Botanical Authenticity of Miraruira Sold in the Amazonas State, Brazil, Based on Chemical Profiling Using DI-MS and Chemometric Analyses
Abstract
1. Introduction
2. Results and Discussion
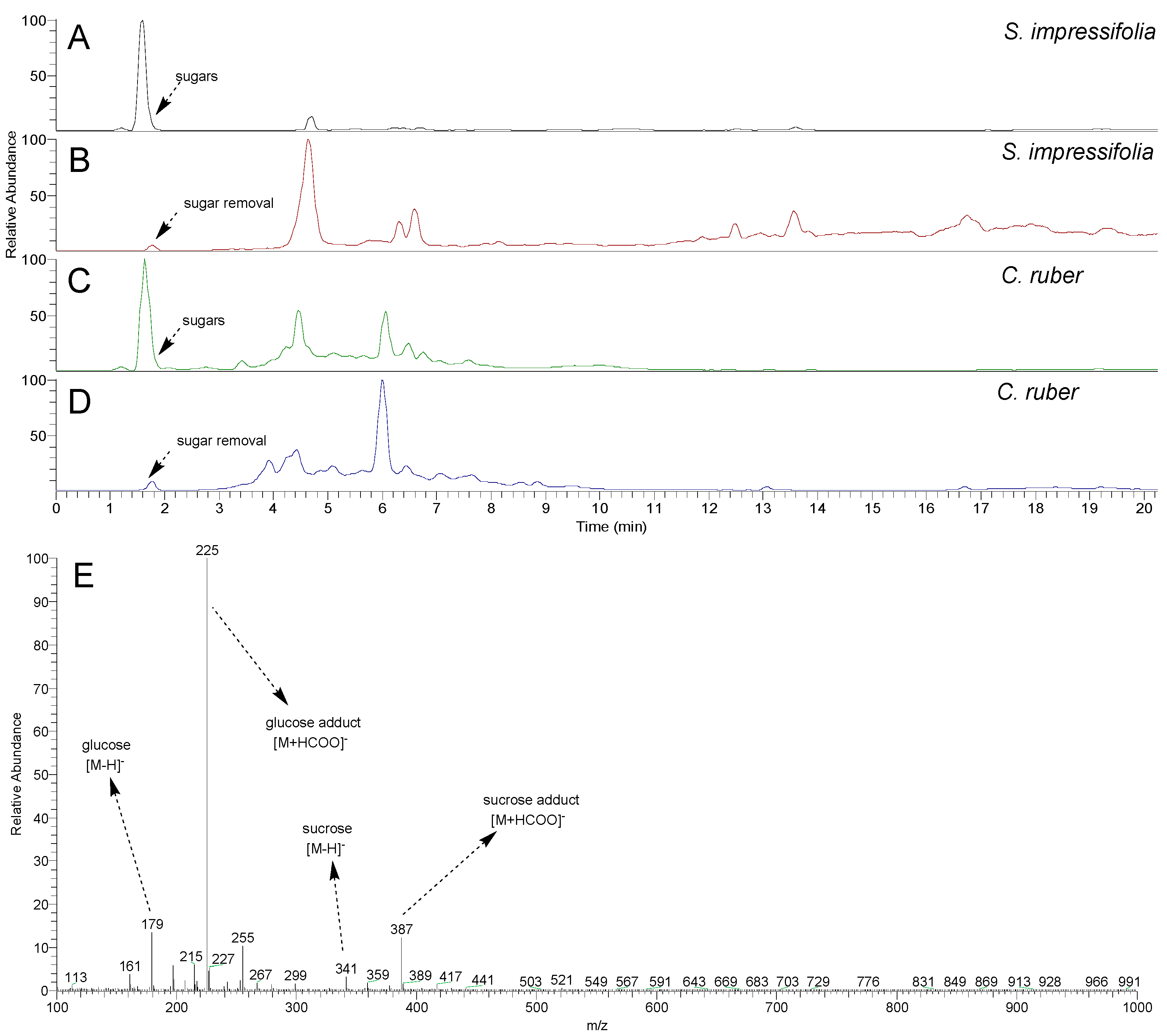
2.1. Clean-Up Evaluation Using HPLC-MS Analysis
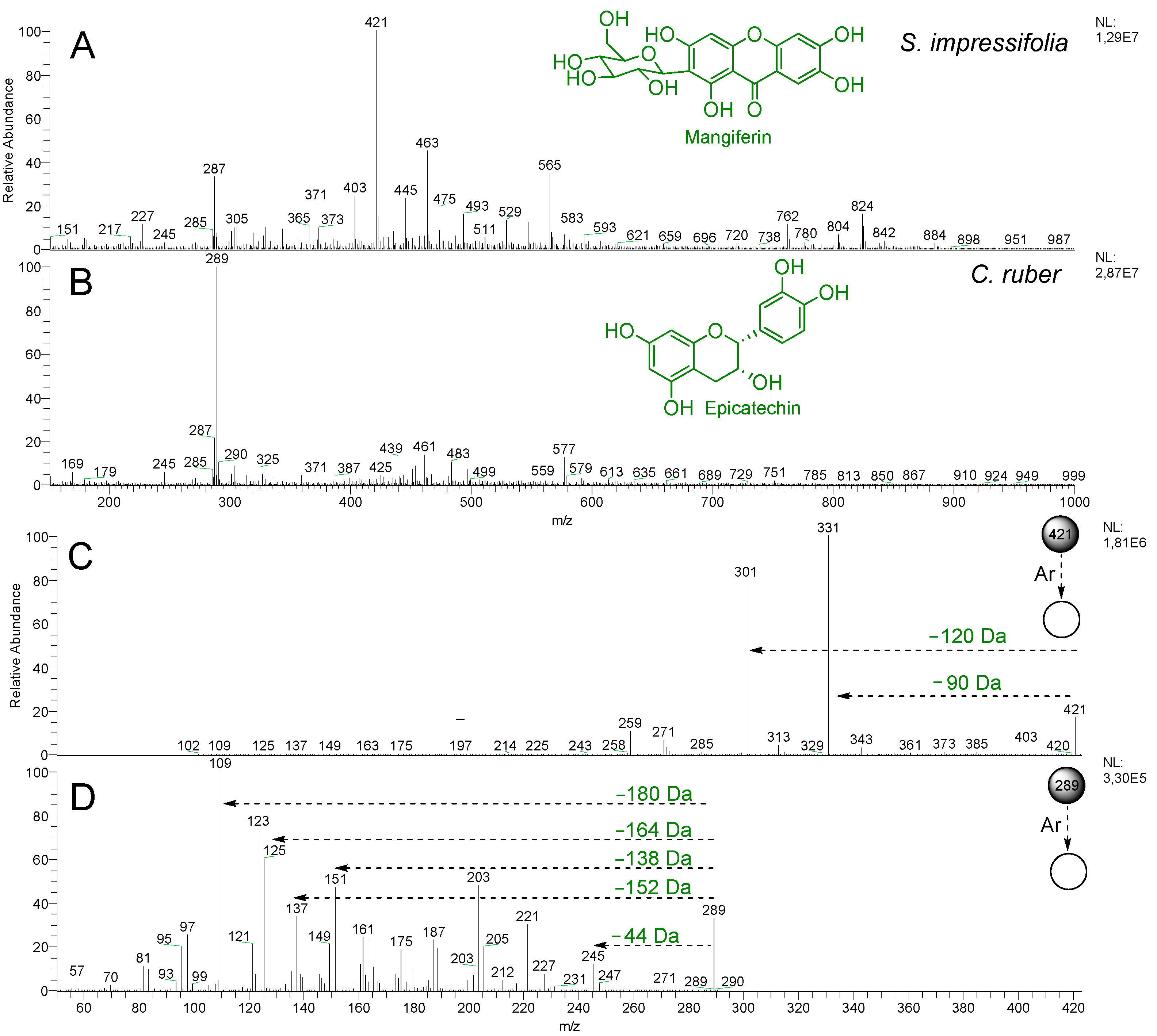
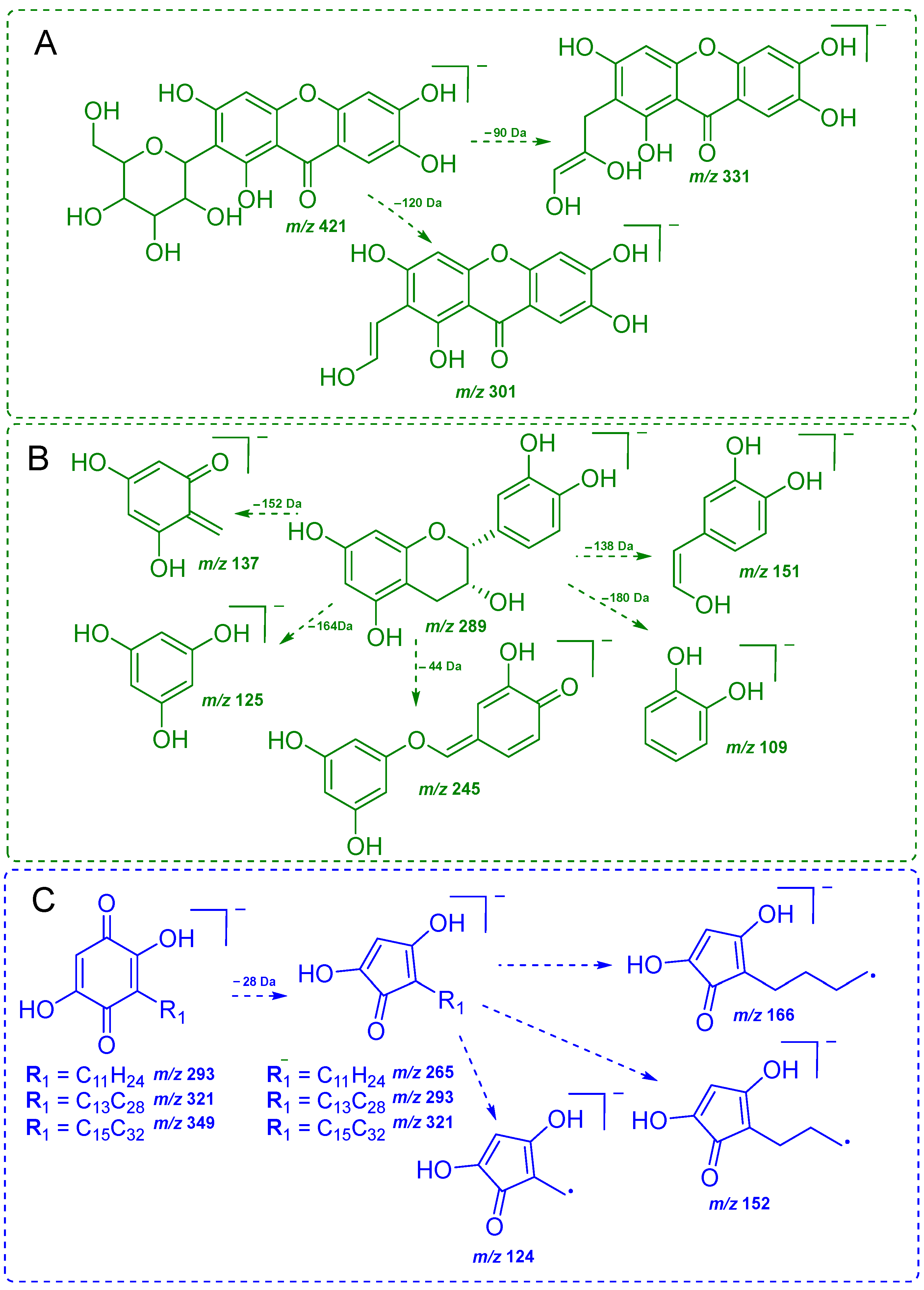
2.2. Chemical Profile via DI-MS and Chemometric Analysis
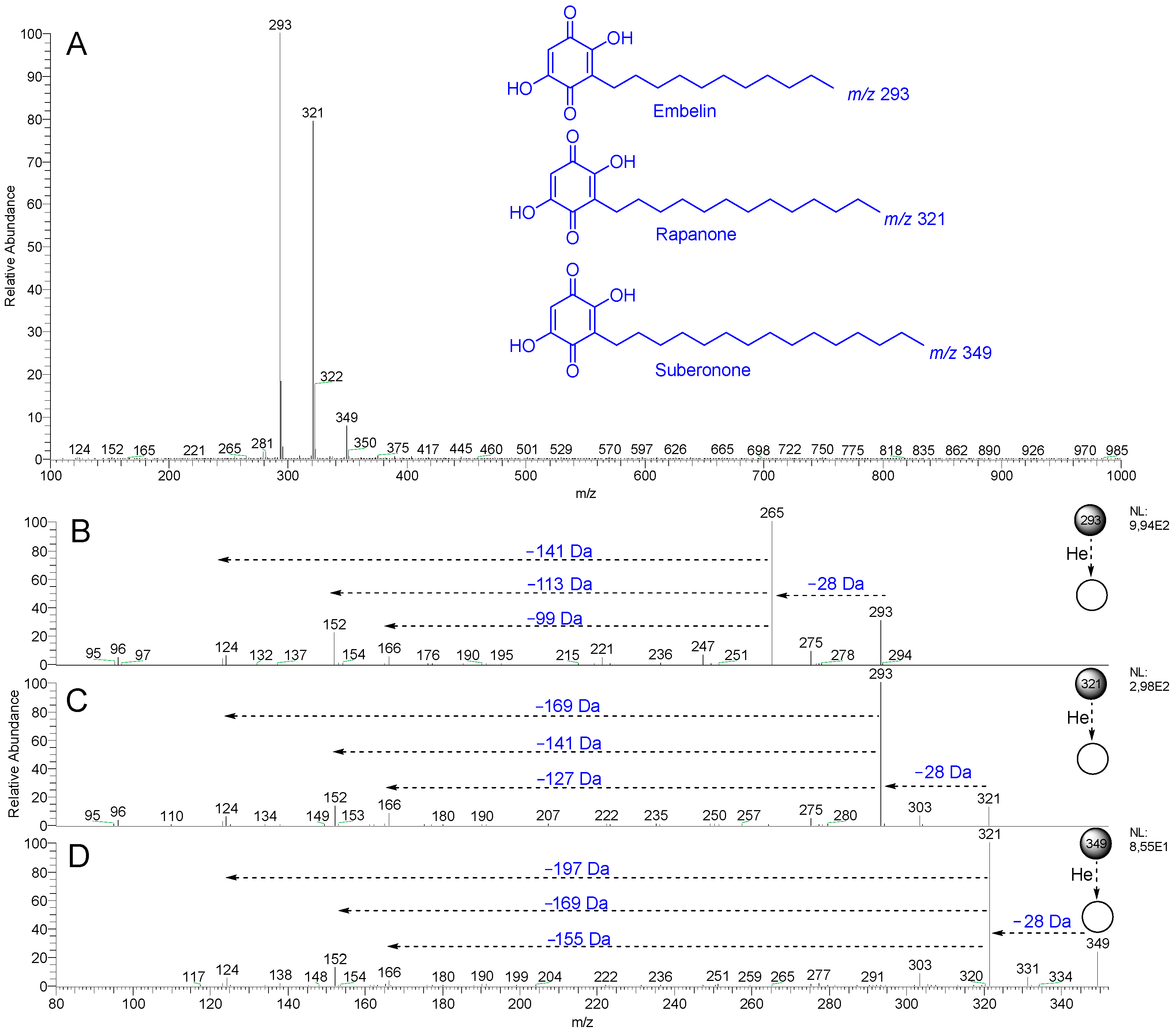
2.3. Screening of Benzoquinones in C. ruber and Commercial Samples of Miraruira
2.4. Quality Control Challenges in the Commercialization of MPBPs: Lessons from the Case of Miraruira
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Aqueous Extraction and Clean-Up Procedures
3.4. HPLC-MS and DI-MS Analyses
3.5. Chemometric Analysis
3.6. Screening of Benzoquinones in C. ruber and Commercial Samples of Miraruira
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPBP | Medicinal Plant-Based Product |
| DI-MS | Direct-Injection Mass Spectrometry |
| PCA | Principal Component Analysis |
| HCA | Hierarchical Cluster Analysis |
References
- Rodrigues, A.C.B.C.; Oliveira, F.P.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Costa, E.V.; Silva, F.M.A.; Rocha, W.C.; Koolen, H.H.F.; et al. In vitro and in vivo anti-leukemia activity of the stem bark of Salacia impressifolia (Miers) A.C. Smith (Celastraceae). J. Ethnopharmacol. 2019, 231, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Takenokuchi, M.; Matsumoto, K.; Nitta, Y.; Takasugi, R.; Inoue, Y.; Iwai, M.; Kadoyama, K.; Yoshida, K.; Takano-Ohmuro, H.; Taniguchi, T. In Vitro and In Vivo Antiglycation Effects of Connarus ruber Extract. Planta Medica 2022, 88, 1026–1035. [Google Scholar] [CrossRef]
- Silva, F.M.; Paz, W.H.; Vasconcelos, L.S.F.; Silva, A.L.; Silva-Filho, F.A.; Almeida, R.A.; Souza, A.D.L.; Pinheiro, M.L.B.; Koolen, H.H. Chemical constituents from Salacia impressifolia (Miers) AC Smith collected at the Amazon rainforest. Biochem. Syst. Ecol. 2016, 68, 77–80. [Google Scholar] [CrossRef]
- Nakamura, T.; Ishida, Y.; Ainai, K.; Nakamura, S.; Shirata, S.; Murayama, K.; Kurimoto, S.; Saigo, K.; Murashige, R.; Tsuda, S.Y.F. Genotoxicity-suppressing Effect of Aqueous Extract of Connarus ruber Cortex. Genes Environ. 2011, 33, 81–88. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef]
- Ota, A.; Ulrih, N.P. An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.G.C.; Coelho–Ferreira, M.; Santos, R.S. Perspectives on Medicinal Plants in Public Markets across the Amazon: A Review. Econ. Bot. 2016, 70, 64–78. [Google Scholar] [CrossRef]
- Brandão, M.G.L.; Cosenza, G.P.; Pereira, F.L.; Vasconcelos, A.S.; Fagg, C.W. Changes in the trade in native medicinal plants in Brazilian public markets. Environ. Monit. Assess. 2013, 185, 7013–7023. [Google Scholar] [CrossRef]
- Palhares, R.M.; Baratto, L.C.; Scopel, M.; Mügge, F.L.B.; Brandão, M.G.L. Medicinal Plants and Herbal Products From Brazil: How Can We Improve Quality? Front. Pharmacol. 2021, 11, 606623. [Google Scholar] [CrossRef]
- Ichim, M.C.; Booker, A. Chemical Authentication of Botanical Ingredients: A Review of Commercial Herbal Products. Front. Pharmacol. 2021, 12, 666850. [Google Scholar] [CrossRef]
- Grazina, L.; Mafra, I.; Monaci, L.; Amaral, J.S. Mass spectrometry-based approaches to assess the botanical authenticity of dietary supplements. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3870–3909. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.; Engelhard, C. Characteristics of Low-Temperature Plasma Ionization for Ambient Mass Spectrometry Compared to Electrospray Ionization and Atmospheric Pressure Chemical Ionization. Anal. Chem. 2012, 84, 10657–10664. [Google Scholar] [CrossRef] [PubMed]
- Rijke, E.; Zappey, H.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Liquid chromatography with atmospheric pressure chemical ionization and electrospray ionization mass spectrometry of flavonoids with triple-quadrupole and ion-trap instruments. J. Chromatogr. A 2003, 984, 45–58. [Google Scholar] [CrossRef]
- Silva, F.M.A.; Silva-Filho, F.A.; Lima, B.R.; Almeida, R.A.; Soares, E.R.; Koolen, H.H.F.; Souza, A.D.L.; Pinheiro, M.L.B. Chemotaxonomy of the Amazonian Unonopsis Species Based on Leaf Alkaloid Fingerprint Direct Infusion ESI-MS and Chemometric Analysis. J. Braz. Chem. Soc. 2016, 27, 599–604. [Google Scholar]
- Guignard, C.; Jouve, L.; Bogéat-Triboulot, M.B.; Dreyer, E.; Hausman, J.F.; Hoffmann, L. Analysis of carbohydrates in plants by high-performance anion-exchange chromatography coupled with electrospray mass spectrometry. J. Chromatogr. A 2005, 1085, 137–142. [Google Scholar] [CrossRef]
- Trevisan, M.T.S.; De Almeida, R.F.; Soto, G.; Virginio Filho, E.M.; Ulrich, C.M.; Owen, R.W. Quantitation by HPLC-UV of Mangiferin and Isomangiferin in Coffee (Coffea arabica) Leaves from Brazil and Costa Rica After Solvent Extraction and Infusion. Food Anal. Methods 2016, 9, 2649–2655. [Google Scholar] [CrossRef]
- Girón, M.D.; Sevillano, N.; Salto, R.; Haidour, A.; Manzano, M.; Jiménez, M.L.; Rueda, R.; López-Pedrosa, J.M. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: Role of mangiferin. Clin. Nutr. 2009, 28, 565–574. [Google Scholar] [CrossRef]
- Shimada, T.; Nagai, E.; Harasawa, Y.; Watanabe, M.; Negishi, K.; Akase, T.; Sai, Y.; Miyamoto, K.; Aburada, M. Salacia reticulata inhibits differentiation of 3T3-L1 adipocytes. J. Ethnopharmacol. 2011, 136, 67–74. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Arulselvan, P.; Muniappan, B.P.; Fakurazi, S.; Kandasamy, M. Mangiferin from Salacia chinensis Prevents Oxidative Stress and Protects Pancreatic β-Cells in Streptozotocin-Induced Diabetic Rats. J. Med. Food 2013, 16, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kaliappan, I.; Kammalla, A.K.; Ramasamy, M.K.; Aruna, A.; Dubey, G.P. LC-MS Quantification of Mangiferin inhydroalcoholic extract of Salacia oblonga, Salacia roxburghii and polyherbal formulation. Int. J. Phytopharm. 2014, 4, 11–15. [Google Scholar]
- Souza, L.M.; Cipriani, T.R.; Iacomini, M.; Gorin, P.A.; Sassaki, G.L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal. 2008, 47, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, M.M.; Muema, F.W.; Kimutai, F.; Xu, Y.B.; Zhang, H.; Chen, G.L.; Guo, M.Q. Antioxidant and Antiproliferative Potentials of Ficus glumosa and Its Bioactive Polyphenol Metabolites. Pharmaceuticals 2021, 14, 266. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (−)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.Á.; Fernández-Millán, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Sistema de Informação Sobre a Biodiversidade Brasileira. Herbarium—Instituto Nacional de Pesquisas da Amazônia (INPA). Available online: https://collectory.sibbr.gov.br/collectory/public/show/dr799 (accessed on 1 May 2025).
- Silva, R.L.; Demarque, D.P.; Dusi, R.G.; Sousa, J.P.B.; Albernaz, L.C.; Espindola, L.S. Residual Larvicidal Activity of Quinones against Aedes aegypti. Molecules 2020, 25, 3978. [Google Scholar] [CrossRef]
- Mahendran, S.; Badami, S.; Maithili, V. Evaluation of antidiabetic effect of embelin from Embelia ribes in alloxan induced diabetes in rats. Biomed. Prev. Nutr. 2011, 1, 25–31. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, A.K.; Sharma, M.C.; Gupta, R.S. Antioxidant activity and protection of pancreatic β-cells by embelin in streptozotocin-induced diabetes. J. Diabetes 2012, 4, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Niture, N.T.; Ansari, A.A.; Shah, P.D. Anti-diabetic activity of embelin: Involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 2013, 20, 797–804. [Google Scholar] [CrossRef]
- Queiroz, L.C.; Neves, K.O.G.; Monaretto, T.; Costa, L.A.M.A.; Colnago, L.A.; Santos, A.D.C.; Machado, M.B. Determination of adulterants in copaiba Oil-Resin using 1H NMR. Microchem. J. 2025, 213, 113732. [Google Scholar] [CrossRef]
- Carvalho, A.C.B.; Lana, T.N.; Perfeito, J.P.S.; Silveira, D. The Brazilian market of herbal medicinal products and the impacts of the new legislation on traditional medicines. J. Ethnopharmacol. 2018, 212, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.C.A.; Godinho, J.W.L.S.; Ferreira, T.T.D.; Luz, T.R.S.A.; Leite, J.A.C.; Moraes, D.F.C.; Amaral, F.M.M. Trade and quality control of medicinal plants in brazil. Int. J. Pharm. Pharm. Sci. 2016, 8, 32–39. [Google Scholar] [CrossRef]
- Silva, F.M.A.; Hanna, A.C.S.; Souza, A.A.; Silva-Filho, F.A.; Canhoto, O.M.F.; Magalhães, A.; Benevides, P.J.C.; Azevedo, M.B.M.; Siani, A.C.; Pohlit, A.M.; et al. Integrative Analysis Based on HPLC-DAD-MS/MS and NMR of Bertholletia excelsa Bark Biomass Residues: Determination of Ellagic Acid Derivatives. J. Braz. Chem. Soc. 2019, 30, 830–836. [Google Scholar] [CrossRef]
- Bastos, L.M.; Silva, F.M.A.; Souza, L.R.S.; Sá, I.S.C.; Silva, R.M.; Souza, A.D.L.; Nunomura, R.C.S. Integrative Approach Based on Simplex-Centroid Design, ESI-MS and Chemometric Analysis for Comprehensive Characterization of Phenolic Compounds from Endopleura uchi Bark. J. Braz. Chem. Soc. 2020, 31, 351–356. [Google Scholar] [CrossRef]
- Nunes, C.A.; Freitas, M.P.; Pinheiro, A.C.M.; Bastos, S.C. Chemoface: A novel free user-friendly interface for chemometrics. J. Braz. Chem. Soc. 2012, 23, 2003–2010. [Google Scholar] [CrossRef]
- Morais, L.S.; Dusi, R.G.; Demarque, D.P.; Silva, R.L.; Albernaz, L.C.; Bao, S.N.; Merten, C.; Antinarelli, L.M.R.; Coimbra, E.S.; Espindola, L.S. Antileishmanial compounds from Connarus suberosus: Metabolomics, isolation and mechanism of action. PLoS ONE 2020, 15, e0241855. [Google Scholar] [CrossRef]
- Aiyar, S.N.; Jain, M.K.; Krishnamurti, M.; Seshadri, T.R. Chemical components of the roots of Connarus monocarpus. Phytochemistry 1964, 3, 335–339. [Google Scholar] [CrossRef]

| Samples | Origin | Label Information |
|---|---|---|
| A | Market | Therapeutic indication, preparation method, Dosage, and administration |
| B | Fair | Therapeutic indication, dosage, and administration |
| C | Emporium | No information |
| D | Emporium | Botanical name a, therapeutic indication, preparation method, dosage, and administration |
| E | Emporium | Botanical name a, therapeutic indication, preparation method, dosage, and administration |
| F | Fair | Botanical name a, therapeutic indication, preparation method, dosage, and administration |
| G | Emporium | Preparation method, dosage, and administration |
| H | Emporium | Preparation method, dosage, and administration |
| I | Fair | No information |
| J | Market | No information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, S.M.d.R.; da Silva, F.M.A.; Bataglion, G.A.; de Almeida, M.G.A.; de Souza, L.O.; França, R.d.S.; de Souza, C.A.S.; da Silva-Filho, F.A.; de Souza, A.D.L.; Koolen, H.H.F.; et al. Botanical Authenticity of Miraruira Sold in the Amazonas State, Brazil, Based on Chemical Profiling Using DI-MS and Chemometric Analyses. Plants 2025, 14, 2012. https://doi.org/10.3390/plants14132012
Braga SMdR, da Silva FMA, Bataglion GA, de Almeida MGA, de Souza LO, França RdS, de Souza CAS, da Silva-Filho FA, de Souza ADL, Koolen HHF, et al. Botanical Authenticity of Miraruira Sold in the Amazonas State, Brazil, Based on Chemical Profiling Using DI-MS and Chemometric Analyses. Plants. 2025; 14(13):2012. https://doi.org/10.3390/plants14132012
Chicago/Turabian StyleBraga, Shelson M. da R., Felipe M. A. da Silva, Giovana A. Bataglion, Marcia G. A. de Almeida, Larissa O. de Souza, Rebeca dos S. França, Cesar A. S. de Souza, Francinaldo A. da Silva-Filho, Afonso D. L. de Souza, Hector H. F. Koolen, and et al. 2025. "Botanical Authenticity of Miraruira Sold in the Amazonas State, Brazil, Based on Chemical Profiling Using DI-MS and Chemometric Analyses" Plants 14, no. 13: 2012. https://doi.org/10.3390/plants14132012
APA StyleBraga, S. M. d. R., da Silva, F. M. A., Bataglion, G. A., de Almeida, M. G. A., de Souza, L. O., França, R. d. S., de Souza, C. A. S., da Silva-Filho, F. A., de Souza, A. D. L., Koolen, H. H. F., & Pinheiro, M. L. B. (2025). Botanical Authenticity of Miraruira Sold in the Amazonas State, Brazil, Based on Chemical Profiling Using DI-MS and Chemometric Analyses. Plants, 14(13), 2012. https://doi.org/10.3390/plants14132012







