Genetic and Morphological Variation Among Populations of Duckweed Species in Thailand
Abstract
1. Introduction
2. Results
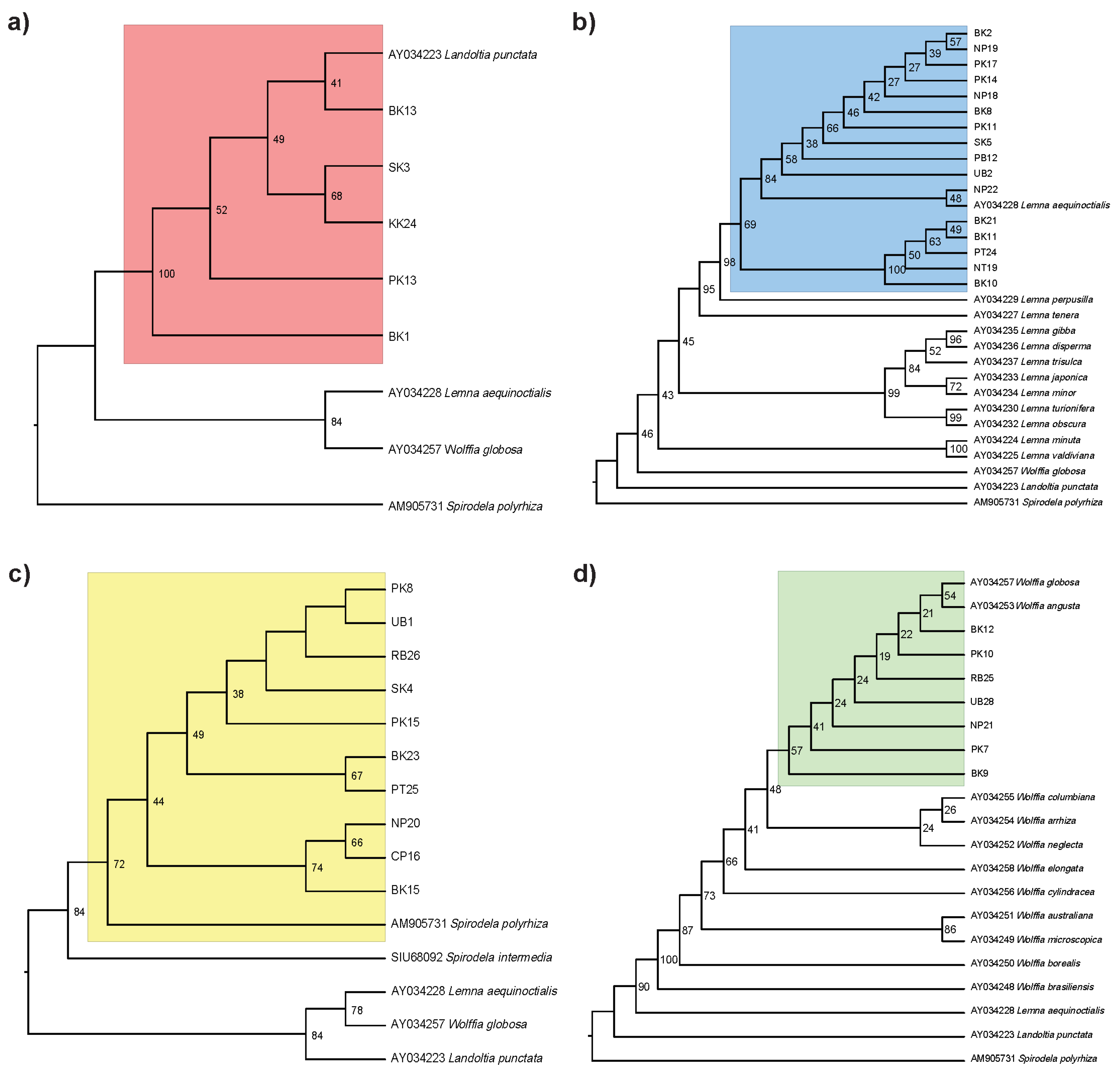
2.1. Species Identification of Thai Duckweeds
2.2. Genetic Diversity of Thai Duckweeds
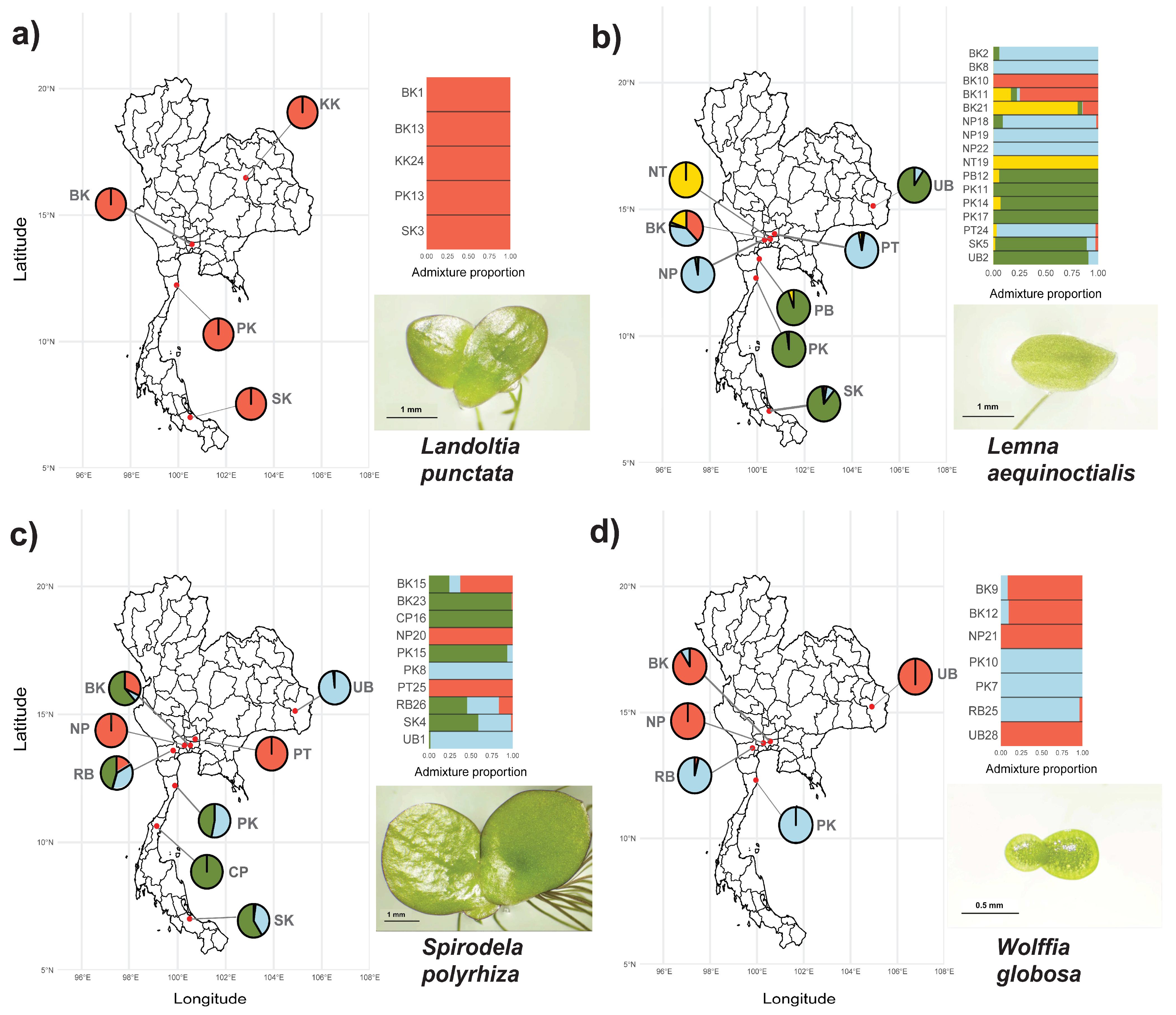
2.3. Population Structures of Thai Duckweeds
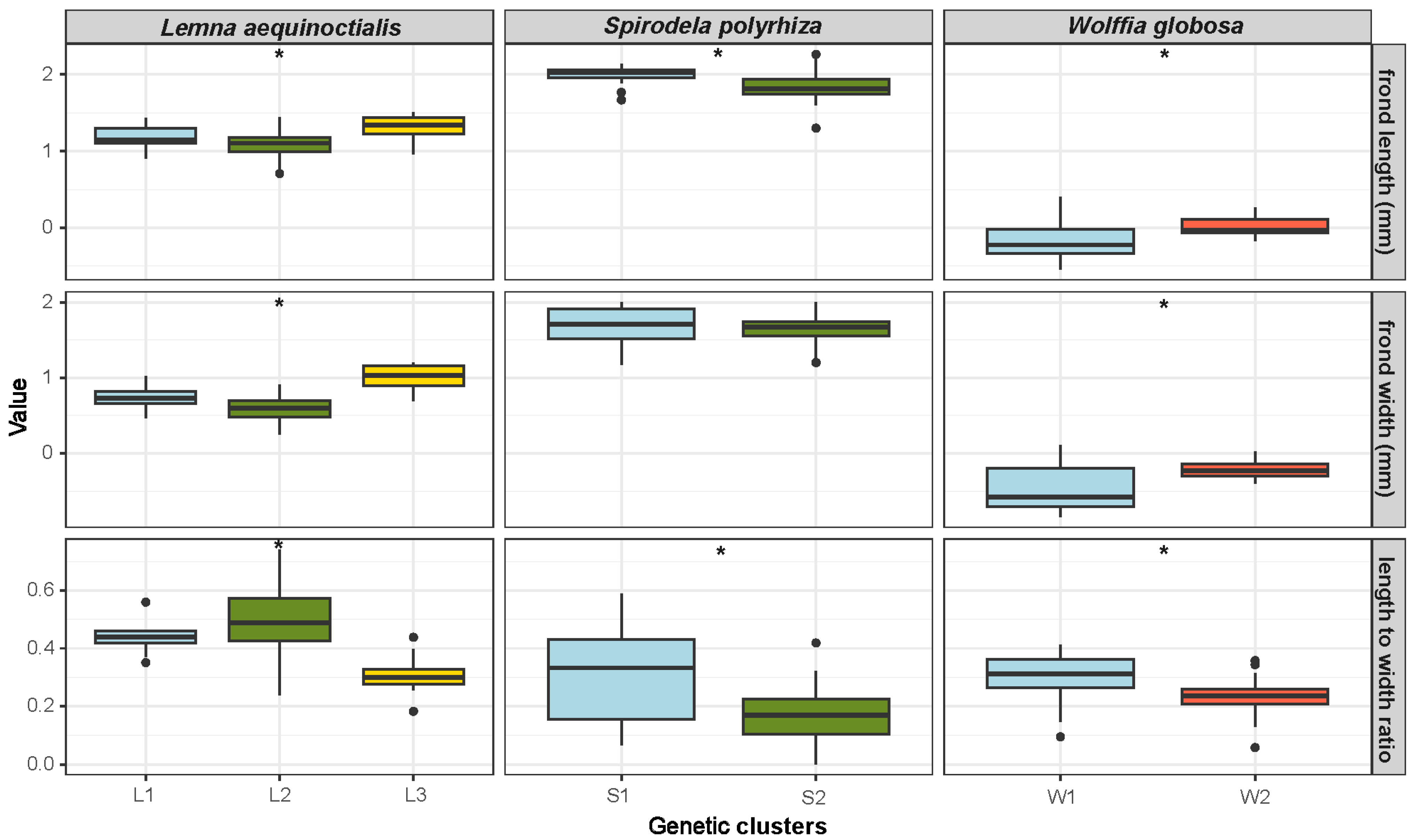
2.4. Morphological Variation: Univariate Analysis
2.5. Morphological Variation: Bivariate Analysis
3. Discussion
3.1. Phylogenetic Placement of Thai Duckweeds
3.2. Genetic Diversity and Population Structures of Thai Duckweeds
4. Materials and Methods
4.1. Sampling Sites and Sample Preparation
4.2. DNA Extraction, PCR Amplification, and Sequencing
4.3. Phylogenetic Analysis
4.4. Population Structure and Genetic Data Analysis
4.5. Morphological Study and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bog, M.; Appenroth, K.J.; Sree, K.S. Key to the determination of taxa of Lemnaceae: An update. Nord. J. Bot. 2020, 38, e02658. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Borisjuk, N.; Eric, L. Telling duckweed apart: Genotyping technologies for the Lemnaceae. Chin. J. Appl. Environ. Biol. 2013, 19, 1–10. [Google Scholar] [CrossRef]
- Fourounjian, P.; Fakhoorian, T.; Cao, H. Importance of duckweeds in basic research and their industrial applications. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–17. [Google Scholar]
- Sree, K.S.; Bog, M.; Appenroth, K.J. Taxonomy of duckweeds (Lemnaceae), potential new crop plants. Emir. J. Food Agric. 2016, 28, 291–302. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.-J.; Sree, K.S. Duckweed (Lemnaceae): Its Molecular Taxonomy. Front. Sustain. Food Syst. 2019, 3, 117. [Google Scholar] [CrossRef]
- Appenroth, K.; Jansen, M.; Lam, E.; Shoham, T.; Sree, K. Important terms in duckweed research defined. ISCDRA Newsl. 2024, 12, 90–94. [Google Scholar]
- Sree, K.S.; Sudakaran, S.; Appenroth, K.-J. How fast can angiosperms grow? Species and clonal diversity of growth rates in the genus Wolffia (Lemnaceae). Acta Physiol. Plant. 2015, 37, 204. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, K.; Li, W.; Yan, B.; Yu, Y.; Li, J.; Zhang, Y.; Xia, S.; Cheng, Z.; Lin, F.; et al. A review on bioenergy production from duckweed. Biomass Bioenergy 2022, 161, 106468. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Johari, M.A.A.M.; Yahya, H.N.; Yahya, H. Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses. Future Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Herawati, V.E.; Pinandoyo, P.; Darmanto, Y.; Rismaningsih, N.; Windarto, S.; Radjasa, O.K. The effect of fermented duckweed (Lemna minor) in feed on growth and nutritional quality of tilapia (Oreochromis niloticus). Biodiversitas J. Biol. Divers. 2020, 21, 3350–3358. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional value of the duckweed species of the Genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Duckweed: An effective tool for phyto-remediation. Toxicol. Environ. Chem. 2013, 95, 1256–1266. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Huang, M.; Guo, L.; Zhang, Y.; Zhao, H. Improving biomass and starch accumulation of bioenergy crop duckweed (Landoltia punctata) by abscisic acid application. Sci. Rep. 2018, 8, 9544. [Google Scholar] [CrossRef] [PubMed]
- Thi Da, C.; Lundh, T.; Lindberg, J. Digestibility of dietary components and amino acids in plant protein feed ingredients in striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquac. Nutr. 2013, 19, 741–750. [Google Scholar] [CrossRef]
- Verma, R.; Suthar, S. Utility of duckweeds as source of biomass energy: A review. BioEnergy Res. 2015, 8, 1589–1597. [Google Scholar] [CrossRef]
- Tippery, N.P.; Les, D.H. Tiny plants with enormous potential: Phylogeny and evolution of duckweeds. In The Duckweed Genomes; Cao, X.H., Fourounjian, P., Wang, W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–38. [Google Scholar]
- Yan, Y.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a Δ6-desaturase responsible for the production of γ-linolenic and stearidonic acids in Lemna gibba. BMC Plant Biol. 2013, 13, 201. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Clark, W.D.; Sharma, J.G.; Goswami, R.K.; Shrivastav, A.K.; Tocher, D.R. Mass production of Lemna minor and its amino acid and fatty acid profiles. Front. Chem. 2018, 6, 479. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Sree, K.S.; Adelmann, K.; Garcia, C.; Lam, E.; Appenroth, K.-J. Natural variance in salt tolerance and induction of starch accumulation in duckweeds. Planta 2015, 241, 1395–1404. [Google Scholar] [CrossRef]
- Xu, S.; Stapley, J.; Gablenz, S.; Boyer, J.; Appenroth, K.J.; Sree, K.S.; Gershenzon, J.; Widmer, A.; Huber, M. Low genetic variation is associated with low mutation rate in the giant duckweed. Nat. Commun. 2019, 10, 1243. [Google Scholar] [CrossRef]
- Xue, H.; Xiao, Y.; Jin, Y.; Li, X.; Fang, Y.; Zhao, H.; Zhao, Y.; Guan, J. Genetic diversity and geographic differentiation analysis of duckweed using inter-simple sequence repeat markers. Mol. Biol. Rep. 2012, 39, 547–554. [Google Scholar] [CrossRef]
- Yuan, J.-X.; Pan, J.; Wang, B.-S.; Zhang, D.-M. Genetic differentiation of Wolffia globosa in China. J. Syst. Evol. 2011, 49, 509–517. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Yan, Y.; Ermakova, M.; Kerstetter, R.; Messing, J. DNA barcoding of the Lemnaceae, a family of aquatic monocots. BMC Plant Biol. 2010, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Landolt, E. Lemnaceae. In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; Forest Herbarium, Royal Forest Department: Bangkok, Thailand, 2001; Volume 7, pp. 394–399. [Google Scholar]
- Borisjuk, N.; Chu, P.; Gutierrez, R.; Zhang, H.; Acosta, K.; Friesen, N.; Sree, K.S.; Garcia, C.; Appenroth, K.J.; Lam, E.; et al. Assessment, validation and deployment strategy of a two-barcode protocol for facile genotyping of duckweed species. Plant Biol. 2015, 17, 42–49. [Google Scholar] [CrossRef]
- Bhanthumnavin, K.; McGarry, M.G. Wolffia arrhiza as a possible source of inexpensive protein. Nature 1971, 232, 495. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Huang, M.; Peng, M.; Bog, M.; Sree, K.S.; Appenroth, K.-J.; Zhang, J. Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae). Hydrobiologia 2015, 743, 75–87. [Google Scholar] [CrossRef]
- On-nom, N.; Promdang, P.; Inthachat, W.; Kanoongon, P.; Sahasakul, Y.; Chupeerach, C.; Suttisansanee, U.; Temviriyanukul, P. Wolffia globosa-Based Nutritious Snack Formulation with High Protein and Dietary Fiber Contents. Foods 2023, 12, 2647. [Google Scholar] [CrossRef]
- Stepanenko, A.; Braglia, L.; Fuchs, J.; Schubert, V.; Hoang, P.T.; Lee, Y.; Chen, G.; Gianì, S.; Morello, L.; Schubert, I. Genome diversity and phylogeny of the section Alatae of genus Lemna (Lemnaceae), comprising the presumed species Lemna aequinoctialis, Le. perpusilla and Le. aoukikusa. bioRxiv 2025, bioRxiv:2025.2001.2017.632899. [Google Scholar] [CrossRef]
- Bog, M.; Lautenschlager, U.; Landrock, M.F.; Landolt, E.; Fuchs, J.; Sowjanya Sree, K.; Oberprieler, C.; Appenroth, K.-J. Genetic characterization and barcoding of taxa in the genera Landoltia and Spirodela (Lemnaceae) by three plastidic markers and amplified fragment length polymorphism (AFLP). Hydrobiologia 2015, 749, 169–182. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, F.; Cui, W.; Ma, J. Genetic structure of duckweed population of Spirodela, Landoltia and Lemna from Lake Tai, China. Planta 2014, 239, 1299–1307. [Google Scholar] [CrossRef]
- Crawford, D.J.; Landolt, E. Allozyme studies in Spirodela (Lemnaceae): Variation among conspecific clones and divergence among the species. Syst. Bot. 1993, 18, 389–394. [Google Scholar] [CrossRef]
- Landolt, E. Key to the determination of taxa within the family of Lemnaceae. In Veröffentlichungen des Geobotanischen Institutes der Eidg. Techn.; Hochschule, Stiftung Rübel: Zürich, Switzerland, 1980; Volume 70, pp. 13–21. [Google Scholar]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; CBOL Plant Working Group; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Katoh, K.; Kuma, K.-i.; Toh, H.; Miyata, T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005, 33, 511–518. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. Figtree, 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 11 November 2021).
- François, O. Running structure-like population genetic analyses with R. In R Tutorials in Population Genetics; Université Grenoble Alpes: Saint-Martin-d'Hères, France, 2016; pp. 1–9. [Google Scholar]
- Frichot, E.; François, O. LEA: An R Package for Landscape and Ecological Association Studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Paradis, E. pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

| Species | Sample Size | Length (bp) | GC Content (%) | Haplotype Diversity (h) | Segregating Sites (S) | Nucleotide Diversity (π) | Tajima’s D | p-Value |
|---|---|---|---|---|---|---|---|---|
| Landoltia punctata | 5 | 1475 | 33.40% | 1.000 | 152 | 0.038 | −1.625 | 0.104 |
| Lemna aequinoctialis | 16 | 1434 | 33.56% | 1.000 | 502 | 0.048 | −2.276 | 0.023 * |
| Spirodela polyrhiza | 10 | 1484 | 33.19% | 1.000 | 452 | 0.032 | −3.344 | 0.001 * |
| Wolffia globosa | 7 | 1340 | 34.00% | 1.000 | 418 | 0.099 | −1.271 | 0.204 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senayai, A.; Harnvanichvech, Y.; Vajrodaya, S.; Oyama, T.; Kraichak, E. Genetic and Morphological Variation Among Populations of Duckweed Species in Thailand. Plants 2025, 14, 2030. https://doi.org/10.3390/plants14132030
Senayai A, Harnvanichvech Y, Vajrodaya S, Oyama T, Kraichak E. Genetic and Morphological Variation Among Populations of Duckweed Species in Thailand. Plants. 2025; 14(13):2030. https://doi.org/10.3390/plants14132030
Chicago/Turabian StyleSenayai, Athita, Yosapol Harnvanichvech, Srunya Vajrodaya, Tokitaka Oyama, and Ekaphan Kraichak. 2025. "Genetic and Morphological Variation Among Populations of Duckweed Species in Thailand" Plants 14, no. 13: 2030. https://doi.org/10.3390/plants14132030
APA StyleSenayai, A., Harnvanichvech, Y., Vajrodaya, S., Oyama, T., & Kraichak, E. (2025). Genetic and Morphological Variation Among Populations of Duckweed Species in Thailand. Plants, 14(13), 2030. https://doi.org/10.3390/plants14132030








