparation for reprogramming the fate of stem cell niche in cotton lateral meristem. Genome Biol. 2023, 24, 194. [Google Scholar] [CrossRef]
- Yang, W.; Zhai, H.; Wu, F.; Deng, L.; Chao, Y.; Meng, X.; Chen, Q.; Liu, C.; Bie, X.; Sun, C.; et al. Peptide REF1 is a local wound signal promoting plant regeneration. Cell 2024, 187, 3024–3038.e14. [Google Scholar] [CrossRef] [PubMed]
- Babic, V.; Datla, R.S.; Scoles, G.J.; Keller, W.A. Development of an efficient Agrobacterium-mediated transformation system for Brassica carinata. Plant Cell Rep. 1998, 17, 183–188. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Huang, C.; Guo, X.; Nie, Y. Somatic embryogenesis and plant regeneration from different wild diploid cotton (Gossypium) species. Plant Cell Rep. 2006, 25, 289–296. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 71, 63–72. [Google Scholar] [CrossRef]
- Juturu, V.N.; Mekala, G.K.; Kirti, P.B. Current status of tissue culture and genetic transformation research in cotton (Gossypium spp.). Plant Cell Tissue Organ Cult. 2014, 120, 813–839. [Google Scholar] [CrossRef]
- Ge, X.; Xu, J.; Yang, Z.; Yang, X.; Wang, Y.; Chen, Y.; Wang, P.; Li, F. Efficient genotype-independent cotton genetic transformation and genome editing. J. Integr. Plant Biol. 2023, 65, 907–917. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Lourenço-Tessutti, I.T.; de Melo, B.P.; Morgante, C.V.; Filho, A.S.; Lins, C.B.J.; Ferreira, G.F.; Mello, G.N.; Macedo, L.L.P.; Lucena, W.A.; et al. Improved cotton transformation protocol mediated by Agrobacterium and biolistic combined-methods. Planta 2021, 254, 20. [Google Scholar] [CrossRef]
- Wojtania, A.; Skrzypek, E.; Gabryszewska, E. Effect of cytokinin, sucrose and nitrogen salts concentrations on the growth and development and phenolics content in Magnolia × soulangiana ‘Coates’ shoots in vitro. Acta Sci. Pol. Hortorum Cultus 2015, 14, 51–62. [Google Scholar]
- Liu, C.; Fan, H.; Zhang, J.; Wu, J.; Zhou, M.; Cao, F.; Tao, G.; Zhou, X. Combating browning: Mechanisms and management strategies in in vitro culture of economic woody plants. For. Res. 2024, 4, e032. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, P.; Yu, Y.; Xiong, Y. Glucose-TOR signaling regulates PIN2 stability to orchestrate auxin gradient and cell expansion in Arabidopsis root. Proc. Natl. Acad. Sci. USA 2020, 117, 32223–32225. [Google Scholar] [CrossRef]
- Sochacki, D.; Marciniak, P.; Ciesielska, M.; Zaród, J.; Sutrisno. The influence of selected plant growth regulators and carbohydrates on in vitro shoot multiplication and bulbing of the tulip (Tulipa L.). Plants 2023, 12, 1134. [Google Scholar] [CrossRef]
- Harada, H.; Murai, Y. Micropropagation of Prunus mume. Plant Cell Tissue Organ Cult. 1996, 46, 265–267. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jürgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef]
- Aregawi, K.; Shen, J.; Pierroz, G.; Sharma, M.K.; Dahlberg, J.; Owiti, J.; Lemaux, P.G. Morphogene-assisted transformation of Sorghum bicolor allows more efficient genome editing. Plant Biotechnol. J. 2022, 20, 748–760. [Google Scholar] [CrossRef]
- Zeng, D.; Si, C.; Zhang, M.; Duan, J.; He, C. ERF5 enhances protocorm-like body regeneration via enhancement of STM expression in Dendrobium orchid. J. Integr. Plan. Biol. 2023, 65, 2071–2085. [Google Scholar] [CrossRef] [PubMed]
- Irsyadi, M.B. Factors that effect of the optimal plantlet growth from tissue culture on the acclimatization stage. Proc. Int. Conf. Sci. Eng. 2021, 4, 100–104. Available online: https://sunankalijaga.org/prosiding/index.php/icse/article/view/632 (accessed on 27 February 2021).

| Media | Basal Media | PGRs (mg·L−1) | No. of Callus Induction * | Callus Induction Rate (%) * | |||
|---|---|---|---|---|---|---|---|
| 2,4-D | KIN | 6-BA | NAA | ||||
| CIM1 | MS | 0.1 | 0.5 | 0 | 0 | 18.7 ± 0.6 d | 62.2 ± 3.8 d |
| CIM2 | MS | 0 | 0 | 0.5 | 2 | 0 e | 0 e |
| CIM3 | MS | 0.1 | 0 | 0.2 | 0 | 26.3 ± 0.6 c | 87.8 ± 3.8 c |
| CIM4 | MS | 0.1 | 0 | 0.5 | 0 | 30.7 ± 1.2 b | 102.2 ± 7.7 b |
| CIM5 | ½ MS | 0.1 | 0 | 0.5 | 0 | 42.0 ± 2.0 a | 140.0 ± 6.7 a |
| Media | PGRs (mg·L−1) | Character of Callus Differentiation | ||
|---|---|---|---|---|
| IBA | KIN | 6-BA | ||
| DFM1 | 0 | 0 | 0.5 | Callus browning is not obvious, but no differentiation is observed. |
| DFM2 | 0 | 0.15 | 2 | Slight callus browning, and differentiation is limited; only a few calluses form adventitious buds. |
| DFM3 | 0 | 0.5 | 1 | Callus proliferates and browns, but no differentiation is observed. |
| DFM4 | 0.5 | 0 | 2 | Callus browning is not obvious, and no differentiation occurs. |
| DFM5 | 0.5 | 0.15 | 1 | Callus browns at the sprouting site and differentiates into 2–3 adventitious shoots. |
| DFM6 | 0.5 | 0.5 | 0.5 | Callus turns black at the sprouting site, with no adventitious shoot formation. |
| DFM7 | 1 | 0 | 1 | Callus gradually browns, and no differentiation is observed. |
| DFM8 | 1 | 0.15 | 0.5 | Callus shows no proliferation and undergoes browns. |
| DFM9 | 1 | 0.5 | 2 | Callus browns, with no differentiation. |
| Media | PRGs (mg·L−1) | Callus Differentiation Rate (%) * | Differentiation Coefficient * | ||
|---|---|---|---|---|---|
| IBA | KIN | 6-BA | |||
| DFM2 | 0 | 0.15 | 2 | 4.4 ± 1.9 b | 1.3 ± 0.6 a |
| DFM5 | 0.5 | 0.15 | 1 | 51.1 ± 1.9 a | 1.8 ± 0.1 a |
| Medium | PRGs (mg·L−1) | No. of Buds | Sprouting Rate (%) | No. of Proliferation Shoots † | Proliferation Coefficient * | ||
|---|---|---|---|---|---|---|---|
| IBA | KIN | 6-BA | |||||
| DFM5 | 0.5 | 0.15 | 1 | 15 | 100 | 48 | 3.2 ± 0.1 |
| 15 | 49 | ||||||
| 15 | 47 | ||||||
| DFM10 | 0.5 | 0 | 1 | 15 | 100 | 48 | 3.2 ± 0.0 |
| 15 | 49 | ||||||
| 15 | 48 | ||||||
| Medium | Plant Growth Regulators | No. of Shoots | No. of Rooted Shoots | Rooting Rate (%) * | No. of Roots | Rooting Coefficient * |
|---|---|---|---|---|---|---|
| R1 | 0.5 mg·L−1 NAA | 30 | 29 | 96.7 ± 3.3 a | 79 | 2.7 ± 0.1 a |
| 30 | 28 | 78 | ||||
| 15 | 15 | 40 | ||||
| R2 | 0.5 mg·L−1 NAA + 0.5 mg·L−1 KIN | 30 | 21 | 64.4 ± 5.1 b | 31 | 1.5 ± 0.1 b |
| 30 | 19 | 27 | ||||
| 15 | 9 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, Q.; Cha, L.; Lin, F.; Tang, F.; Kang, Y.; Yang, G.; Huang, S.; Guo, Y.; Yin, J. Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants. Plants 2025, 14, 2033. https://doi.org/10.3390/plants14132033
Li Y, Jiang Q, Cha L, Lin F, Tang F, Kang Y, Yang G, Huang S, Guo Y, Yin J. Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants. Plants. 2025; 14(13):2033. https://doi.org/10.3390/plants14132033
Chicago/Turabian StyleLi, Yamei, Qionghai Jiang, Lisha Cha, Fei Lin, Fenling Tang, Yong Kang, Guangsui Yang, Surong Huang, Yuhua Guo, and Junmei Yin. 2025. "Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants" Plants 14, no. 13: 2033. https://doi.org/10.3390/plants14132033
APA StyleLi, Y., Jiang, Q., Cha, L., Lin, F., Tang, F., Kang, Y., Yang, G., Huang, S., Guo, Y., & Yin, J. (2025). Highly Efficient Regeneration of Bombax ceiba via De Novo Organogenesis from Hypocotyl and Bud Explants. Plants, 14(13), 2033. https://doi.org/10.3390/plants14132033






