Influence of Light Spectrum on Bread Wheat Head Colonization by Fusarium graminearum and on the Accumulation of Its Secondary Metabolites
Abstract
1. Introduction
2. Results
2.1. FHB Symptom Development in Bread Wheat Heads
2.2. F. graminearum Quantification in Bread Wheat Heads
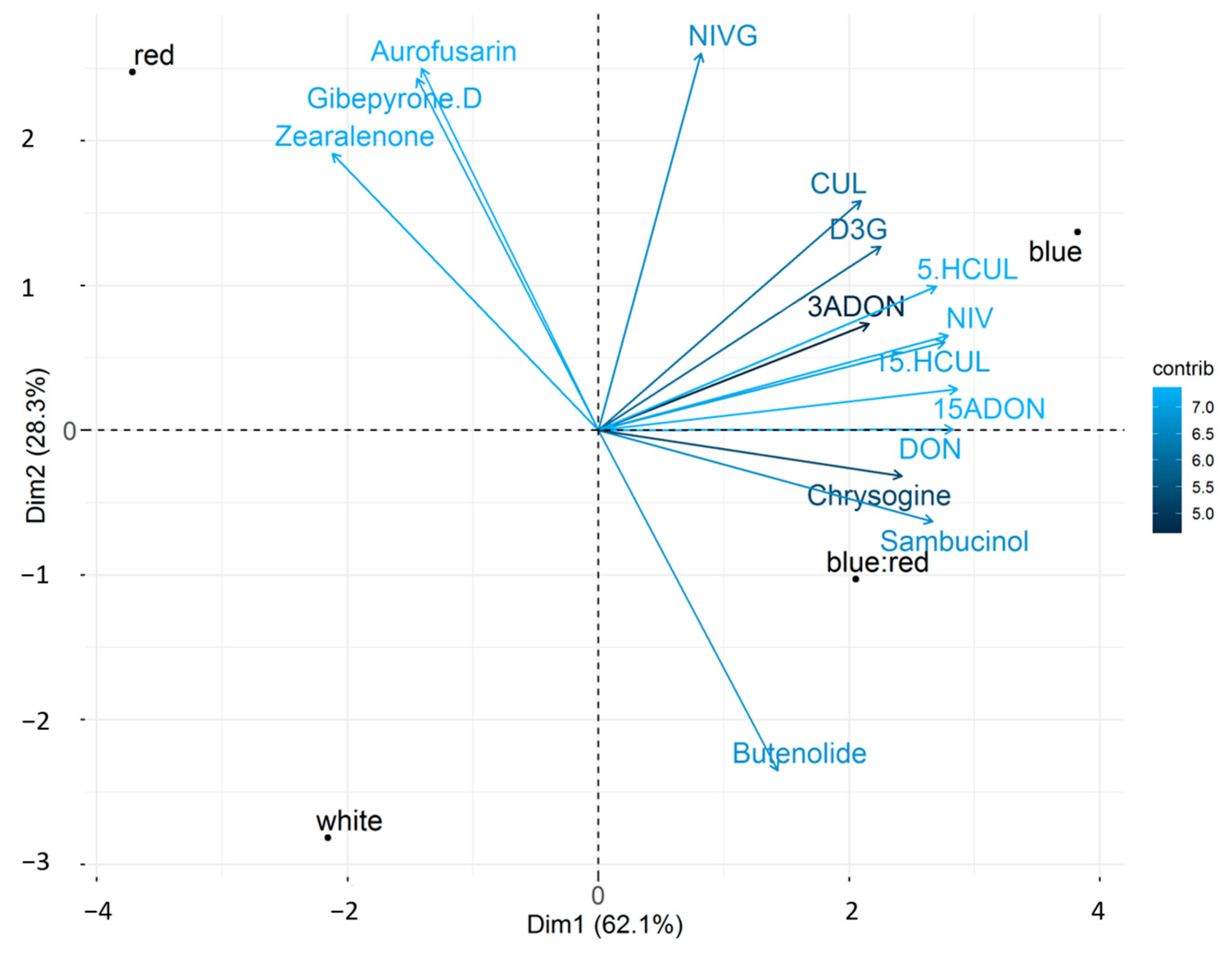
2.3. F. graminearum Secondary Metabolite Accumulation in Bread Wheat Heads
2.4. Correlation Between Fungal Biomass and Secondary Metabolites in Bread Wheat Heads
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Inoculum Obtainment
4.2. Plant Material and Artificial Inoculation
4.3. Light Treatments
4.4. Evaluation of FHB Symptoms
4.5. Fusarium graminearum Quantification
4.6. Determination of F. graminearum Secondary Metabolites in Wheat Heads
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 15ADON | 15 acetyl-deoxynivalenol |
| 15.HCUL | 15-hydroxyculmorin |
| 3ADON | 3 acetyl-deoxynivalenol |
| 5.HCUL | 5-hydroxyculmorin |
| Ct | Cycle threshold |
| CUL | Culmorin |
| D3G | Deoxynivalenol 3-glucoside |
| DON | Deoxynivalenol |
| dpi | Days post inoculation |
| FFP | Farnesyl pyrophosphate |
| FHB | Fusarium head blight |
| GLC | β-1,3-glucanase |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LED | Light-emitting diode |
| LOD | Limit of detection |
| NIV | Nivalenol |
| NIVG | Nivalenol glucoside |
| NPR1 | Nonexpressor of pathogenesis-related genes-1 |
| PAL | Phenylalanine ammonia-lyase |
| PCA | Principal component analysis |
| PDA | Potato dextrose agar |
| POX | Class III plant peroxidase |
| PKS | Polyketide synthase |
| qPCR | Quantitative real-time PCR |
| THSD | Tukey’s honestly significant difference |
| UTGs | Uridine diphosphate glycosyltransferases |
References
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LED’s effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Livadariu, O.; Maximilian, C.; Rahmanifar, B.; Cornea, C.P. LED technology applied to plant development for promoting the accumulation of bioactive compounds: A review. Plants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ballaré, C.L. Light regulation of plant defense. Ann. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; McLellan, H.; Birch, P.R.J.; Gilory, E.M. Tuning the wavelength: Manipulation of light signaling to control plant defense. Int. J. Mol. Sci. 2023, 24, 3803. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.D.; Castillo, J.A. Influence of light on plant–phyllosphere interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Gallé, A.; Czékus, Z.; Tóth, L.; Galgóczy, L.; Poór, P. Pest and disease management by red light. Plant Cell Environ. 2021, 44, 3197–3210. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Ong, M.M.X.; Lee, Y.Y.; Teo, E.J.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Wang, D.; Dawadi, B.; Qu, J.; Ye, J. Light-engineering technology for enhancing plant disease resistance. Front. Plant Sci. 2022, 12, 805614. [Google Scholar] [CrossRef]
- Dieleman, J.A.; Kruidhof, H.M.; Weerheim, K.; Leiss, K. LED lighting strategies affect physiology and resilience to pathogens and pests in eggplant (Solanum melongena L.). Front. Plant Sci. 2021, 11, 610046. [Google Scholar] [CrossRef]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Blue LED (light-emitting diodes)- mediated growth promotion and control of Botrytis disease in lettuce. Acta Agric. Scand. B Soil Plant Sci. 2013, 63, 271–277. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Belwal, T.; Javed, M.; Luo, Z. Influence of the Red LEDs light irradiation on the quality anc chemical attributes of postharvest table grape (Vitis vinifera L.) during storage. Food Bioprocess Technol. 2022, 15, 1436–1447. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Y.; Nan, L.; Lai, T.; Wang, R. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. J. Integr. Agric. 2017, 16, 106–114. [Google Scholar] [CrossRef]
- Chiang, C.; Olsen, J.E.; Basler, D.; Bankestad, D.; Hoch, G. Latitude and weather influences on sun light quality and the relationship to tree growth. Forests 2019, 10, 610. [Google Scholar] [CrossRef]
- Kotilainen, T.; Aphalo, P.J.; Brelsford, C.C.; Böök, H.; Devraj, S.; Heikkilä, A.; Hernández, R.; Kylling, A.; Lindfors, A.V.; Robson, T.M. Patterns in the spectral composition of sunlight and biologically meaningful spectral photon ratios as affected by atmospheric factors. Agric. For. Meteorol. 2020, 291, 108041. [Google Scholar] [CrossRef]
- Spitschan, M.; Aguirre, G.K.; Brainard, D.H.; Sweeney, A.M. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep. 2016, 6, 26756. [Google Scholar] [CrossRef]
- Cerón-Bustamante, M.; Balducci, E.; Beccari, G.; Nicholson, P.; Covarelli, L.; Benincasa, P. Effect of light spectra on cereal fungal pathogens, a review. Fungal Biol. Rev. 2022, 43, 100291. [Google Scholar] [CrossRef]
- Kilaru, S.; Fantozzi, E.; Cannon, S.; Schuster, M.; Chaloner, T.M.; Guiu-Aragones, C.; Gurr, S.J.; Steinberg, G. Zymoseptoria tritici white-collar complex integrates light, temperature and plant cues to initiate dimorphism and pathogenesis. Nat. Comm. 2022, 13, 5625. [Google Scholar] [CrossRef]
- Solanki, T.; Aphalo, P.J.; Neimane, S.; Hartikainen, S.M.; Pieristè, M.; Shapiguzov, A.; Porcar-Castell, A.; Atherton, J.; Heikkilä, A.; Robson, T.M. UV-screening and springtime recovery of photosynthetic capacity in leaves of Vaccinium vitis-idaea above and below the snow pack. Plant Physiol. Biochem. 2019, 134, 40–52. [Google Scholar] [CrossRef]
- Tiley, A.M.M.; Lawless, C.; Pilo, P.; Karki, S.J.; Lu, J.; Long, Z.; Gibriel, H.; Bailey, A.M.; Feechan, A. The Zymoseptoria tritici white collar-1 gene, ZtWco-1, is required for development and virulence on wheat. Fungal Genet. Biol. 2022, 161, 103715. [Google Scholar] [CrossRef]
- Bluhm, B.H.; Dunkle, L.D. PHL1 of Cercospora zeae-maydis encodes a member of the photolyase/cryptochrome family involved in UV protection and fungal development. Fungal Genet. Biol. 2008, 45, 1364–1372. [Google Scholar] [CrossRef]
- Castrillo, M.; García-Martínez, J.; Avalos, J. Light-dependent functions of the Fusarium fujikuroi CryD DASH cryptochrome in development and secondary metabolism. Appl. Environ. Microbiol. 2013, 79, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Geisen, R.; Schmidt-Heydt, M.; Logrieco, A.F.; Mulè, G. Light regulation of mycotoxin biosynthesis: New perspectives for food safety. World Mycotoxin J. 2016, 9, 129–145. [Google Scholar] [CrossRef]
- Johnson, S.W.; Coolbaugh, R.C. Light-stimulated gibberellin biosynthesis in Gibberella fujikuroi. Plant Physiol. 1990, 94, 1696–1701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.; Ridenour, J.B.; Dunkle, L.D.; Bluhm, B.H. Regulation of stomatal tropism and infection by light in Cercospora zeae-maydis: Evidence for coordinated host/pathogen responses to photoperiod? PLoS Pathog. 2011, 7, e1002113. [Google Scholar] [CrossRef]
- Lee, K.; Singh, P.; Chung, W.C.; Ash, J.; Kim, T.S.; Hang, L.; Park, S. Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae. Fungal Genet. Biol. 2006, 43, 694–706. [Google Scholar] [CrossRef]
- McCorison, C.B.; Goodwin, S.B. The wheat pathogen Zymoseptoria tritici senses and responds to different wavelengths of light. BMC Genomics 2020, 21, 513. [Google Scholar] [CrossRef]
- Prado, M.M.; Prado-Cabrero, A.; Fernández-Martín, R.; Avalos, J. A gene of the opsin family in the carotenoid gene cluster of Fusarium fujikuroi. Curr. Genet. 2004, 46, 47–58. [Google Scholar] [CrossRef]
- Cerόn-Bustamante, M.; Tini, F.; Beccari, G.; Benincasa, P.; Covarelli, L. Effect of different light wavelengths on Zymoseptoria tritici development and leaf colonization in bread wheat. J. Fungi 2023, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Parada, R.Y.; Mon-nai, W.; Ueno, M.; Kihara, J.; Arase, S. Red-light-induced resistance to brown spot disease caused by Bipolaris oryzae in rice. J. Phytopathol. 2015, 163, 116–123. [Google Scholar] [CrossRef]
- Shirasawa, H.; Ueno, M.; Kihara, J.; Arase, S. Protective effect of red light against blast disease caused by Magnaporthe oryzae in rice. Crop Prot. 2012, 39, 41–44. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Valenti, I.; Tini, F.; Sevarika, M.; Agazzi, A.; Beccari, G.; Bellezza, I.; Ederli, L.; Grottelli, S.; Pasquali, M.; Romani, R.; et al. Impact of enniatin and deoxynivalenol co-occurrence on plant, microbial, insect, animal and human systems: Current knowledge and future perspectives. Toxins 2023, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotox J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Bin-Umer, M.A.; McLaughlin, J.E.; Basu, D.; McCormick, S.; Tumer, N.E. Trichothecene mycotoxins inhibit mitochondrial translation-implication for the mechanism of toxicity. Toxins 2011, 3, 1484–1501. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Beccari, G.; Senatore, M.T.; Tini, F.; Sulyok, M.; Covarelli, L. Fungal community, Fusarium head blight complex and secondary metabolites associated with malting barley grains harvested in Umbria, central Italy. Int. J. Food Microbiol. 2018, 273, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple fungal metabolites including mycotoxins in naturally infected and Fusarium-inoculated wheat samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.-R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Ansari, K.I.; Doyle, S.M.; Kacprzyk, J.; Khan, M.R.; Walter, S.; Brennan, J.M.; Arunachalam, C.S.; McCabe, P.F.; Doohan, F.M. Light influences how the fungal toxin deoxynivalenol affects plant cell death and defense responses. Toxins 2014, 6, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.B.M.; Karsaï, I.; Schepers, J.; Snijders, C.H.A. Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for Fusarium head blight resistance. Plant Sci. 1993, 94, 195–206. [Google Scholar] [CrossRef]
- Ederli, L.; Beccari, G.; Tini, F.; Bergamini, I.; Bellezza, I.; Romani, R.; Covarelli, L. Enniatin B and deoxynivalenol activity on bread wheat and on Fusarium species development. Toxins 2021, 13, 728. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, Á.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef]
- Jansen, C.; Von Wettstein, D.; Schäfer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection pattern in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Kim, H.; Son, H.; Lee, Y.-W. Effects of light on secondary metabolism and fungal development of Fusarium graminearum. J. Appl. Microbiol. 2014, 116, 380–389. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Rüfer, C.; Raupp, F.; Bruchmann, A.; Perrone, G.; Geisen, R. Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int. J. Food Microbiol. 2011, 145, 229–237. [Google Scholar] [CrossRef]
- Thind, T.S.; Schilder, A.C. Understanding photoreception in fungi and its role in fungal development with focus on phytopathogenic fungi. Indian Phytopathol. 2018, 71, 169–182. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef]
- Fanelli, F.; Schmidt-Heydt, M.; Haidukowski, M.; Geisen, R.; Logrieco, A.; Mulè, G. Influence of light on growth, fumonisin biosynthesis and FUM1 gene expression by Fusarium proliferatum. Int. J. Food Microbiol. 2012, 153, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Schmidt-Heydt, M.; Haidukowski, M.; Susca, A.; Geisen, R.; Logrieco, A.; Mulè, G. Influence of light on growth, conidiation and fumonisin production by Fusarium verticillioides. Fungal Biol. 2012, 116, 241–248. [Google Scholar] [CrossRef]
- Yu, S.M.; Ramkumar, G.; Lee, Y.H. Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. J. Appl. Microbiol. 2013, 115, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Yang, Q.; Yang, X.; Zheng, Y.; Liu, Y.; Xing, F. Effects of light on the ochratoxigenic fungi Aspergillus ochraceus and A. carbonarius. Toxins 2021, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Canessa, P.; Schumacher, J.; Hevia, M.A.; Tudzynski, P.; Larrondo, L.F. Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: Characterization of the white collar complex. PLoS ONE 2013, 8, e84223. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Carter-Wientjes, C.; Williams, K.A.; Bhatnagar, D. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Lett. Appl. Microbiol. 2012, 55, 460–466. [Google Scholar] [CrossRef]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the comparative susceptibility of microbial species to photoinactivation using 380-480 nm violet-blue light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef]
- Trzaska, W.J.; Wrigley, H.E.; Thwaite, J.E.; May, R.C. Species-specific antifungal activity of blue light. Sci. Rep. 2017, 7, 4605. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: Phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol. 2008, 147, 790–801. [Google Scholar] [CrossRef]
- Xie, X.Z.; Xue, Y.J.; Zhou, J.J.; Zhang, B.; Chang, H.; Takano, M. Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol. Plant 2011, 4, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.Z.; Xiong, D.C.; Guo, F.; Li, Q.; Duan, J.; Ye, X.S. Light-driven highly efficient glycosylation reactions. Org. Chem. Front. 2016, 3, 737–743. [Google Scholar] [CrossRef]
- Sangwan, R.; Mandal, P.K. Recent advances in photoinduced glycosylation: Oligosaccharides, glycoconjugates and their synthetic applications. RSC Adv. 2017, 7, 26256–26321. [Google Scholar] [CrossRef]
- Spell, M.L.; Deveaux, K.; Bresnahan, C.G.; Bernard, B.L.; Sheffield, W.; Kumar, R.; Ragains, J.R. A visible-light-promoted o-glycosylation with a thioglycoside donor. Angew. Chemie Int. Ed. 2016, 55, 6515–6519. [Google Scholar] [CrossRef] [PubMed]
- Wever, W.J.; Cinelli, M.A.; Bowers, A.A. Visible light mediated activation and o-glycosylation of thioglycosides. Org. Lett. 2013, 15, 30–33. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, 40119656. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl. Environ. Microbiol. 2010, 76, 136–141. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Effect of fungicide application to control Fusarium head blight and 20 Fusarium and Alternaria mycotoxins in winter wheat (Triticum aestivum L.). World Mycotoxin J. 2015, 8, 499–510. [Google Scholar] [CrossRef]
- Sunic, K.; Kovac, T.; Loncaric, A.; Babic, J.; Sulyok, M.; Krska, R.; Drezner, G.; Spanic, V. Fusarium secondary metabolite content in naturally produced and artificially provoked FHB pressure in winter wheat. Agronomy 2021, 11, 2239. [Google Scholar] [CrossRef]
- Wipfler, R.; McCormick, S.P.; Proctor, R.; Teresi, J.; Hao, G.; Ward, T.; Alexander, N.; Vaughan, M. Synergistic phytotoxic effects of culmorin and trichothecene mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef]
- Dvorska, J.E.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Antioxidant systems of the developing quail embryo are compromised by mycotoxin aurofusarin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Jarolim, K.; Wolters, K.; Woelflingseder, L.; Pahlke, G.; Beisl, J.; Puntscher, H.; Braun, D.; Sulyok, M.; Warth, B.; Marko, D. The secondary Fusarium metabolite aurofusarin induces oxidative stress, cytotoxicity and genotoxicity in human colon cells. Toxicol. Lett. 2018, 284, 170–183. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research progress of safety of zearalenone: A review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Malz, S.; Grell, M.N.; Thrane, C.; Maier, F.J.; Rosager, P.; Felk, A.; Albertsen, K.S.; Salomon, S.; Bohn, L.; Schäfer, W.; et al. Identification of a gene cluster responsible for the biosynthesis of aurofusarin in the Fusarium graminearum species complex. Fungal Genet. Biol. 2005, 42, 420–433. [Google Scholar] [CrossRef]
- Janevska, S.; Arndt, B.; Niehaus, E.M.; Burkhardt, I.; Rösler, S.M.; Brock, N.L.; Humpf, H.U.; Dickschat, J.S.; Tudzynski, B. Gibepyrone biosynthesis in the rice pathogen Fusarium fujikuroi is facilitated by a small Polyketide Synthase gene cluster. J. Biol. Chem. 2016, 291, 27403–27420. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.T.; Gardiner, D.M.; Lysøe, E.; Fuertes, P.R.; Tudzynski, B.; Wiemann, P.; Sondergaard, T.E.; Giese, H.; Brodersen, D.E.; Sørensen, J.L. An update to polyketide synthase and non-ribosomal synthetase genes and nomenclature in Fusarium. Fungal Genet. Biol. 2015, 75, 20–29. [Google Scholar] [CrossRef]
- Tini, F.; Covarelli, L.; Cowger, C.; Sulyok, M.; Benincasa, P.; Beccari, G. Infection timing affects Fusarium poae colonization of bread wheat spikes and mycotoxin accumulation in the grain. J. Sci. Food Agric. 2022, 102, 6358–6372. [Google Scholar] [CrossRef]
- Miedaner, T.; Moldovan, M.; Ittu, M. Comparison of spray and point inoculation to assess resistance to Fusarium head blight in a multienvironment wheat. Trial Phytopathol. 2003, 93, 1068–1072. [Google Scholar] [CrossRef]
- Tosti, G.; Benincasa, P.; Cortona, R.; Falcinelli, B.; Farneselli, M.; Guiducci, M.; Onofri, A.; Pannacci, E.; Tei, F.; Giulietti, M. Growing lettuce under multispectral light-emitting diodes lamps with adjustable light intensity. Ital. J. Agron. 2017, 11, 57–62. [Google Scholar] [CrossRef]
- Tini, F.; Beccari, G.; Onofri, A.; Ciavatta, E.; Gardiner, D.M.; Covarelli, L. Fungicides may have differential efficacies towards the main causal agents of Fusarium head blight of wheat. Pest Manag. Sci. 2020, 76, 3738–3748. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Suproniene, S.; Nielsen, L.K.; Lazzaro, I.; Spliid, N.H.; Justesen, A.F. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Methods 2009, 76, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to ‘ggplot2’, R package version 2.1.2; CRAN; Wien, Austria, 2021. Available online: https://CRAN.R-project.org/package=GGally (accessed on 16 March 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 16 March 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón-Bustamante, M.; Tini, F.; Beccari, G.; Onofri, A.; Balducci, E.; Sulyok, M.; Covarelli, L.; Benincasa, P. Influence of Light Spectrum on Bread Wheat Head Colonization by Fusarium graminearum and on the Accumulation of Its Secondary Metabolites. Plants 2025, 14, 2013. https://doi.org/10.3390/plants14132013
Cerón-Bustamante M, Tini F, Beccari G, Onofri A, Balducci E, Sulyok M, Covarelli L, Benincasa P. Influence of Light Spectrum on Bread Wheat Head Colonization by Fusarium graminearum and on the Accumulation of Its Secondary Metabolites. Plants. 2025; 14(13):2013. https://doi.org/10.3390/plants14132013
Chicago/Turabian StyleCerón-Bustamante, Minely, Francesco Tini, Giovanni Beccari, Andrea Onofri, Emilio Balducci, Michael Sulyok, Lorenzo Covarelli, and Paolo Benincasa. 2025. "Influence of Light Spectrum on Bread Wheat Head Colonization by Fusarium graminearum and on the Accumulation of Its Secondary Metabolites" Plants 14, no. 13: 2013. https://doi.org/10.3390/plants14132013
APA StyleCerón-Bustamante, M., Tini, F., Beccari, G., Onofri, A., Balducci, E., Sulyok, M., Covarelli, L., & Benincasa, P. (2025). Influence of Light Spectrum on Bread Wheat Head Colonization by Fusarium graminearum and on the Accumulation of Its Secondary Metabolites. Plants, 14(13), 2013. https://doi.org/10.3390/plants14132013







